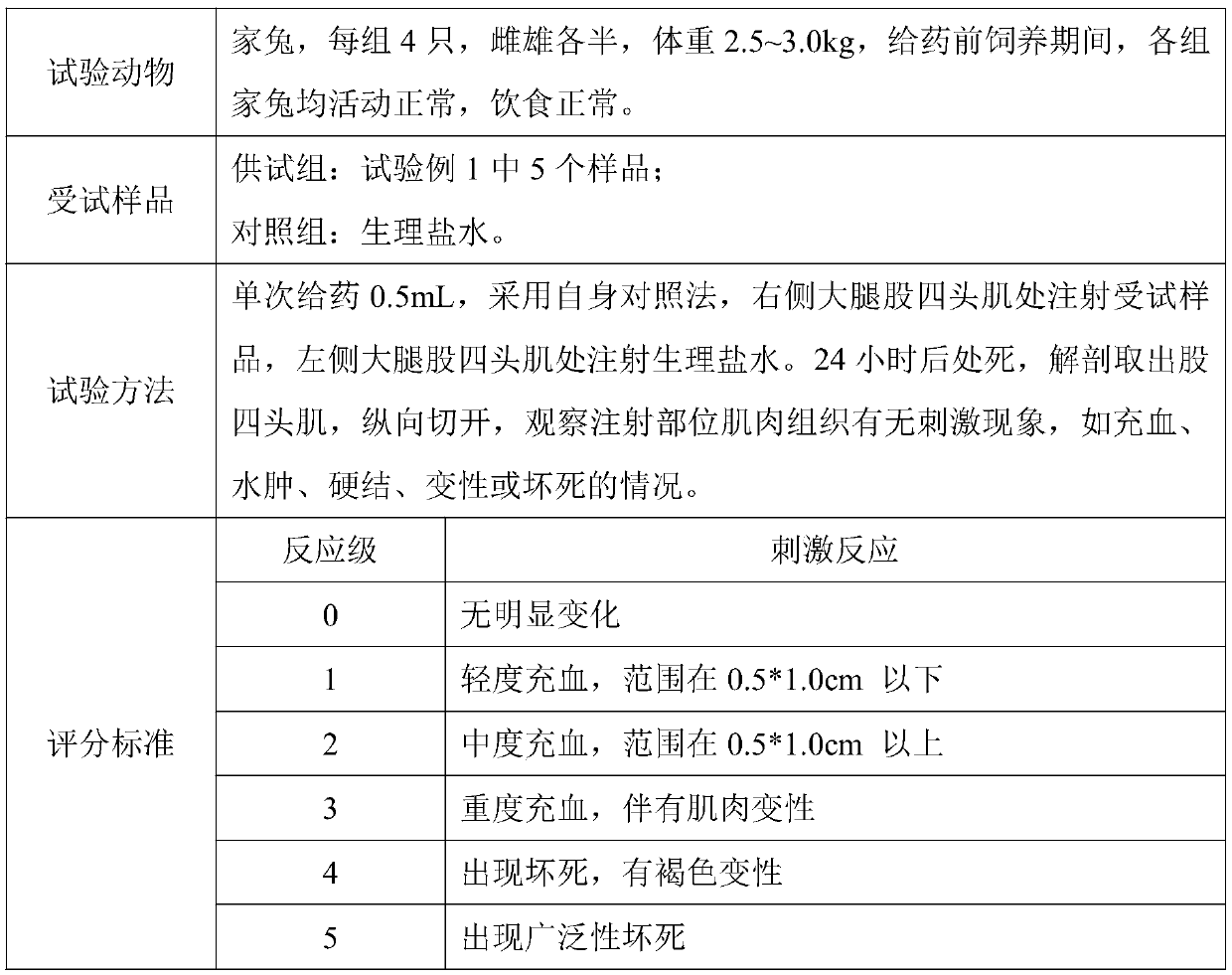
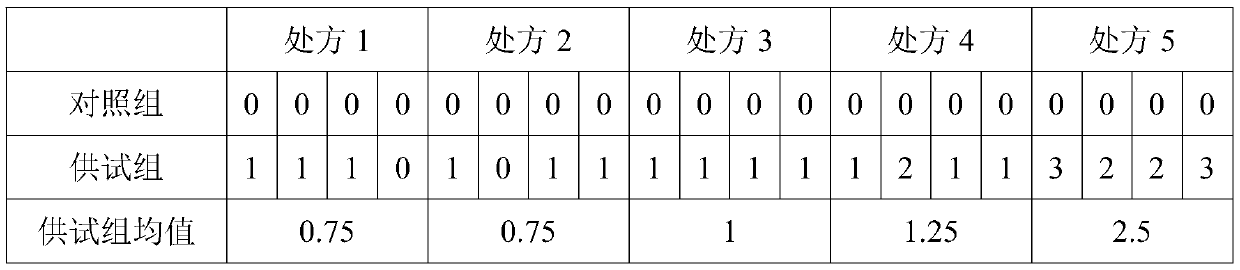
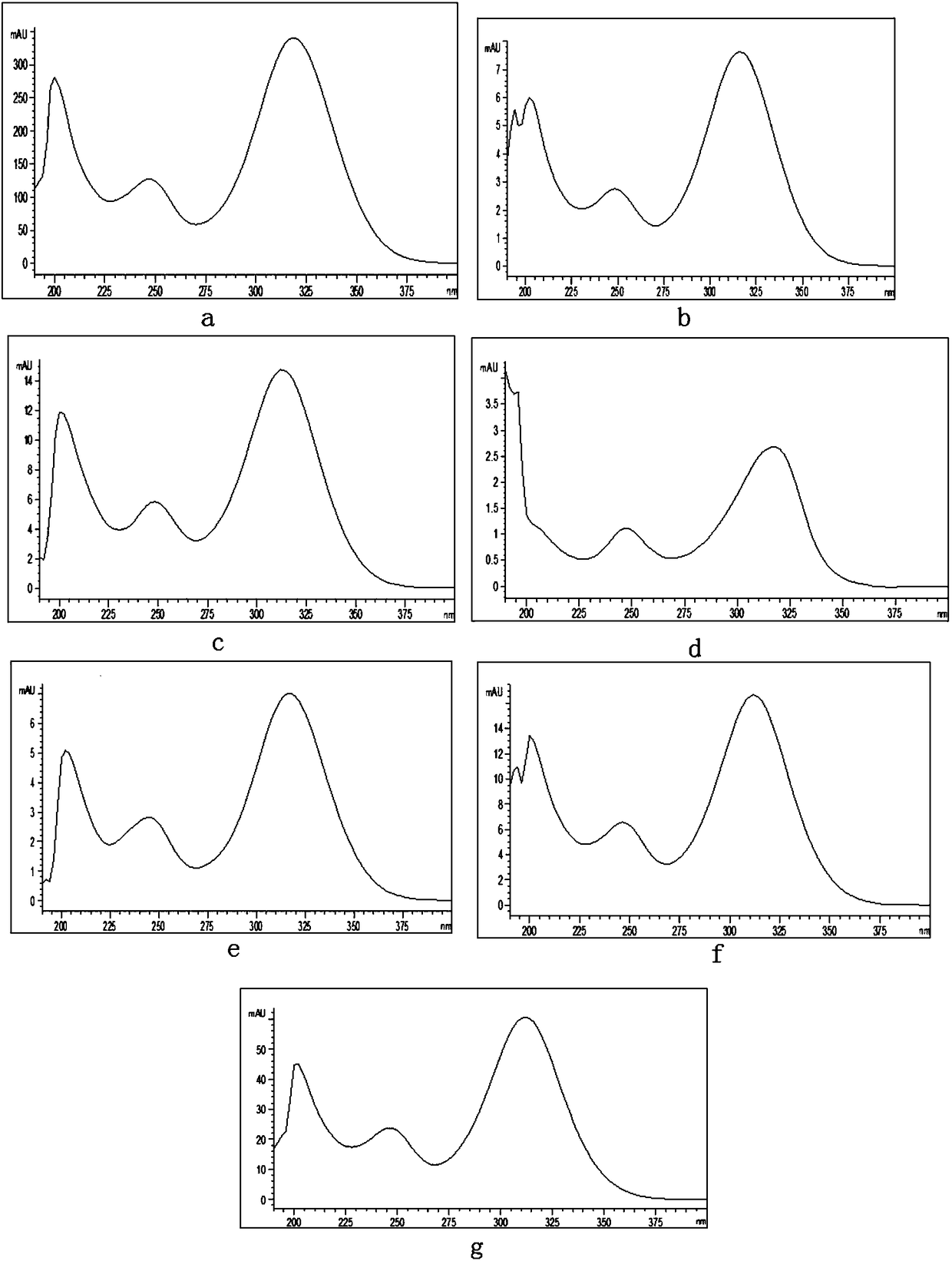
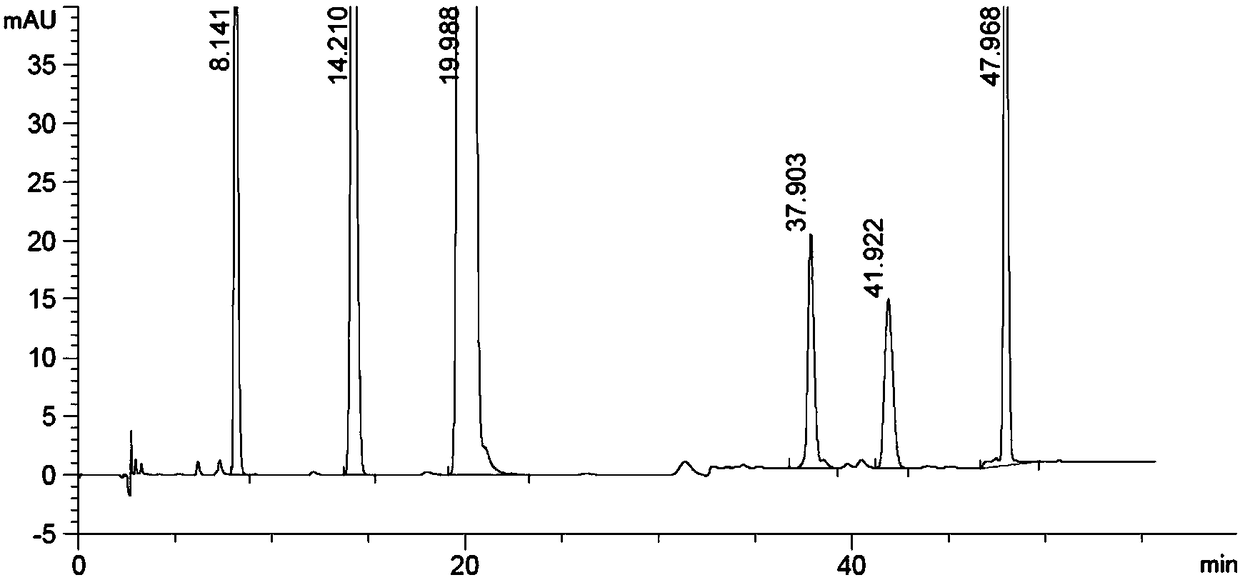
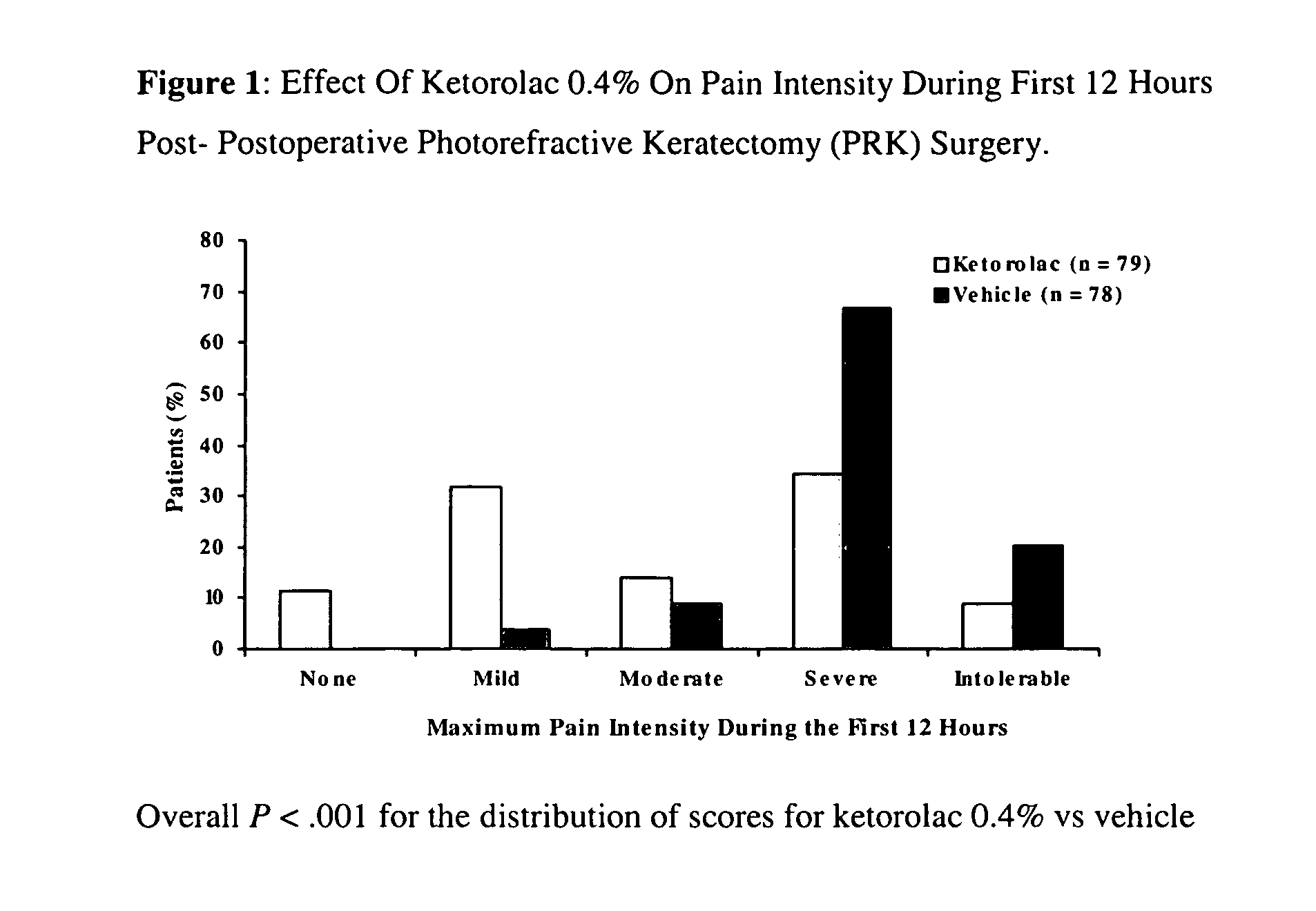
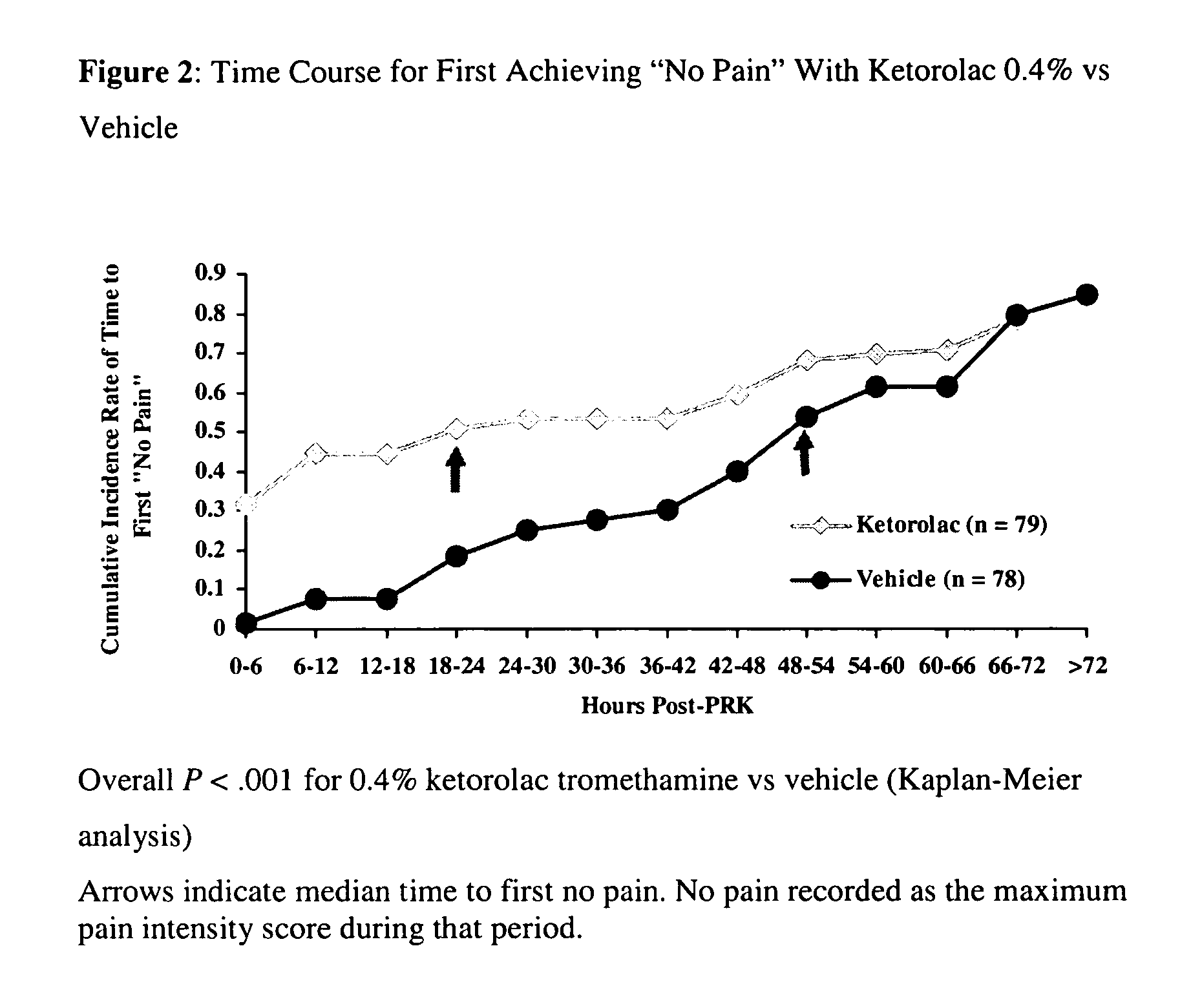
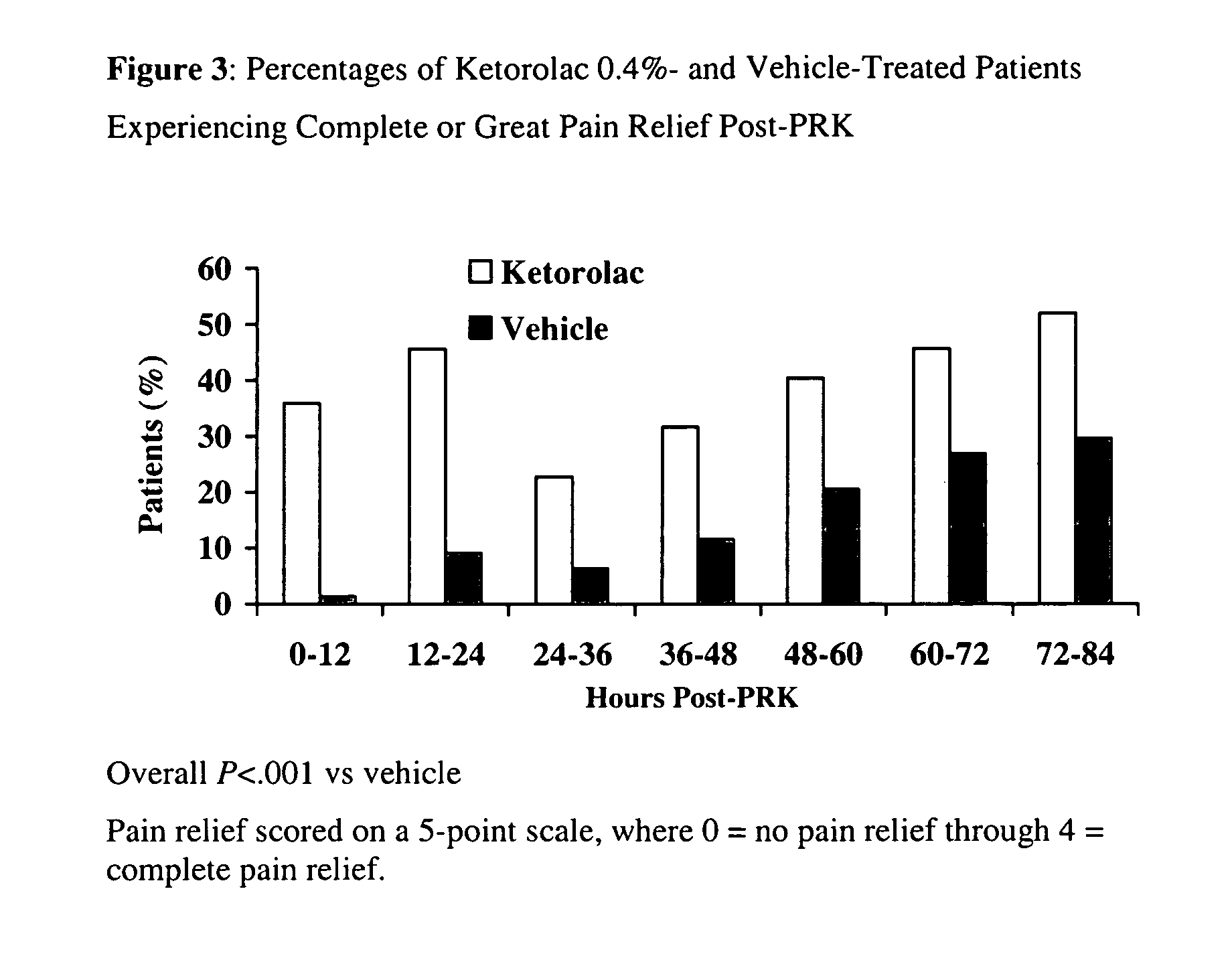
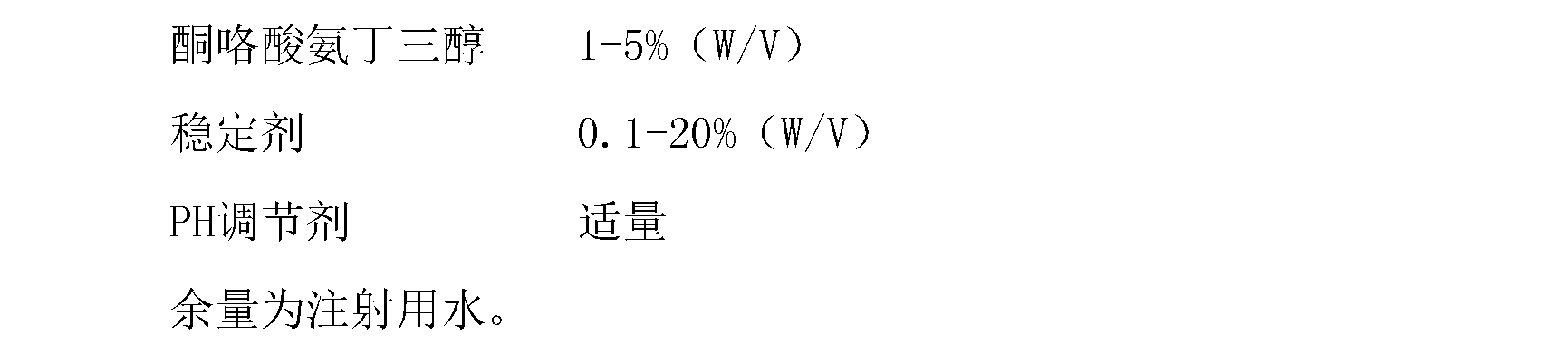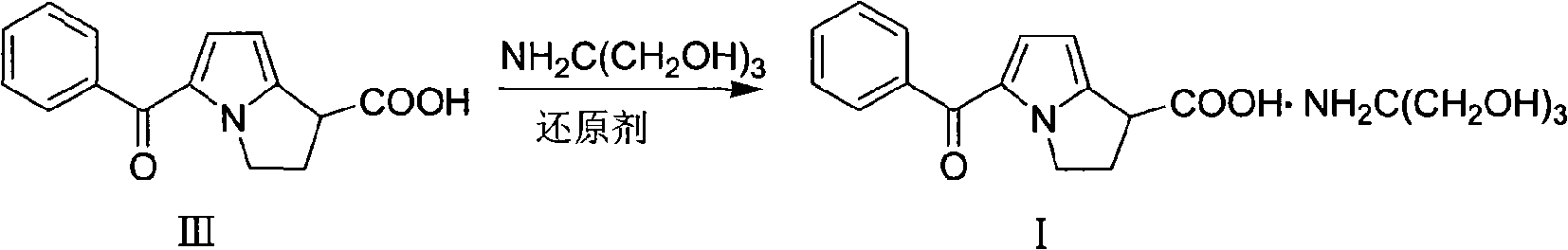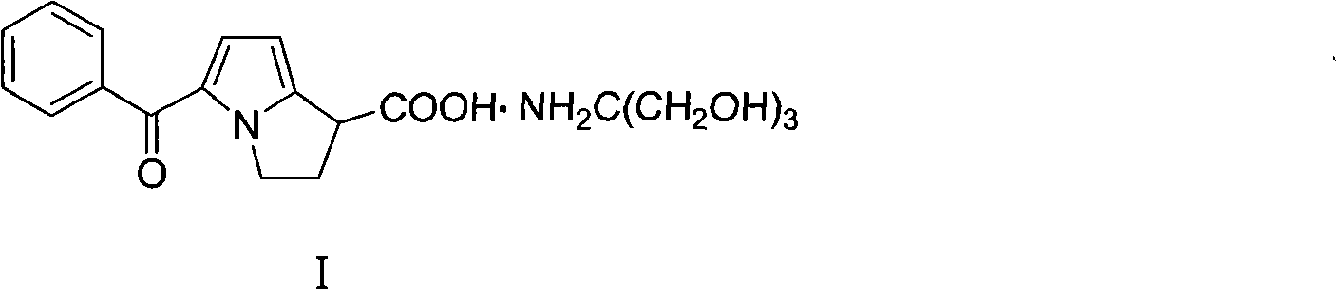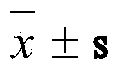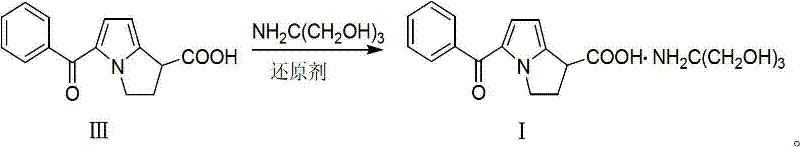Patents
Literature
63 results about "Ketorolac Tromethamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
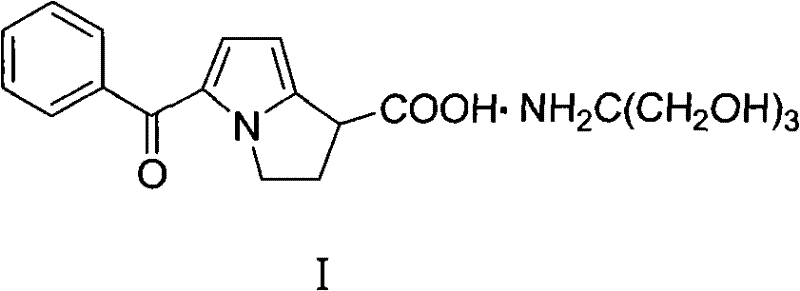
The tromethamine salt of ketorolac, a synthetic pyrrolizine carboxylic acid derivative with anti-inflammatory, analgesic and antipyretic properties. Ketorolac tromethamine, a non-selective inhibitor of the cyclooxygenases (COX), inhibits both COX-1 and COX-2 enzymes. This agent exerts its anti-inflammatory effect by preventing conversion of arachidonic acid to prostaglandins at inflammation site mediated through inhibition of COX-2, which is undetectable in most tissues but is up-regulated at the inflammation sites. Since COX-1 is expressed virtually in all tissues, inhibition of COX-1 enzyme by this agent prevents normal state production of prostaglandins, which plays housekeeping roles in the protection of the gastrointestinal tract, regulating renal blood flow, and functioning in platelet aggregation. As a result, inhibition of COX-1 is usually associated with adverse effects such as gastrointestinal toxicity and nephrotoxicity.
