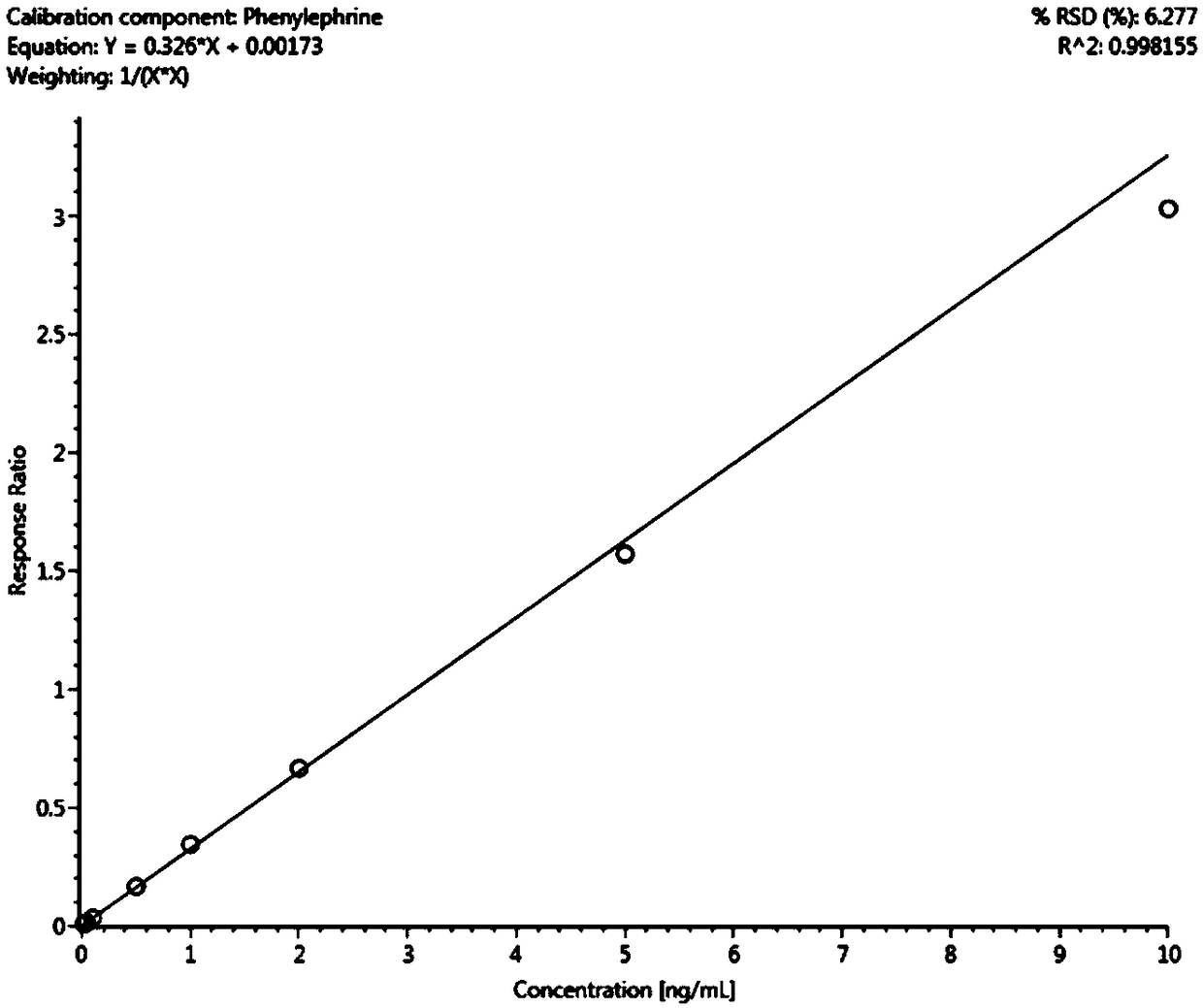
