Medicinal composition for treating hemorrhoids
A technology of composition and medicine, which is applied in the new field of medicine, can solve the problems such as lack of pentoxifylline, achieve good therapeutic effect, promote hemorrhoidal venous return, and improve the effect of varicose veins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
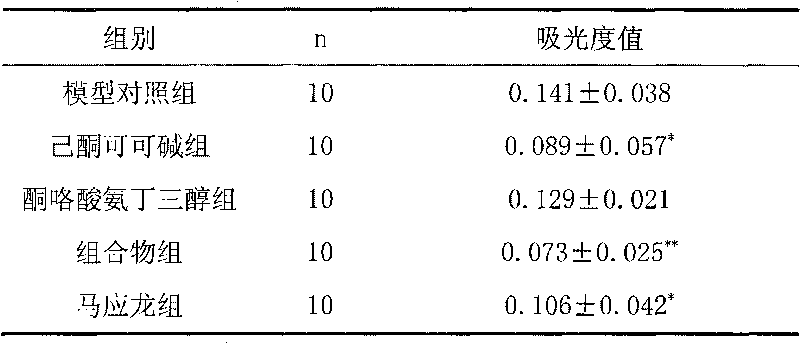
[0014] The experiment of embodiment 1 composition to rat croton oil hemorrhoid model
[0015] 50 SD rats (about 200 grams) were randomly divided into five groups according to body weight, i.e. model control group, pentoxifylline group (containing pentoxifylline 1%), ketorolac tromethamine group (containing ketorolac Tromethamine 0.5%), composition group (pentoxifylline 1%, ketorolac tromethamine 0.5%), Ma Yinglong group, 10 rats in each group.
[0016] experimental method:
[0017] Dosing: Rats are administered rectally two days in advance, twice a day, with 10 mg cotton balls covered with medicine, and the cotton balls are stuffed into the rectum. When administering next time, first check whether there are cotton balls in the rectum (such as Cotton balls should be removed). The last administration should be 2 hours after modeling.
[0018] Modeling: Croton oil is a commonly used inflammatory agent, and the common croton oil mixture is prepared with 1 part of distilled wate...
Embodiment 2
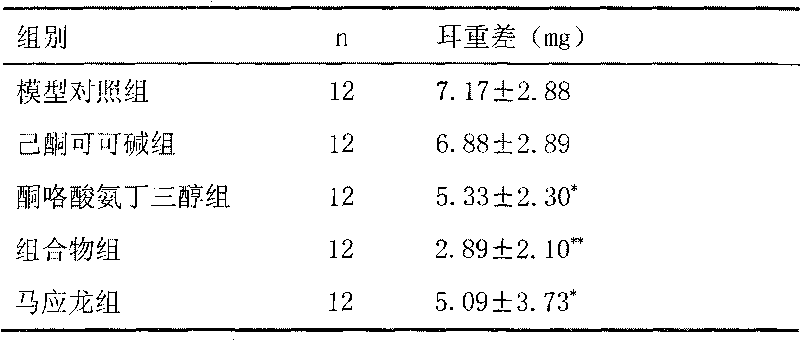
[0025] Example 2 Xylene Anti-inflammatory Experiment in Mice
[0026] 60 KM mice, half male and half female, weighing 25-30 grams, were randomly divided into five groups according to body weight, namely model control group (blank auxiliary material), pentoxifylline group (containing pentoxifylline 0.5%), ketorolac Tromethamine group (containing 0.5% ketorolac tromethamine), composition group (0.5% pentoxifylline, 0.5% ketorolac tromethamine), Ma Yinglong group, 12 rats in each group.
[0027] experimental method:
[0028] Take 20 μl of xylene and apply it to the right ear of each mouse. After 30 minutes, each test drug was evenly applied to the right ear of the mouse, and the right ear was sacrificed after being given xylene for 3.5 hours.
[0029] Experimental indicators:
[0030] Ear weight difference: cut two ears along the base line of the auricle, and use an 8mm punch to punch ear pieces in the same part of the left and right ears, and weigh them. The difference in ear...
Embodiment 3
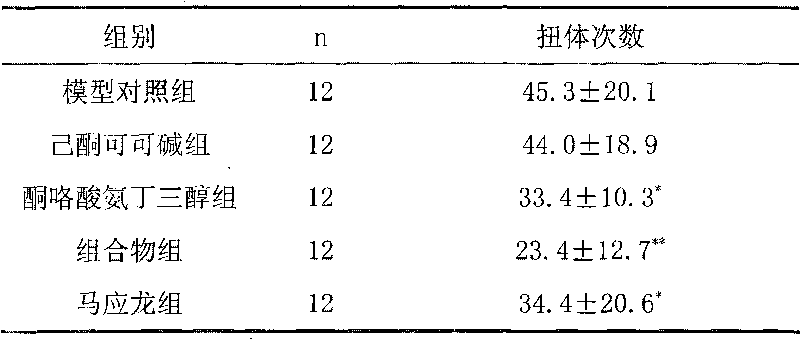
[0036] Example 3 Administration of each group to mice analgesic effect experiment
[0037] 48 KM mice, half male and half female, with a body weight of 25-30 grams, were randomly divided into four groups according to body weight, i.e. model control group (blank auxiliary material), ketorolac tromethamine group (containing ketorolac tromethamine 1 %), composition group (pentoxifylline 0.5%, ketorolac tromethamine 1%), Ma Yinglong group, 12 rats in each group. For anal administration of mice, 10 mg cotton balls are soaked with the drug, and the cotton balls are stuffed into the rectum, once a day, for three consecutive days, 30 minutes after the last administration, 5% of the rectal wall is injected at a distance of 3.5 cm from the anus Formalin 0.2ml. The number of writhing times in 20 minutes was recorded. The specific results are shown in Table 3
[0038] Table 3 Comparison of writhing times in each experimental group
[0039]
[0040] Composition compared with the con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com