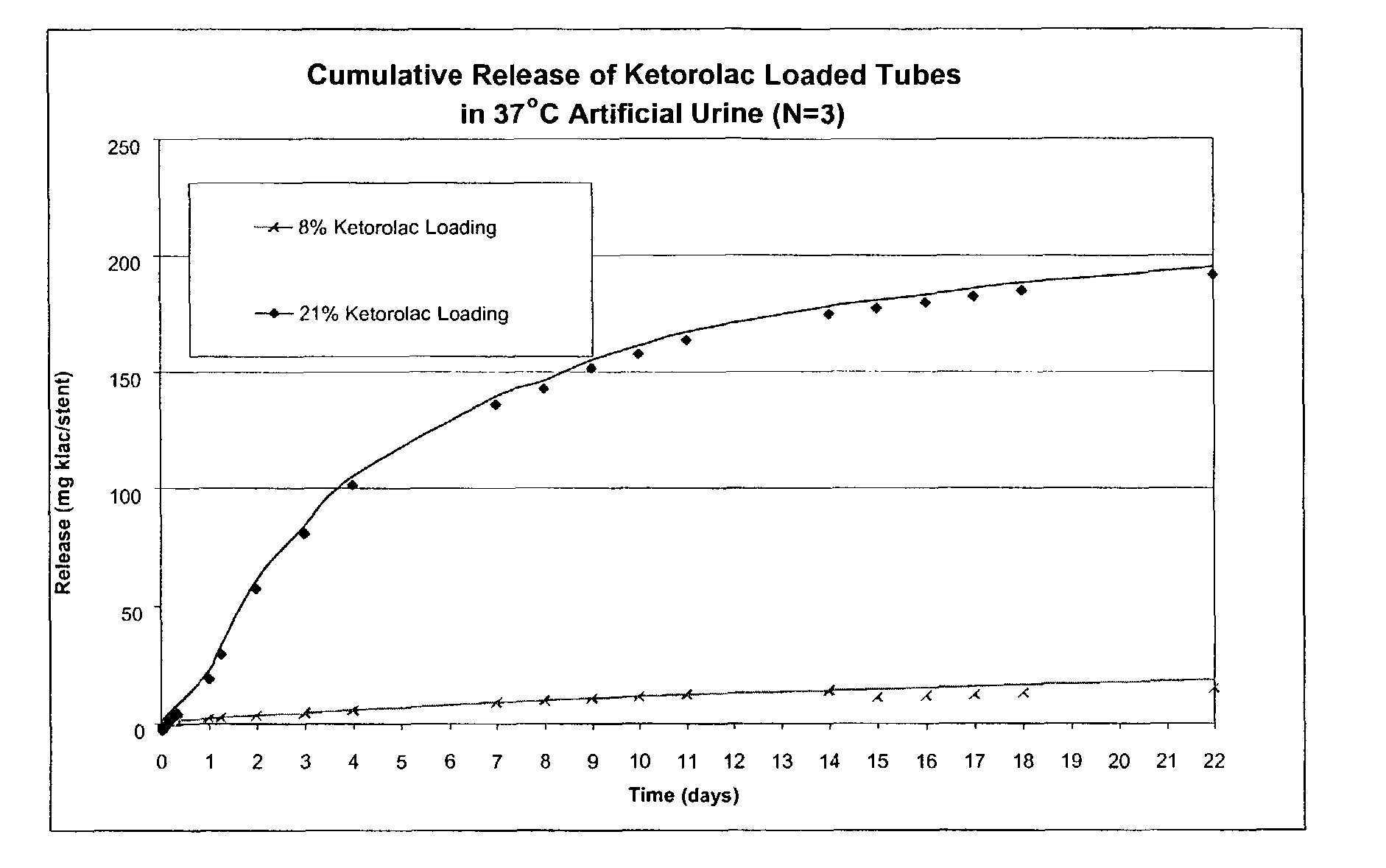
Implantable or insertable medical devices for controlled drug delivery
Implantable or insertable medical devices are provided, which comprises: (a) a biocompatible polymer; and (b) at least one therapeutic agent selected from an anti-inflammatory agent, an analgesic agent, an anesthetic agent, and an antispasmodic agent. The medical devices are adapted for implantation or insertion at a site associated with pain or discomfort upon implantation or insertion. In many embodiments, the therapeutic will be selected from at least one of (i) ketorolac and pharmaceutically acceptable salts thereof (e.g., ketorolac tromethamine) and (ii) 4-diethylamino-2-butynylphenylcyclohexyl glycolate and pharmaceutically acceptable salts thereof (e.g., oxybutynin chloride). Also provided are uses for the implantable or insertable medical devices, which uses comprise reducing pain or discomfort accompanying the implantation or insertion of such devices. Further uses may comprise reducing microbial buildup along the device. Methods for manufacturing implantable or insertable medical devices are also provided.
Owner:BOSTON SCI SCIMED INC
Implantable or insertable medical devices for controlled drug delivery
Implantable or insertable medical devices are provided, which comprises: (a) a biocompatible polymer; and (b) at least one therapeutic agent selected from an anti-inflammatory agent, an analgesic agent, an anesthetic agent, and an antispasmodic agent. The medical devices are adapted for implantation or insertion at a site associated with pain or discomfort upon implantation or insertion. In many embodiments, the therapeutic will be