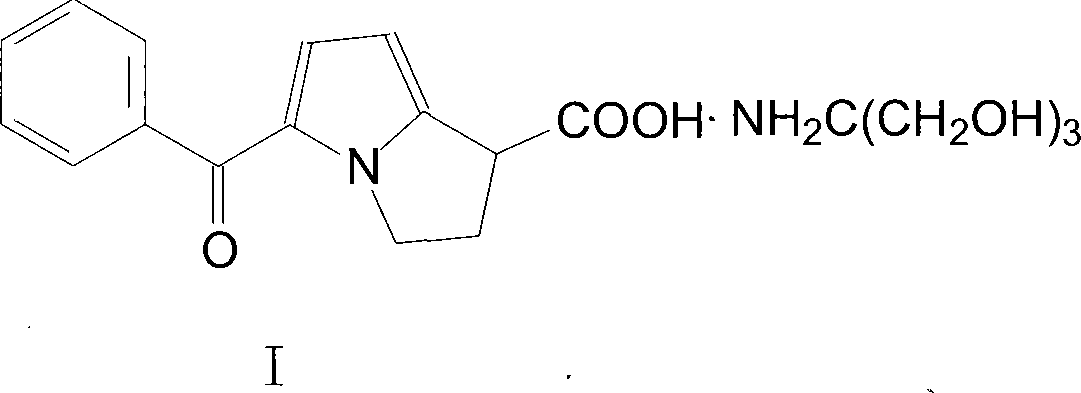
Method for preparing ketorolac tromethamine
A technology of ketorolac tromethamine and triethyl ester, applied in directions such as organic chemistry, can solve problems such as unsatisfactory yield, high cost of industrialized production, insufficient simplification of steps, etc., and achieves reduced labor intensity, reduced loss, Yield-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Put 13g of triethyl 5-benzoylpyrrole-2-methanetricarboxylate, K 2 CO 3 30g, 10g of n-tetrabutylammonium bromide, and 1.5L of 1,2-dichloroethane were placed in a reaction flask, stirred and refluxed for 24 hours, cooled to 10°C, filtered, concentrated under reduced pressure, and then added 300ml of methanol and 150ml of 25gNaOH Aqueous solution, reflux and stir for 12 hours, evaporate methanol, lower to room temperature, wash twice with diethyl ether, adjust pH value to ph=1 with concentrated hydrochloric acid, precipitate oil, dissolve 5 g of tromethamine in 100 ml of methanol and add to the oil, Then add 3g of activated carbon, heat to reflux for 2h, cool to 05°C, an off-white solid precipitates, filter with suction, wash twice with ethanol and ethyl acetate respectively, and dry to obtain 6g of off-white compound I solid, mp164~166°C, crude product Yield 89%.
Embodiment 2
[0015] Put 28g of triethyl 5-benzoylpyrrole-2-methanetricarboxylate, K 2 CO 3 56g, 23g of n-tetrabutylammonium bromide, and 3.0L of 1,2-dichloroethane were placed in a reaction flask, stirred and refluxed for 24 hours, cooled to 5°C, filtered, concentrated under reduced pressure, and then added 300ml of 500ml of methanol and 43g of NaOH Aqueous solution, reflux and stir for 12h, evaporate methanol, lower to room temperature, wash twice with ether, adjust pH value to 2 with concentrated hydrochloric acid, precipitate oil, dissolve tromethamine 12g in 300ml methanol and add to the oil, then add Activated carbon 8g, heated to reflux for 2h, cooled to 0-5°C, an off-white solid precipitated, filtered with suction, washed twice with ethanol and ethyl acetate respectively, and dried to obtain 13g of off-white compound I solid, mp164-166°C, crude product Yield 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com