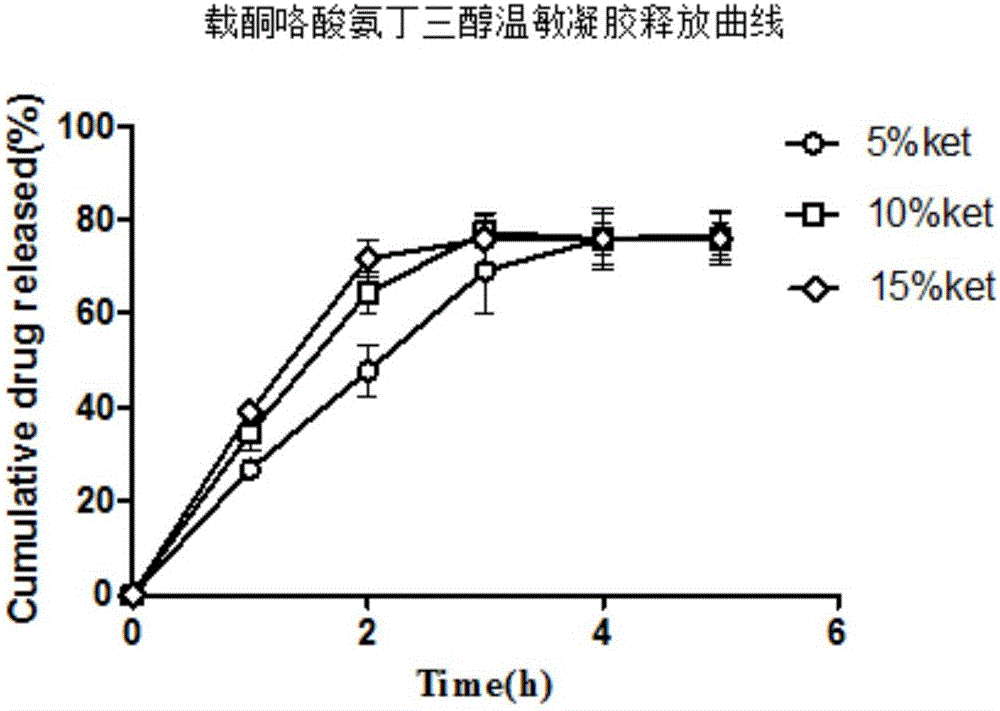
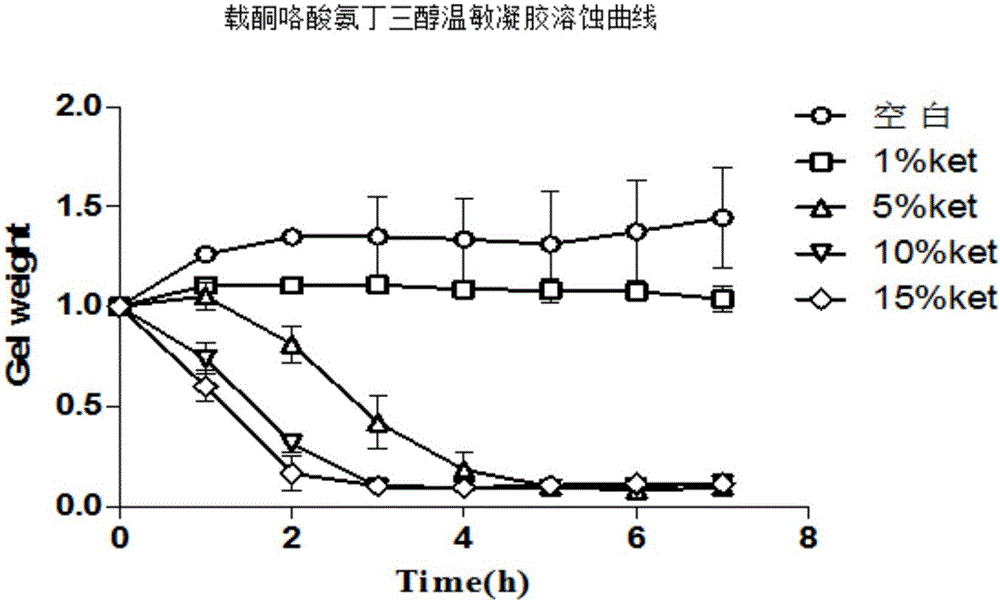
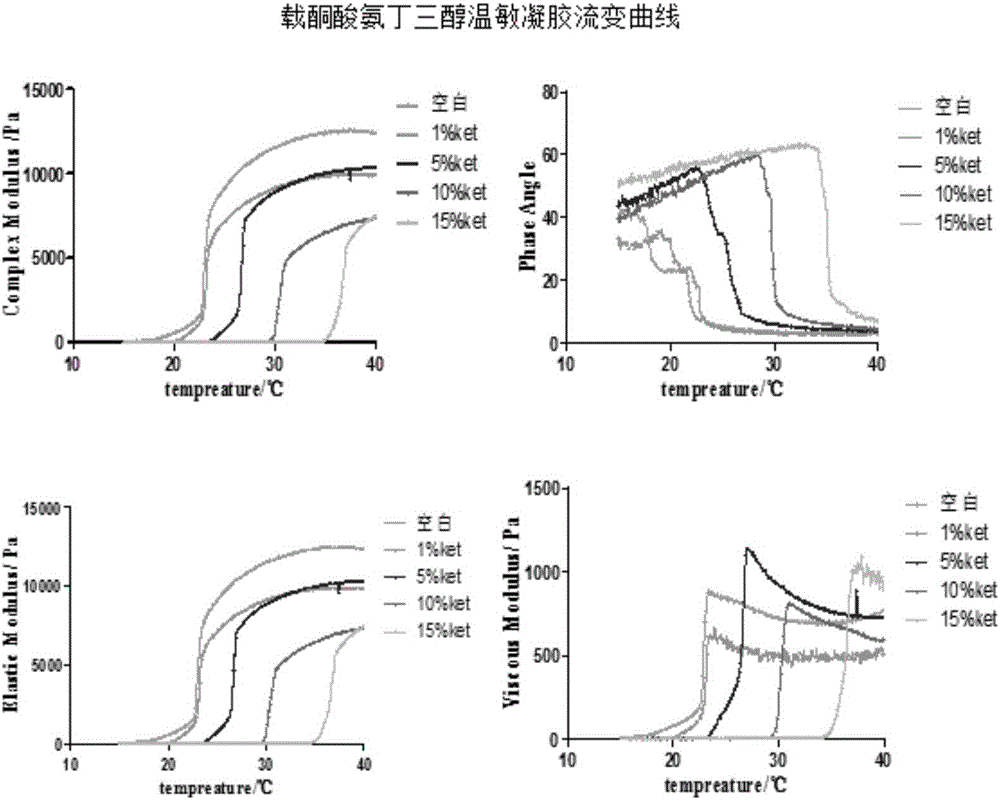
Nasal-delivery temperature-sensitive in-situ gel sustained-release preparation comprising ketorolac tromethamine
A technology of ketorolac tromethamine and in-situ gel, which is applied in the directions of non-central analgesics, antipyretics, anti-inflammatory agents, etc., can solve the problems of inconvenient use, insufficient absorption, and frequent administration of patients. , to achieve the effect of less irritation, convenient administration and outstanding sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of Blank Gel I
[0042] Weigh an appropriate amount of κ-carrageenan and dissolve it in pure water at 70-80°C, stir to fully dissolve it, and cool to room temperature to obtain a carrageenan stock solution (0.25%, w / w). Add 1 part of Poloxamer 407 to 4 parts (by weight) of 0.25% carrageenan stock solution, leave it to stand at 4 ℃ and stir evenly after the solid components are dissolved to obtain a uniform mixture, which is blank gel I; The gelling temperature is about 22-23°C, and the weight percentage of each component is shown in Table 1:
[0043] Table 1
[0044] Reagent
[0045] .
Embodiment 2
[0046] Example 2 Preparation of Blank Gel II
[0047] The experimental procedure is the same as in the above-mentioned Example 1, but adding an appropriate amount of methyl p-hydroxybenzoate while adding Poloxamer407 to the carrageenan stock solution, the resulting gel is blank gel II; the gelation temperature of this embodiment About 22~23°C, wherein the weight percentage of each component is shown in Table 2:
[0048] Table 2
[0049] Reagent
[0050] .
Embodiment 3
[0051] Example 3 Ketorolac tromethamine bioadhesive thermosensitive in situ gel I
[0052] Experimental procedure is identical with above-mentioned Example 1; In gained blank gel I, add ketorolac trometamol (Ket) in right amount and stir evenly, make its final medicine content be 150 mg ACV / g gel; The The gelling temperature of embodiment is about 34 ℃, and wherein each component weight percent is as shown in table 3:
[0053] table 3
[0054]
[0055] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com