selected from at least one of (i) ketorolac and pharmaceutically acceptable salts thereof (e.g., ketorolac tromethamine) and (ii) 4-diethylamino-2-butynylphenylcyclohexyl glycolate and pharmaceutically acceptable salts thereof (e.g., oxybutynin chloride). Also provided are uses for the implantable or insertable medical devices, which uses comprise reducing pain or discomfort accompanying the implantation or insertion of such devices. Further uses may comprise reducing microbial buildup along the device. Methods for manufacturing implantable or insertable medical devices are also provided.
Owner:BOSTON SCI SCIMED INC
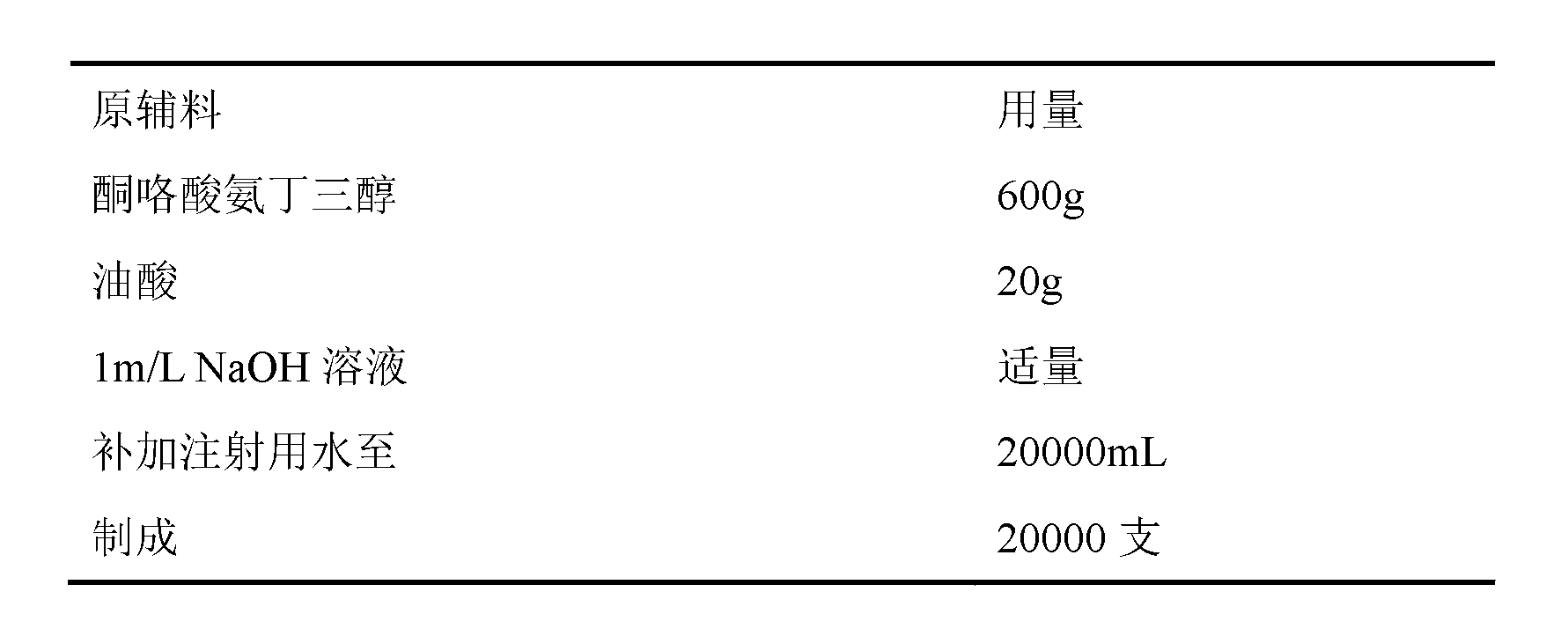
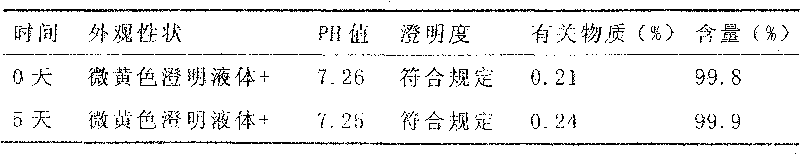
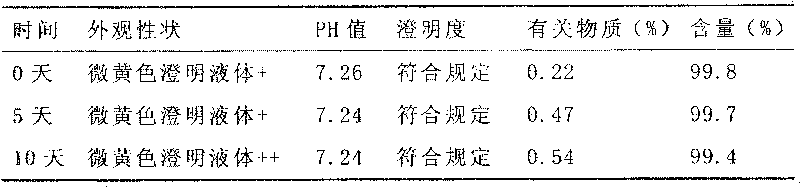
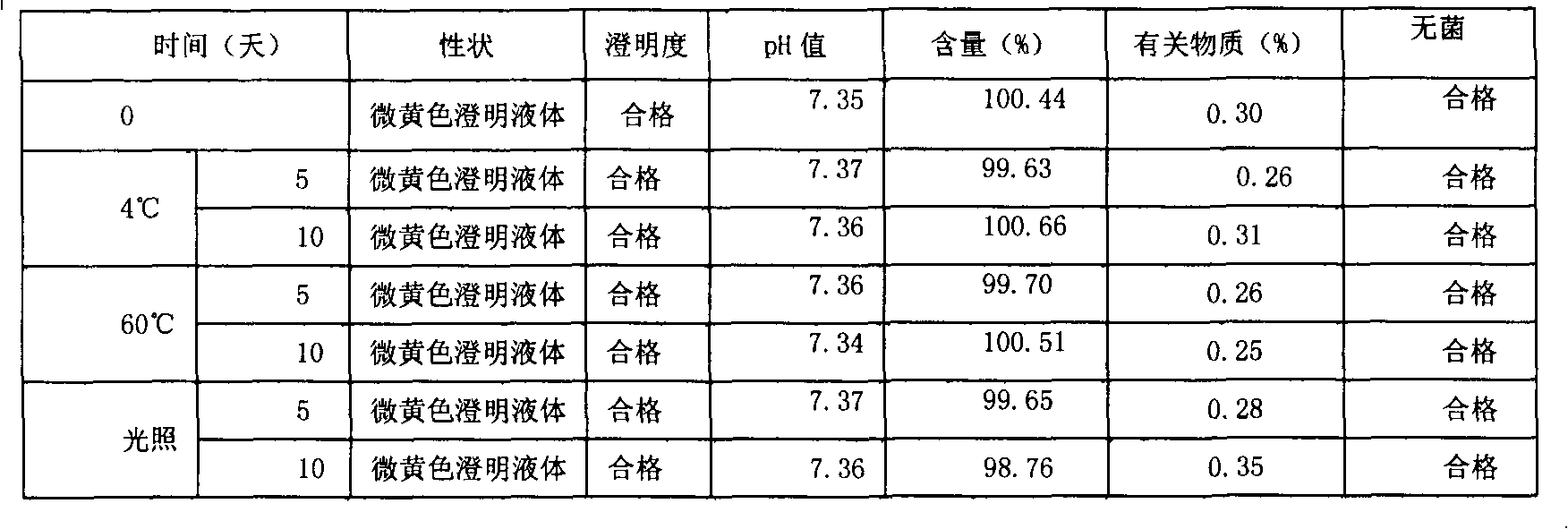
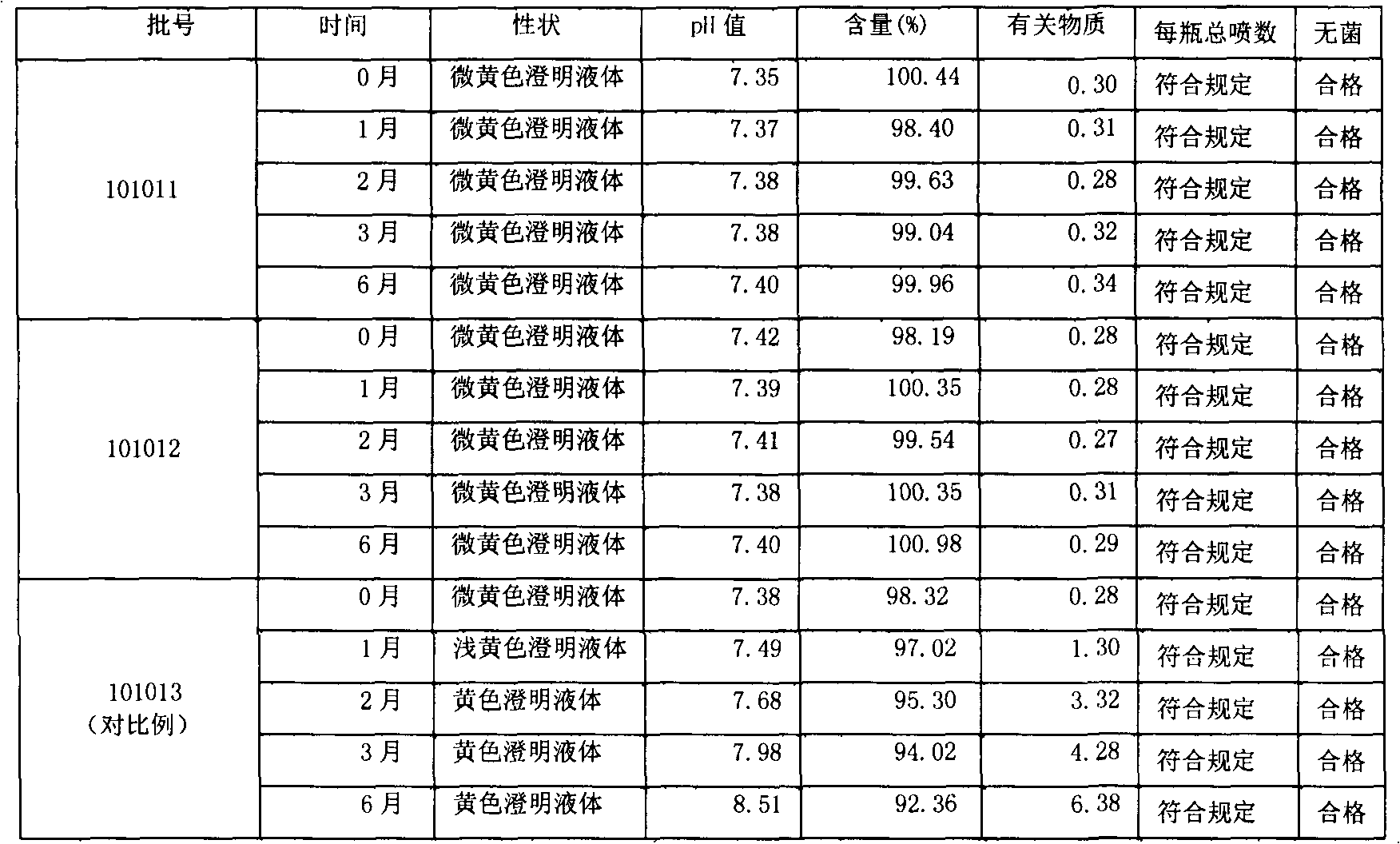
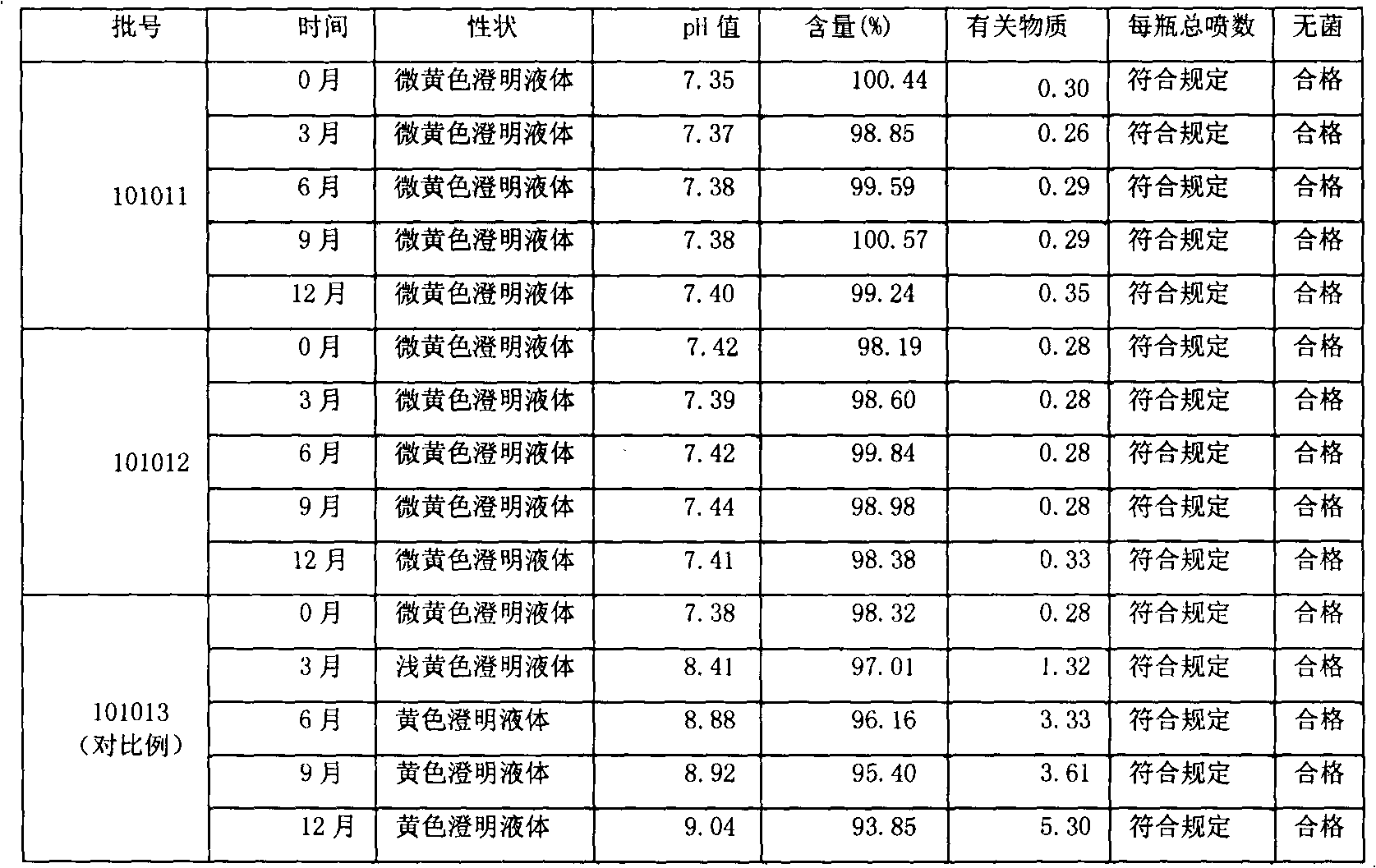
Ketoralac ammonia butanetriol injection and preparing method thereof
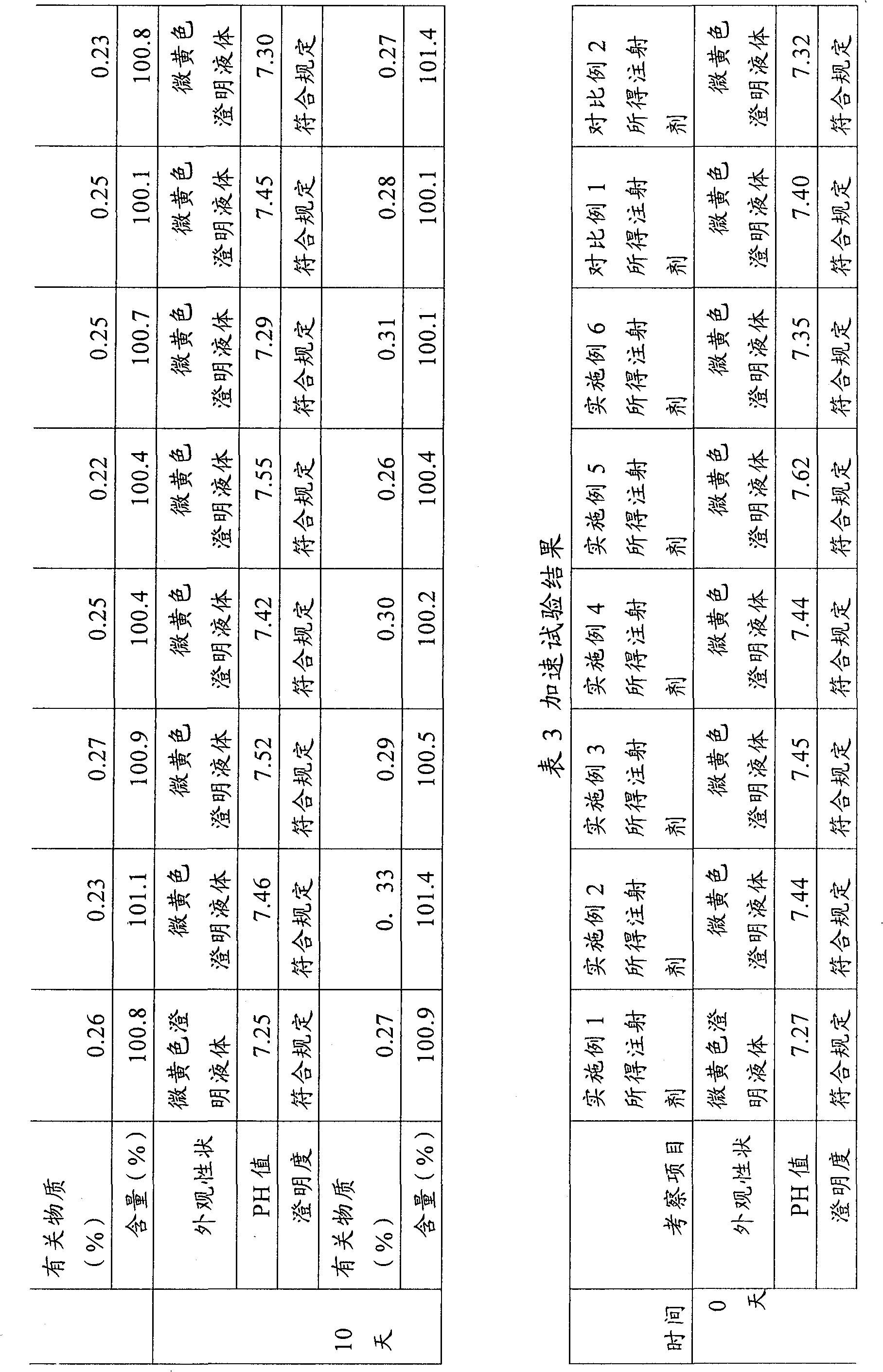
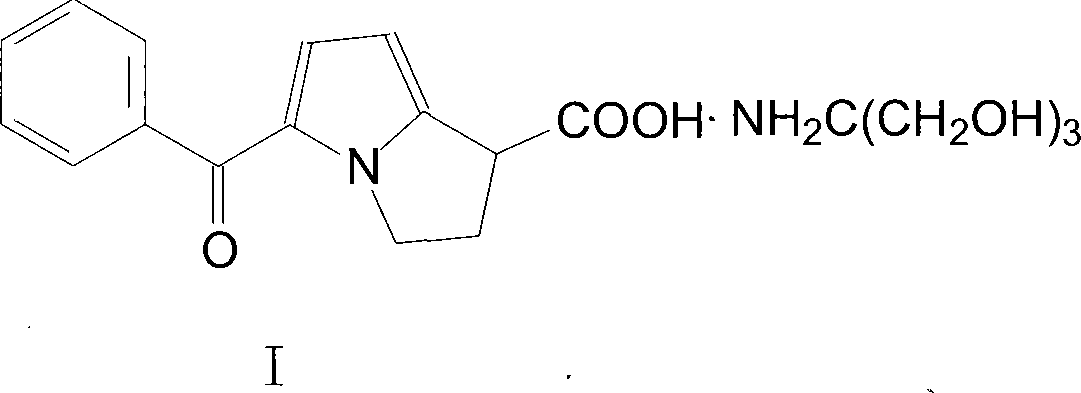
ActiveCN101199514ASolve easy discolorationLow content of related substancesOrganic active ingredientsAntipyreticAcid derivativeKetorolac Tromethamine
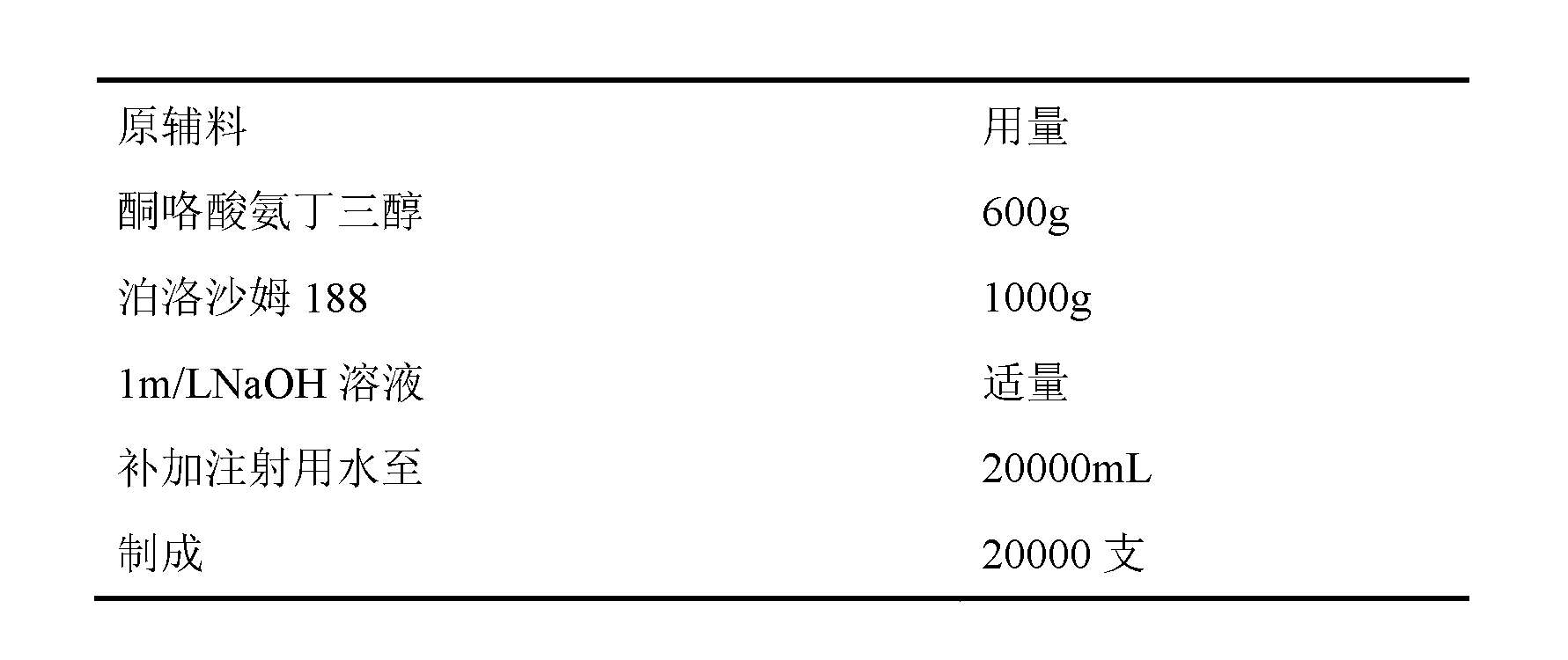
Disclosed is ketorolac tromethamine, which is a pyrrole acid derivative developed by the Syntex company in the U.S. The invention can effectively inhibit prostaglandin (PG) from synthesizing with functions of analgesia, anti-inflammation, antipyresis and inhibiting the platelets from aggregating. The invention provides a preparation method of ketorolac tromethamine injection, which well improves the stability and the clarity of the preparation with stable process.
Owner:鲁南新时代生物技术有限公司
Ketorolac tromethamine injection
The invention discloses a ketorolac tromethamine injection characterized by being prepared from the following components in percentage by weight: 0.1-15 percent of ketorolac tromethamine, 0.01-10 percent of buffering agent and 0.001-5 percent of pH value regulator. In the invention, because a traditional menstruum, such as ethanol is replaced by a phosphate buffer solution, the ketorolac tromethamine injection with good stability, less irritation, safety and more convenience is obtained so as to improve the medicament compliance of patients and the clinical medication convenience.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
Method for preparing ketorolac tromethamine
ActiveCN101143865AThe synthetic route is simpleReduce pollutionOrganic chemistryKetorolac TromethamineNon steroidal anti inflammatory
The present invention relates to a non-steroidal anti-inflammatory drug of ketorolac tromethamine salt, which has the functions of strong acesodyne, moderate anti-inflammation and antipyresis. The present invention applies a synthesis route preparing the compound ketorolac tromethamine salt, the technique applies an one-pot method, the reaction process from 2-benzoyl pyrrole to the ketorolac tromethamine salt can be continuously carried out, extraction and purification are not needed, materials can be easily obtained, labor intensity is reduced, the operational environment is improved, the cost of industrialized production is greatly reduced, environmental pollution is reduced, the loss of materials and products is reduced, and the yield rate is increased.
Owner:LUNAN PHARMA GROUP CORPORATION
Ketorolac tromethamine injection
InactiveCN102846542ASolve easy discolorationConvenient amountOrganic active ingredientsAntipyreticUse medicationIrritation
The invention discloses a prescription of a ketorolac tromethamine injection and a preparation method. The injection provided by the invention can not only effectively solve the problem that the existing ketorolac tromethamine injection containing ethanol causes irritation while being injected and improve the safety of the drugs and the compliance of the drugs, but also completely avoid the white points caused by the traditional technology after sterilization treatment, and thus the ketorolac tromethamine injection is good in stability, high in safety, reliable in quality and significant in efficacy.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Ketorolac tromethamine freezing-dried power injection and preparation method thereof
ActiveCN101167722AOvercoming low bioavailabilityOvercome curative effectOrganic active ingredientsPowder deliveryVisceral painAnalgesics effects
Ketorolac tromethamine is a new type of non-steroidal analgesic and anti-inflammatory drug with strong analgesic effect, which can be administered orally or by injection. It is mainly used to relieve moderate to severe postoperative pain, and Acute renal colic related to trauma, visceral pain related to cancer, anti-inflammatory of local inflammation, etc., the present invention provides a preparation method of ketorolac tromethamine freeze-dried powder preparation, which greatly improves the stability of the main drug, It not only overcomes the shortcoming of slow onset of oral administration, but more importantly, overcomes the problems of unstable main drug of liquid injection and unstable process in actual production.
Owner:LUNAN PHARMA GROUP CORPORATION
Ketoralac ammonia butanetriol injection and preparing method thereof
ActiveCN101199514BSolve easy discolorationLow content of related substancesOrganic active ingredientsAntipyreticAcid derivativeKetorolac Tromethamine
Owner:SHANDONG NEWTIME PHARMA
Ketorolac tromethamine injection capable of reducing irritation and free of organic solvent
ActiveCN111481501ASuppress generationLess irritatingOrganic active ingredientsAntipyreticIrritationOrganosolv
The invention provides a ketorolac tromethamine injection capable of reducing irritation and free of an organic solvent. By using a tromethamine-acidifying agent buffer system is used as a protectiveagent, adverse effects of ethanol and monopotassium phosphate on a packing material and severe irritation at an injection part in the prior art are avoided. The production process is simple and controllable, the physicochemical property of the liquid medicine is stable, and the medication safety and compliance of patients can be improved.
Owner:南京锐志生物医药有限公司
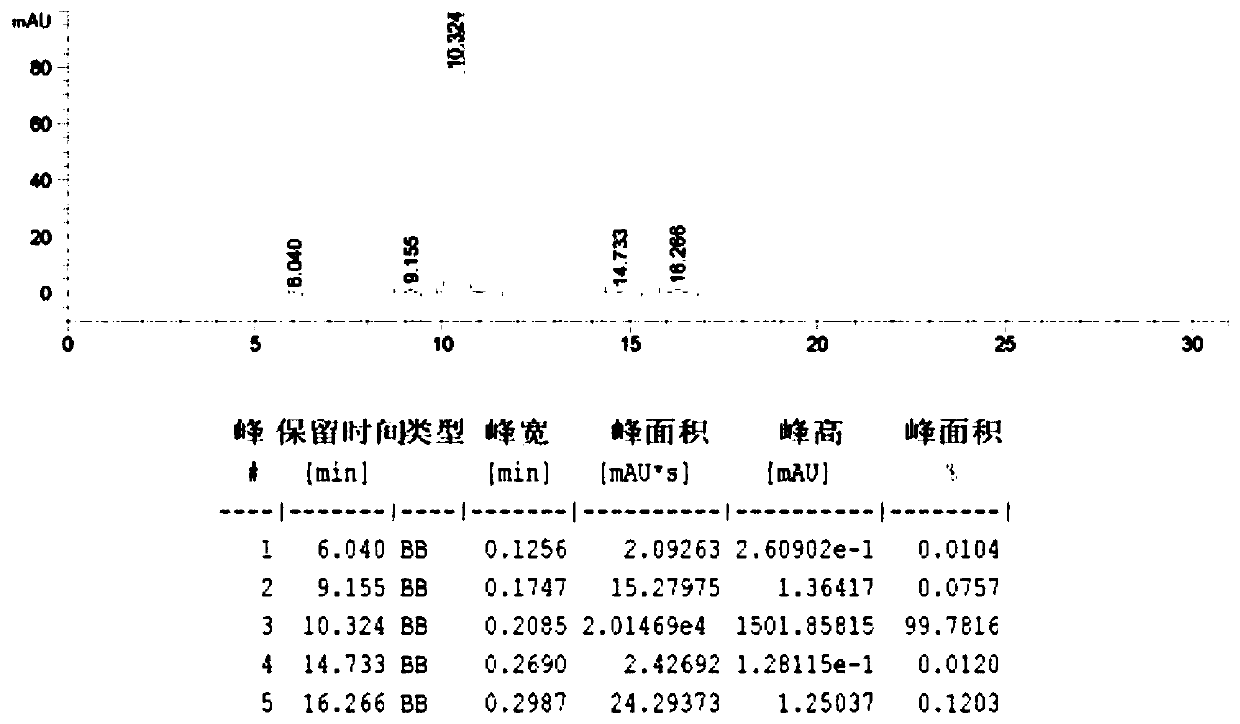
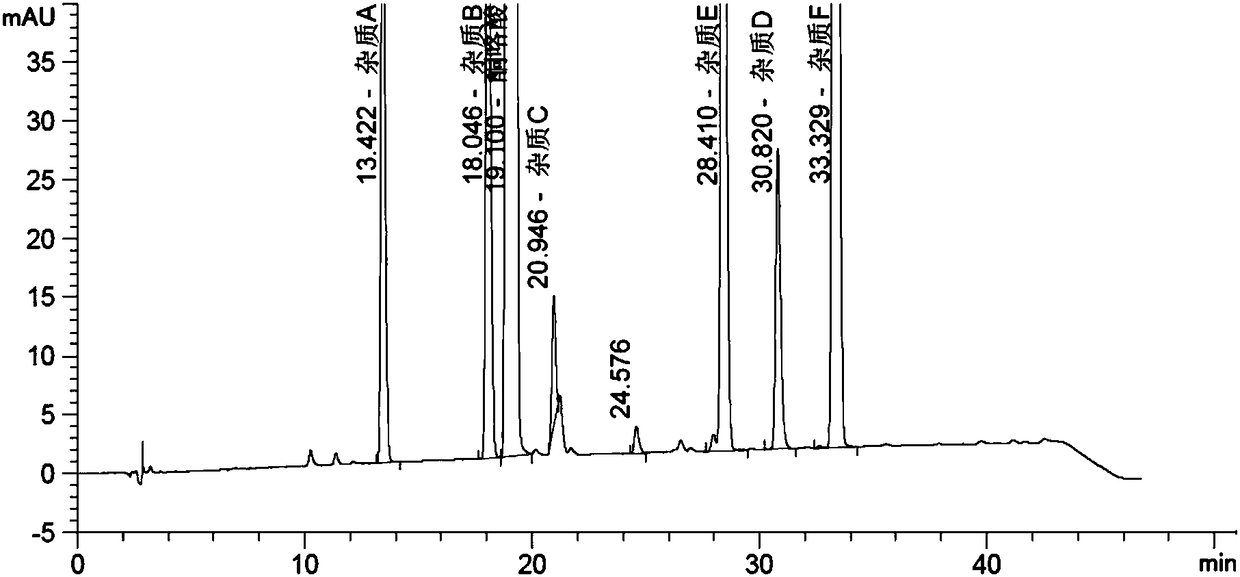
HPLC (high performance liquid chromatography) Detection method of ketorolac tromethamine or ketorolac tromethamine or/and impurities in ketorolac tromethamine preparation
ActiveCN108152418AEfficient separationEfficient detectionComponent separationKetorolac TromethamineLength wave
The invention discloses an HPLC (high performance liquid chromatography) detection method of ketorolac tromethamine or ketorolac tromethamine or / and impurities in ketorolac tromethamine preparation; HPLC is used to detect a solution under detection, and qualitatively or quantitatively determining according to chromatographic results. The HPLC impurity detection method for ketorolac tromethamine bulk pharmaceutical chemicals and related impurities in injections has the advantages that optimizing and modifying are performed in the aspects of detection wavelength and mobile phase system determining, test solution concentration screening and the like, the method is verified in the aspects of system applicability, degrading specificity, detection limit and quantitative limit, linearity and range, precision, accuracy, solution stability, method durability and the like, and the results show that the method has good capacity of detecting impurities.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
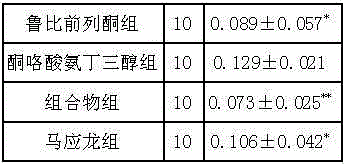
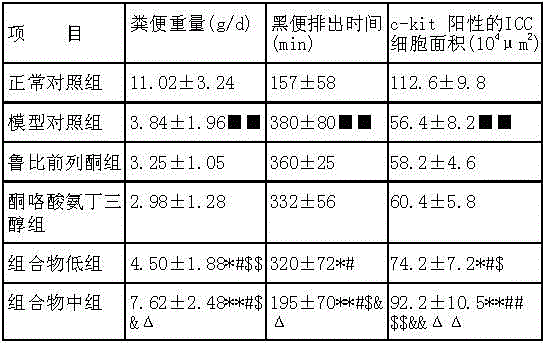
Medicine composition for treating constipation and/or haemorrhoids and application thereof
InactiveCN103550224AClear ingredientsDefinite curative effectElcosanoid active ingredientsDigestive systemPharmaceutical drugCurative effect
Owner:海门市凤城旅游景点开发有限公司
Ketorolac tromethamine nasal spray
InactiveCN103285472AReduced filling equipment requirementsIncrease shipmentsPharmaceutical containersMedical packagingMedicineNasal spray
The invention provides a ketorolac tromethamine nasal spray. A spraying device comprises a spraying bottle and a spraying head, and is characterized in that the spraying bottle comprises a casing (1) and a liner (2) with the volume of 1.5 ml; the liner of the spraying bottle is in a shape of a cylinder, the combination of a cylinder and a cone, or the combination of a cylinder, a cone and a cylinder with a smaller diameter ; the casing of the spraying bottle has the same appearance, size and connector part as those of a conventional 10 ml spray sold in the market; and the spraying head adopts a praying head of the conventional 10 ml spray sold in the market. The ketorolac tromethamine nasal spray has the advantages of few residues, high safety and multiple effective spraying times, the production cost can be reduced, and the medicine waste is prevented.
Owner:北京天衡药物研究院有限公司
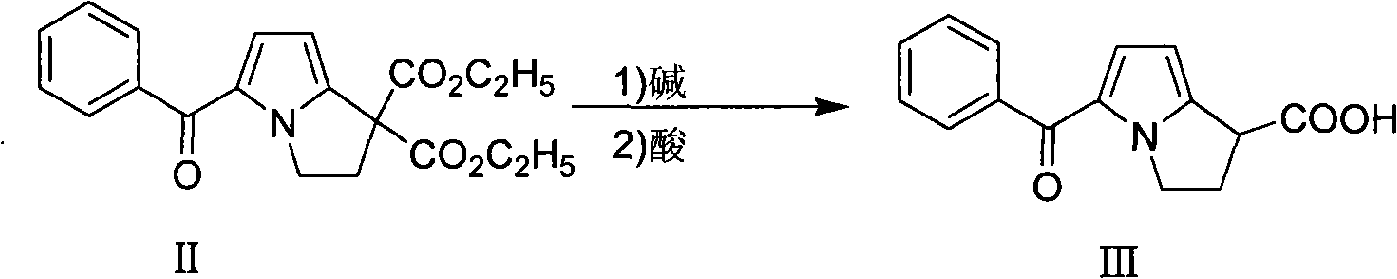
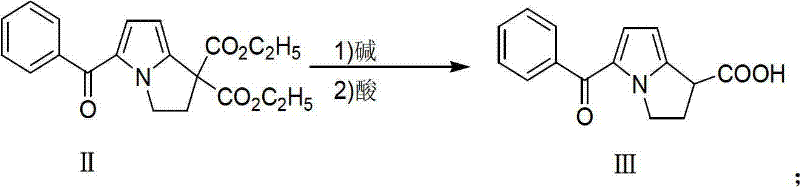
Preparation method of ketorolac tromethamine
ActiveCN101575340AQuality improvementShort reaction timeOrganic chemistryAntipyreticKetorolac TromethamineMedicinal chemistry
The invention provides a preparation method of ketorolac tromethamine. 5-benzoyl-2, 3-dihydro-1H-dilazine-1 and 1-diethyl azodicarboxylate are the starting materials and are prepared into salt by alkaline hydrolysis and acidification when reducers exist, and finally, the salt is prepared into ketorolac tromethamine. The method does not need complicated decarboxylation process, and effectively solves the problems of low purity, poor color and the like caused by the oxidizability of ketorolac. The invention has the advantages of simple operation, high yield and high purity (the total yield in two steps reaches 95% and the purity reaches more than 99.9%); thus, the preparation method is suitable for large-scale commercial process.
Owner:LUNAN PHARMA GROUP CORPORATION
Medical Kits
Provided is a medical kit with all the necessary components for a medical practitioner in the office practice setting to inject a variety of anesthetics. The kits can comprise a holder, such as a box, and active pharmaceutical ingredients (API), with each API placed in a separate container in a form that is ready for injection, the API selected from the group consisting of: a. lidocaine hydrochloride and triamcinolone acetonide with or without a separate container of ammonia; b. bupivacaine hydrochloride (HCl) and lidocaine hydrochloride (HCl), and optionally one or more of triamcinolone acetonide, methylprednisolone acetate, or dexamethasone sodium; c. bupivacaine hydrochloride (HCl) and one or more of triamcinolone acetonide or methylprednisolone acetate; d. Methylprednnisolone acetate and lidocaine hydrochloride (HCl), and optionally bupivacaine hydrochloride (HCl); e. methylprednisolone acetate and lidocaine hydrochloride (HCl), and bupivacaine hydrochloride (HCl); f. lidocaine hydrochloride, triamcinolone acetonide, and ammonia; g. bupivacaine hydrochloride or lidocaine HCl, and betamethasone sodium phosphate and betamethasone acetate; h. bupivacaine hydrochloride or lidocaine HCl, and dexamethasone sodium phosphate; i. ketorolac tromethamine, lidocaine HCl, and optionally bupivacaine hydrochloride; and j. one or more of dexamethasone sodium.
Owner:ASCLEMED USA
Medicinal composition for treating hemorrhoids
ActiveCN101744822ASignificant effectPromote refluxHeterocyclic compound active ingredientsCardiovascular disorderTreatment effectTheobromine
The hemorrhoids are more suitable to be treated conservatively by medicaments mainly. The invention discloses a medicinal composition for treating hemorrhoids, which comprises two components, i.e. pentoxifyllinum and ketorolac tromethamine, wherein the ratio of pentoxifyllinum to ketorolac tromethamine is 0.02-10:1, preferably 0.1-5:1, more preferably 0.5-2:1. A mass of experimental studies show that the medicinal composition has obvious treatment effect on anorectal diseases, such as hemorrhoids and the like.
Owner:LUNAN HOPE PHARM CO LTD
Method for researching abirritation mechanism of Chinese herbal medicinal ingredients of Xinhuang tablets
ActiveCN103432596APain BenefitsGood treatment effectOrganic active ingredientsNervous disorderIndometacinTreatment effect
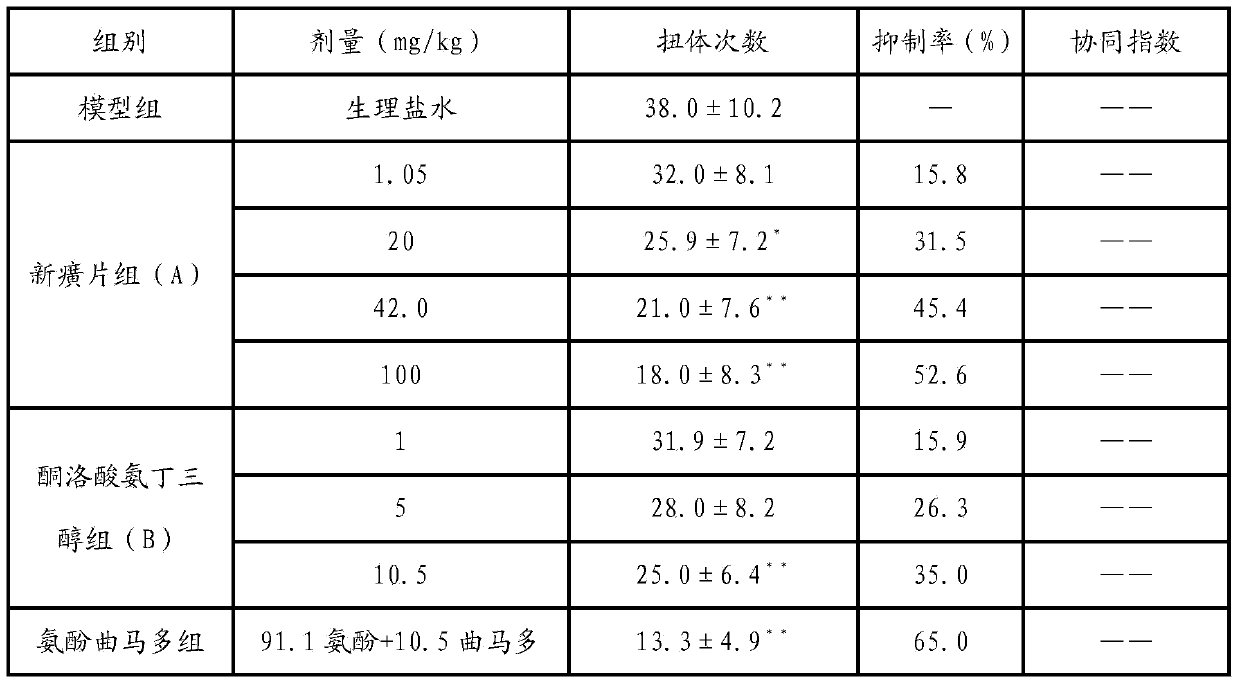
The invention discloses a method for researching an abirritation mechanism of Chinese herbal medicinal ingredients of Xinhuang tablets and a pharmaceutical composition comprising the Xinhuang tablets. The method for researching the abirritation mechanism comprises the following steps of preparing a Xinhuang tablet sample and an indometacin sample in a weight ratio of 95:2, establishing a living acetic acid writhing reaction model and a living planta reaction model to evaluate the drug treatment effects and the synergic index of the Chinese herbal medicinal ingredients of the Xinhuang tablets and indometacin, and establishing a naloxone occlusion reaction model to evaluate the influence of occlusion on the analgesic effect. Based on the research on the mechanism of action, and in order to improve the treatment effect for analgesia, the invention also provides an analgesic composition which is composed of the Xinhuang tablets and ketorolac tromethamine. The method is used for measuring the abirritation mechanism of the Chinese herbal medicinal ingredients of the Xinhuang tablets, is simple and clear; applicable effective drugs can be selected from the known compound medicines by the pharmacological research method.
Owner:XIAMEN TRADITIONAL CHINESE MEDICINE
Ketorolac tromethamine tablet
ActiveCN108451909AEvenly dispersedImprove stabilityOrganic active ingredientsPowder deliveryKetorolac TromethaminePharmacology
The invention belongs to the field of medicine preparation, and particularly relates to a ketorolac tromethamine tablet. According to the ketorolac tromethamine tablet, the ketorolac tromethamine is prepared into a solid dispersion body and is then prepared into the tablet, so that a dissolution ratio of the ketorolac tromethamine is greatly improved, the stability of the ketorolac tromethamine isalso effectively improved, impurities of the ketorolac tromethamine produced by factors such as light heat are effectively avoided, and the security of clinical medicine application is improved.
Owner:LUNAN PHARMA GROUP CORPORATION
Compositions and Methods for Treating Eyes and Methods of Preparation
InactiveUS20180318319A1Patient compliance is goodMinimize the numberOrganic active ingredientsSenses disorderPhenylephrine hclBromfenac
Pharmaceutical compositions, methods for treating various issues of the eyes, and methods of preparing such compositions are described. These pharmaceutical compositions may be for treating glaucoma, in preparation of eye surgery, during eye surgery, various post-op care (e.g., after cataract surgery, laser eye surgery, and the like), for treating dry eyes, and / or for promoting eyelash growth. These pharmaceutical compositions may comprise such active ingredients (APIs) as: timolol, latanoprost, brimonidine tartrate, dorzolamide, moxifloxacin HCl, dexamethasone PO4, phenylephrine HCl, lidocaine HCl, ketorolac tromethamine, bromfenac, prednisolone PO4, gatifloxacin, amniotic cytokine extract (ACE), prostaglandin E2 (PGE2), and combinations thereof.
Owner:OCULAR SCI INC
Ketorolac tromethamine injection and preparation method thereof
ActiveCN103830171AImprove stabilitySimple processOrganic active ingredientsAntipyreticMedicinePhosphate
The invention provides a ketorolac tromethamine injection. The packing volume of the injection is 0.5ml, and the content of ethanol is 10-18 percent (W / V). Furthermore, the preparation method is improved. Compared with the prior art, the injection provided by the invention has the advantages that the usage of the ethanol is reduced, the stability of the injection is improved, and the white spots cannot be separated out from the medicine; the administration safety can be improved because propylene glycol, phosphate and other additional components are not added; the process is simple and feasible, and is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Nasal-delivery temperature-sensitive in-situ gel sustained-release preparation comprising ketorolac tromethamine
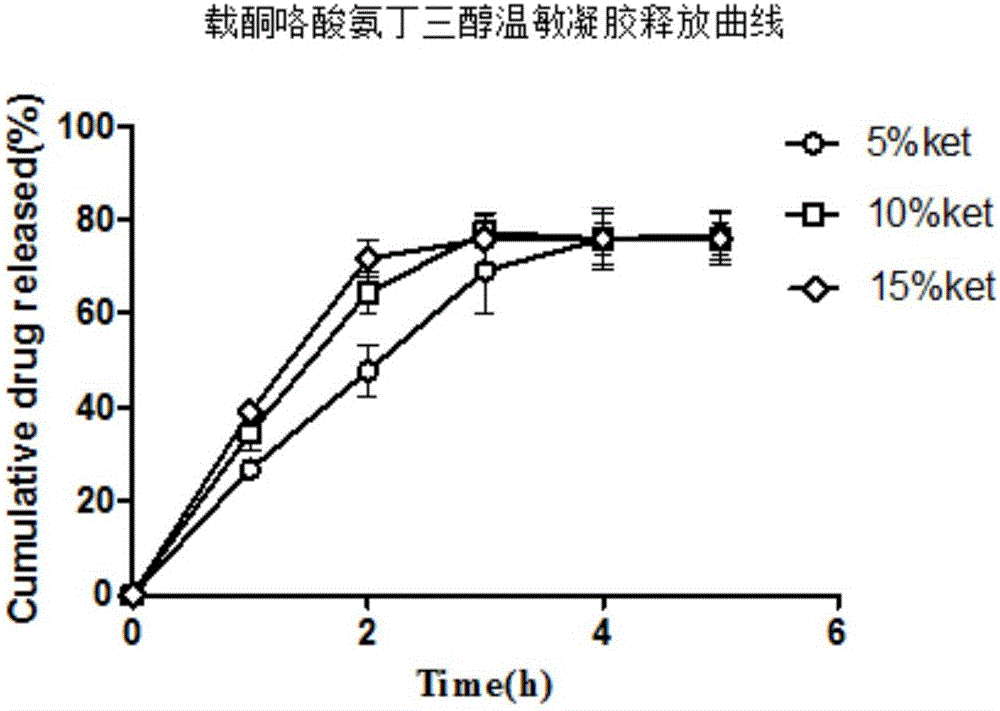
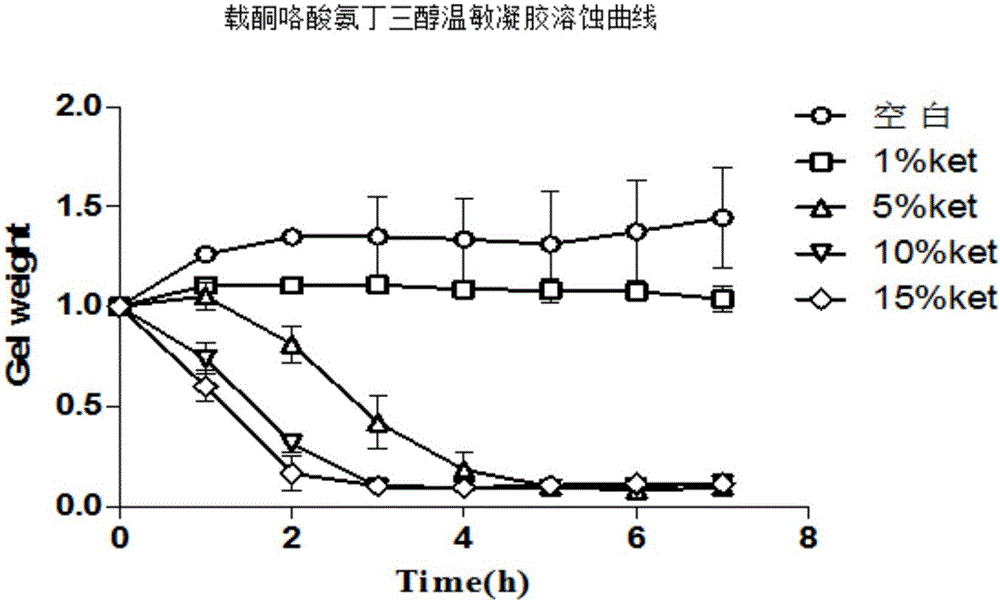
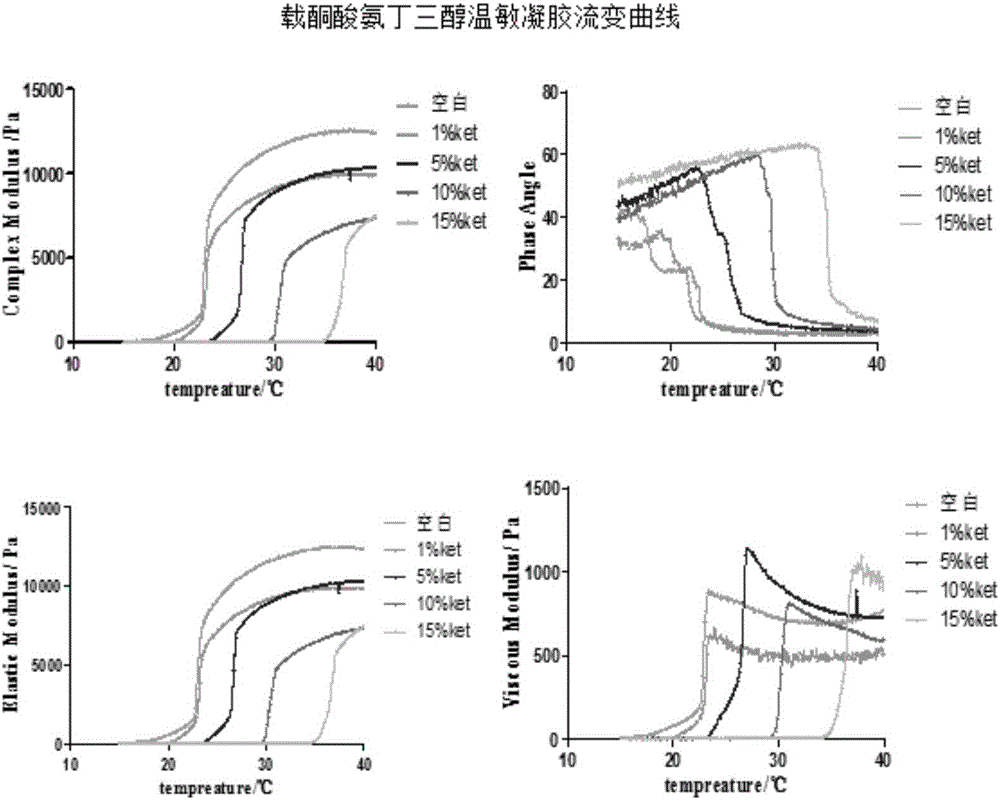
InactiveCN106309354AOvercoming the problem of dissolving too quicklyGood bioadhesionOrganic active ingredientsAntipyreticNasal cavityCarrageenan
The invention belongs to the field of medicine preparations and relates to a nasal-delivery temperature-sensitive in-situ gel sustained-release preparation comprising ketorolac tromethamine and a preparation method thereof. The preparation is composed of poloxamer, carrageenan and ketorolac tromethamine in effective dose. The preparation integrates reverse-phase gelatinization property of the poloxamer and the property that the carrageenan can significantly improve erosion of the temperature-sensitive gel, and improves retention effect of the poloxamer gel in nasal cavity and improves absorption of the ketorolac tromethamine. The preparation is improved in bioavailability of the ketorolac tromethamine through nasal delivery and improves patient compliance.
Owner:FUDAN UNIV
Ketorolac tromethamine compositions for treating or preventing ocular pain
ActiveUS8008338B2Reduce generationMaintain efficacyBiocideSenses disorderKetorolac TromethamineOcular pain
Compositions comprising ketorolac tromethamine at a therapeutically effective concentration of less than 0.5% are disclosed herein. Methods of treating or preventing ocular pain using said compositions are also disclosed herein.
Owner:ALLERGAN SALES LLC
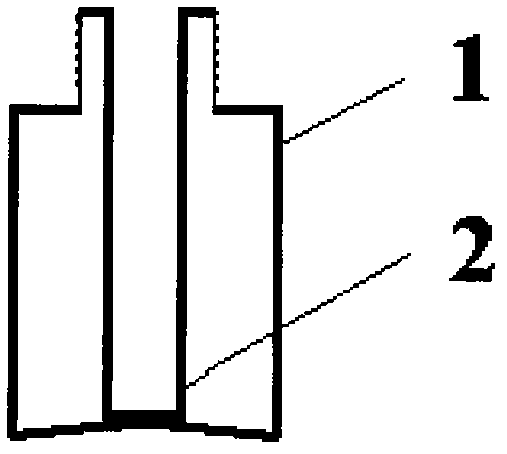
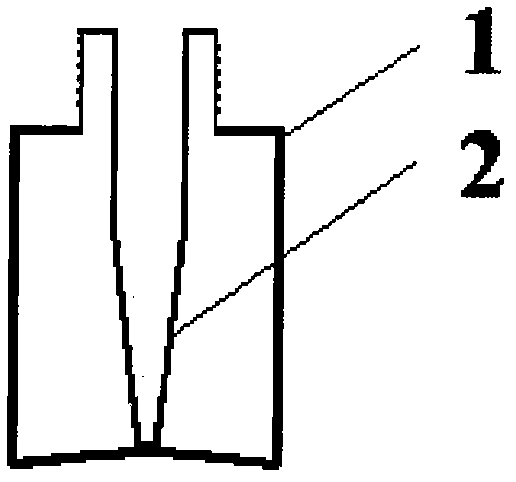
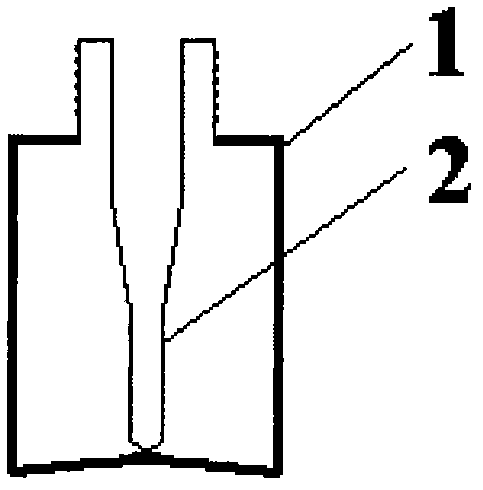
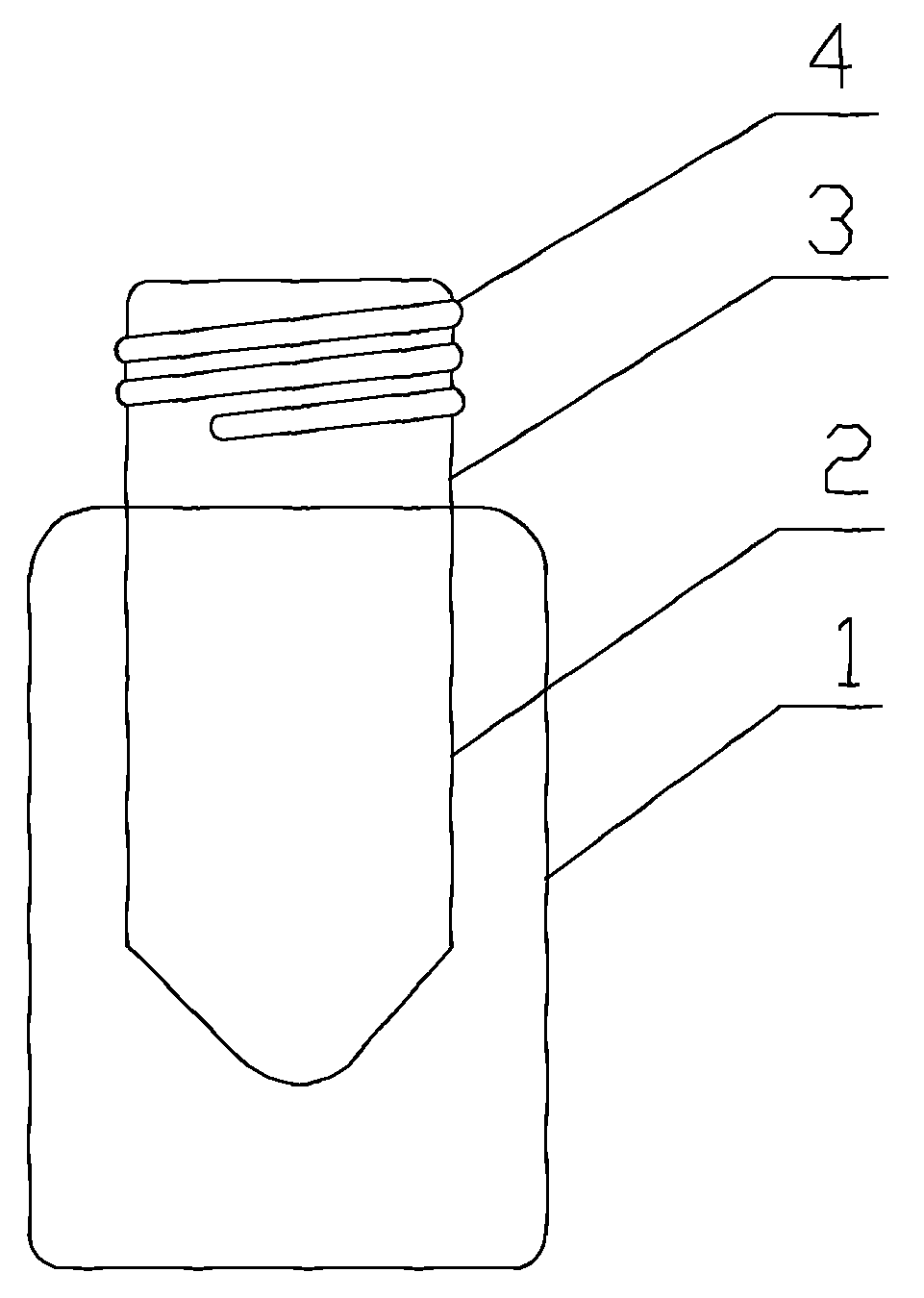
Inner liner type medicine bottle for ketorolac tromethamine spray
InactiveCN103099735ASmall qualityLittle stabilityPharmaceutical containersMedical packagingMedicineKetorolac Tromethamine
The invention discloses an inner liner type medicine bottle for ketorolac tromethamine spray. The inner liner type medicine bottle for the ketorolac tromethamine spray comprises a bottle neck (3), an outer bottle (1) and an inner liner bottle (2) which is arranged inside the outer bottle (1). The inner liner type medicine bottle for the ketorolac tromethamine spray is characterized in that the bottle neck (3) extends to the interior of the bottle body of the outer bottle (1) to form the inner liner bottle (2) in a sealing mode, and a plurality of outer threads (4) are formed in the outer surface of the bottle neck (3). The inner liner type medicine bottle for the ketorolac tromethamine spray is light in weight, and solves the filling problem of small size liquid medicament, and simultaneously improves safety of medicament transportation and stability of long-time placement of the medicament.
Owner:江苏省药物研究所有限公司
Ketorolac tromethamine nasal spray and preparation method thereof
The invention belongs to the field of pharmaceutical preparations, and in particular designs a non-steroid anti-inflammatory drug ketorolac tromethamine nasal delivery system. Compared with conventional troches and injections, the nasal spray provided by the invention provides convenient, efficient and quick-acting analgesic selection for patients with moderately severe pains. Meanwhile, the invention provides a formula of the ketorolac tromethamine nasal spray and a preparation method thereof, so that the stability of the spray product is greatly improved compared with that of conventional injections, and the nasal spray is good in stability.
Owner:NANJING KANGLAIFU MEDICAL TECH
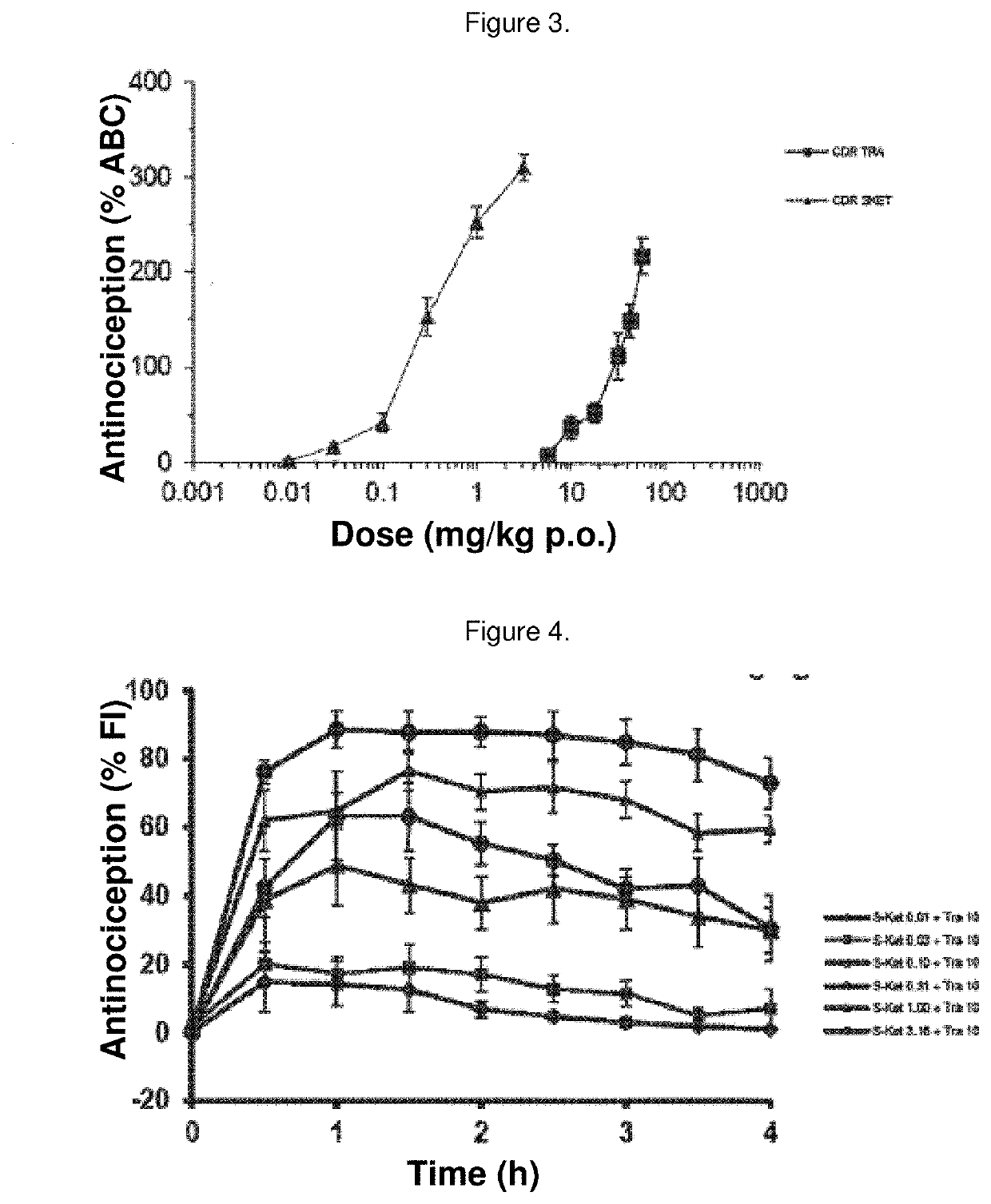
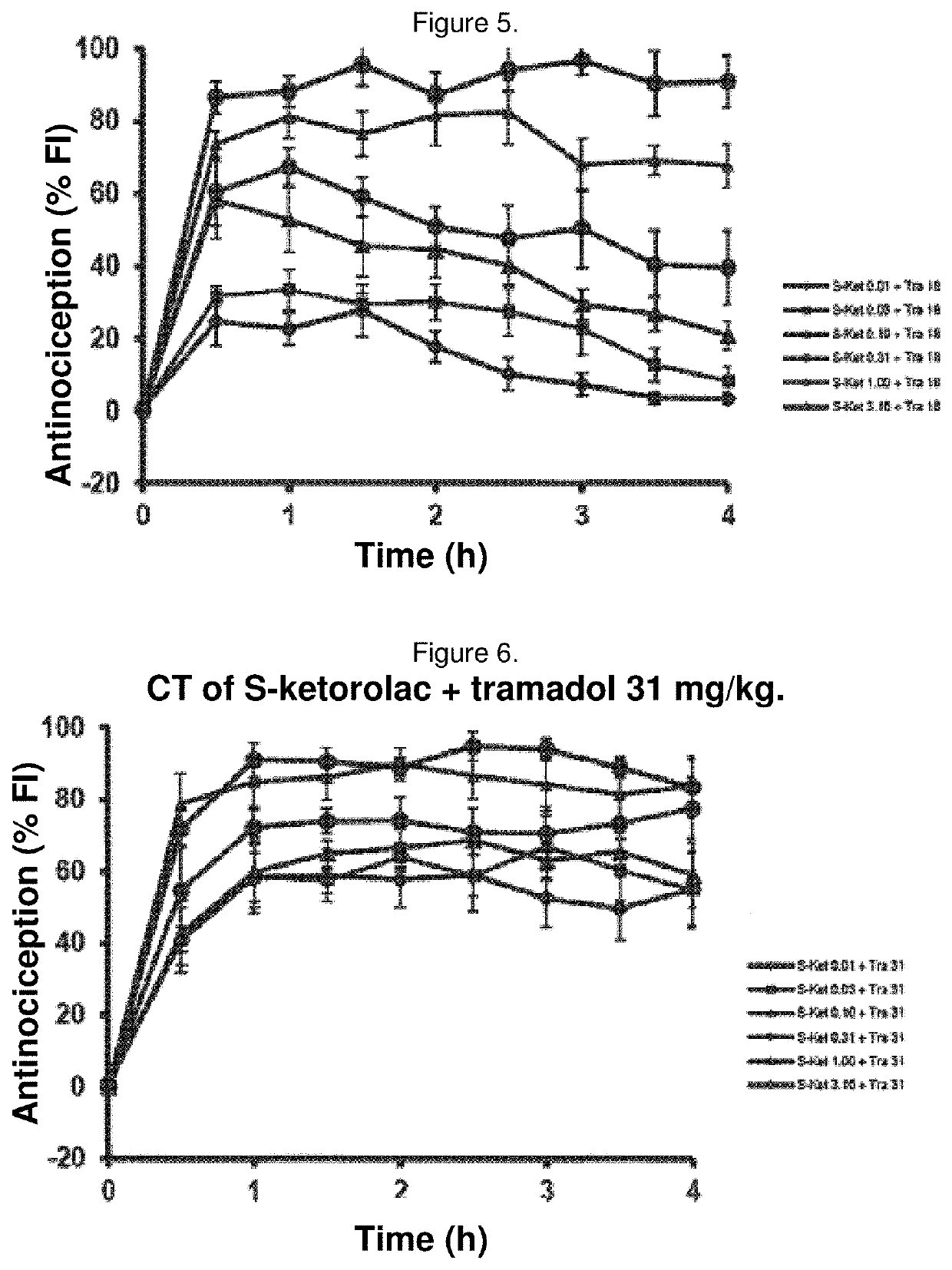
Synergistic pharmaceutical combination of the active enantiomer s-ketorolac tromethamine and tramadol chlorhydrate
PendingUS20210069151A1Decrease in symptomatologyImprove the quality of lifeOrganic active ingredientsNervous disorderOpioidergicNon steroid anti inflammatory drug
The present invention relates to a pharmaceutical composition comprising the synergistic combination of a non-steroidal anti-inflammatory drug (NSAID), such as the active ingredient s-ketorolac tromethamine, and an opioid agent, such as the active ingredient tramadol chlorhydrate, which ingredients are formulated with pharmaceutically acceptable excipients. The composition is used for the control and treatment of mild and / or moderate and / or severe pain.
Owner:AMEZCUA AMEZCUA FEDERICO +1
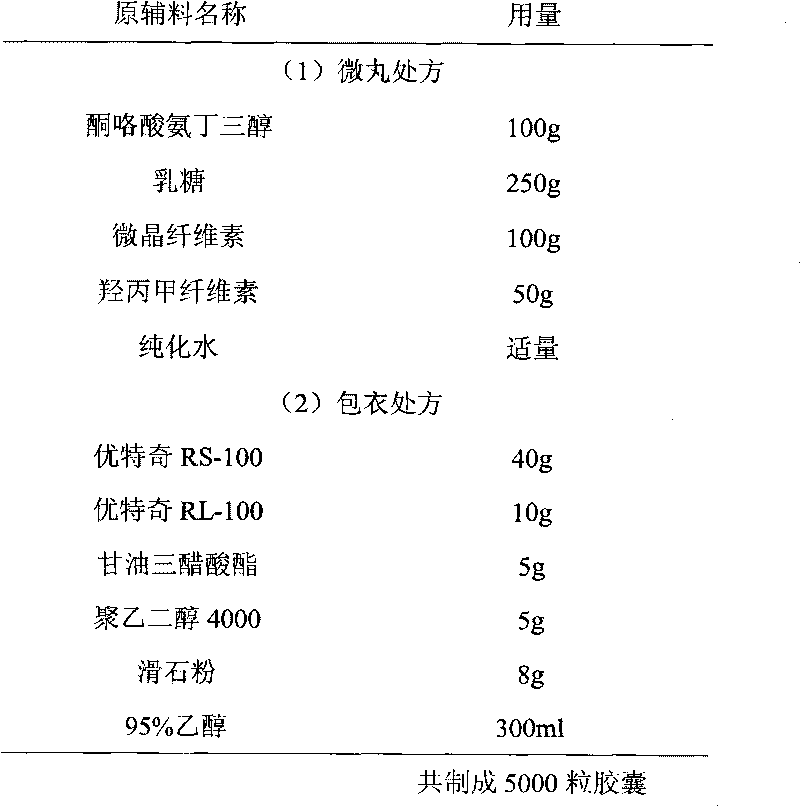
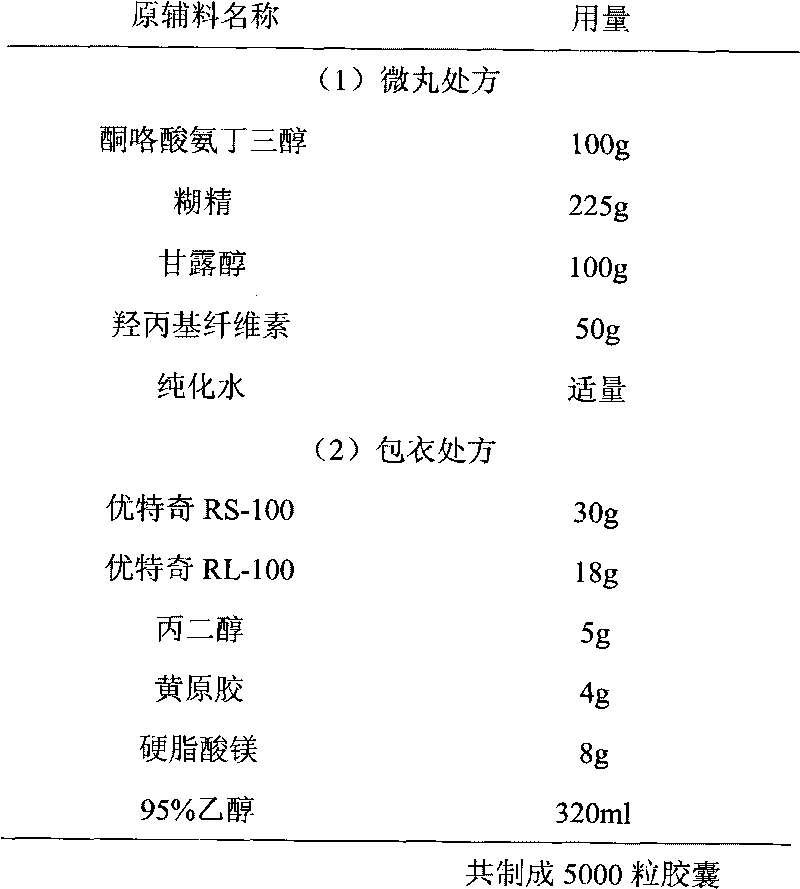
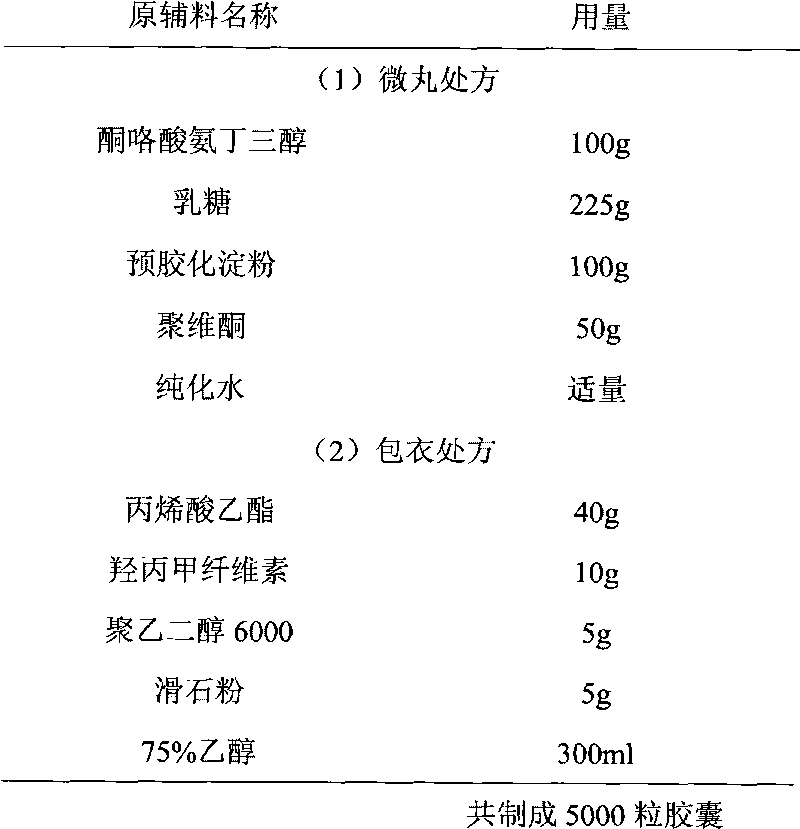
Ketorolac tromethamine capsule and preparation method thereof
ActiveCN101756938AAvoid peaks and valleys in blood concentrationImprove complianceOrganic active ingredientsAntipyreticFast releaseKetorolac Tromethamine
The invention provides a ketorolac tromethamine capsule and a preparation method thereof, which belong to the technical field of medicine preparations. The ketorolac tromethamine capsule is prepared by the following steps: making ketorolac tromethamine into slow-release coated micropills and ordinary micropills; and then, uniformly and proportionally mixing the two kinds of micropills to be put into capsule cases. After the capsule enters the gastrointestinal tract, the ordinary micropills fast release medicine for taking effect, and the slow-release micropills persistently release the medicine through regulating the thickness and the materials of coated films, so the peak valley phenomenon of ordinary capsules is avoided, good release curves and repeatability can be obtained, the occurrence of untoward effects of the medicine is reduced, in addition, the medicine using times are reduced, and the adaptability of patients is improved. The preparation method of the capsule has the advantages of simple process and good controllability, and is suitable for industrial production.
Owner:鲁南新时代生物技术有限公司
Analgesic formulations and methods for reduced postoperative nausea and vomiting and enhanced postoperative pain relief
A multimodal anti-emetic anesthetic / analgesic formulation for pain control not limited to postoperative pain control is described herein. The opioid-free / sparing anesthetic / analgesic formulation comprises a local anesthetic, an N-methyl-D-aspartate (NMDA) receptor antagonist, and a cyclooxygenase (COX) inhibitor such as Bupivacaine Hydrochloride, Ketamine Hydrochloride, and Ketorolac Tromethamine, which is effective to significantly reduce postoperative nausea and vomiting and enhance postoperative pain relief as compared to existing prior art anesthetics / analgesics. The formulation is administered to a mammal in need of anesthesia / analgesia and can be used as a preemptive and preventative multimodal analgesic. The formulation may have a buffer to enhance its shelf life and improve pharmacokinetics. The formulation may further comprise an alpha agonist, a steroid, a Transient Receptor Potential Channel agonist or antagonist, a beta-lactam antibiotic, a protein kinase inhibitor, a competitive or non-competitive glycine or glutamate antagonist, a glutamate or glycine inhibitor, a cyclooxygenase 3 inhibitor, or combinations thereof.
Owner:HUTCHISON HEALTH LLC
Preparation method of ketorolac tromethamine
ActiveCN101575340BQuality improvementShort reaction timeOrganic chemistryAntipyreticReducerEthyl ester
The invention provides a preparation method of ketorolac tromethamine. 5-benzoyl-2, 3-dihydro-1H-dilazine-1 and 1-diethyl azodicarboxylate are the starting materials and are prepared into salt by alkaline hydrolysis and acidification when reducers exist, and finally, the salt is prepared into ketorolac tromethamine. The method does not need complicated decarboxylation process, and effectively solves the problems of low purity, poor color and the like caused by the oxidizability of ketorolac. The invention has the advantages of simple operation, high yield and high purity (the total yield in two steps reaches 95% and the purity reaches more than 99.9%); thus, the preparation method is suitable for large-scale commercial process.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for improving storage stability of ketorolac tromethamine injection
ActiveCN110812325AGood storage stabilityEnsure medication safetyOrganic active ingredientsAntipyreticForeign matterMedicine
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a method for improving storage stability of a ketorolac tromethamine injection. The prepared ketorolac tromethamine injection is filled into a polypropylene plastic ampoule or a cycloolefin polymer plastic bottle, so that the problem that visible foreign matters are unqualified due to the factthat the injection easily generates white dots in the long-term placement process of the ketorolac tromethamine injection is solved, insoluble particles are few, and the quality is stable.
Owner:LUNAN PHARMA GROUP CORPORATION
Application of ketorolac tromethamine in preparation of drug for treating mental diseases
InactiveCN111388468ADrugs work quicklyLong durationOrganic active ingredientsNervous disorderDiseasePharmacometrics
The invention belongs to the technical field of medicine, and particularly relates to application of ketorolac tromethamine in preparation of a drug for treating mental diseases. The medicine is particularly applicable to treatment of depression and delirium patients, pharmacological experiments of animal models find that the drug prepared with ketorolac tromethamine as an active component can significantly improve distraction and depression states of model mice and has significant therapeutic effects on mental disease patients suffering from the symptoms.
Owner:LUNAN PHARMA GROUP CORPORATION
Externally applied ointment of ketorolac tromethamine and its preparation method
InactiveCN100434070CQuick releaseNot stainedNervous disorderAntipyreticKetorolac TromethamineD ointment
The invention relates to an externally applied ointment of ketorolac tromethamine and its preparation method, wherein the ointment comprises 1-5 wt% of ketorolac tromethamine, 0.5-95 wt% of ointment matrix, 0.2-2 wt% of bactericidal agent, 1-4 wt% of penetration enhancer, 1-2 wt% of propylene glycol and aqueous medium. The preparing process comprises (1) preparing ketorolac tromethamine soup, (2) preparing blank ointment, (3) preparing the ointment.
Owner:SHENYANG MINGHUA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com