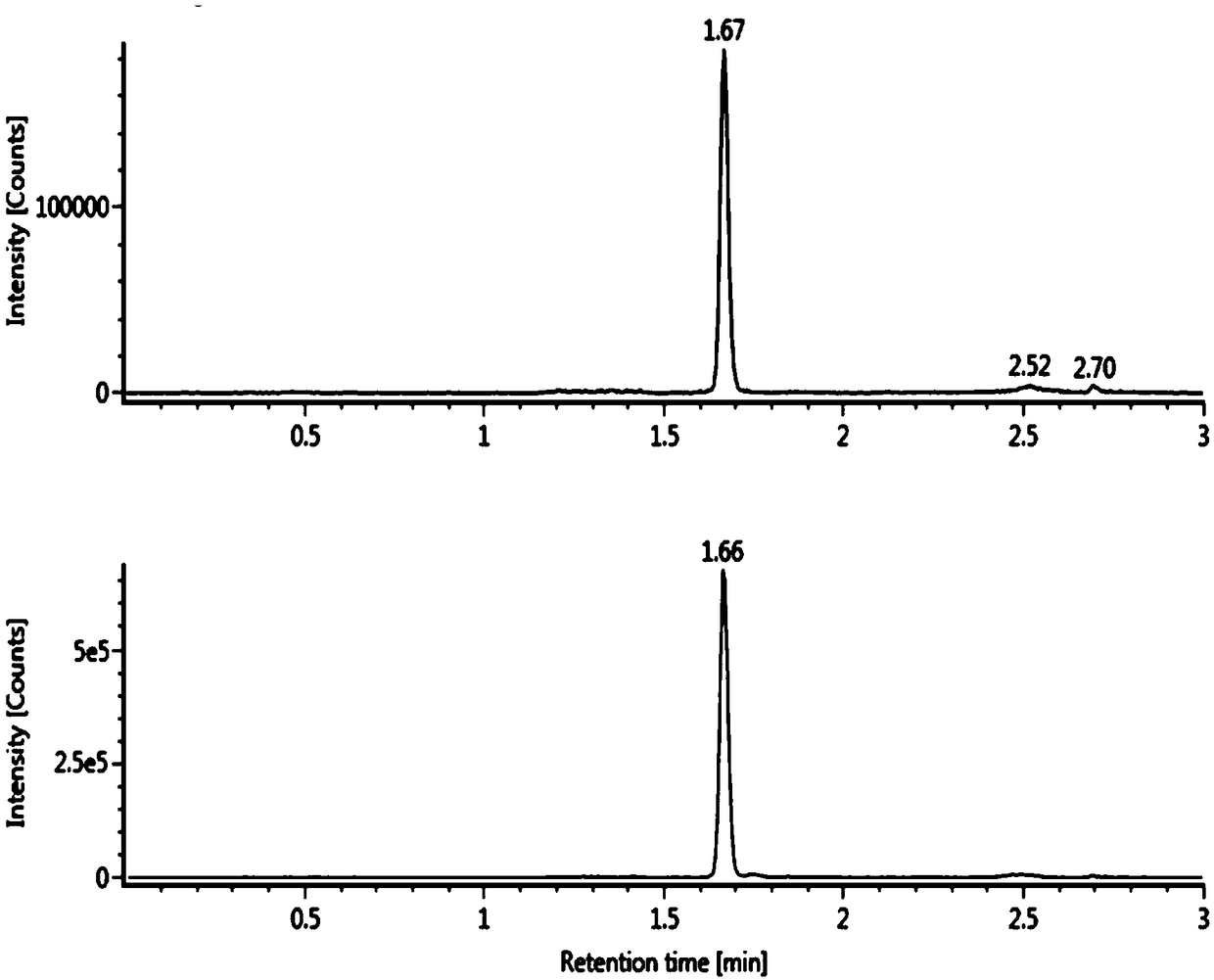
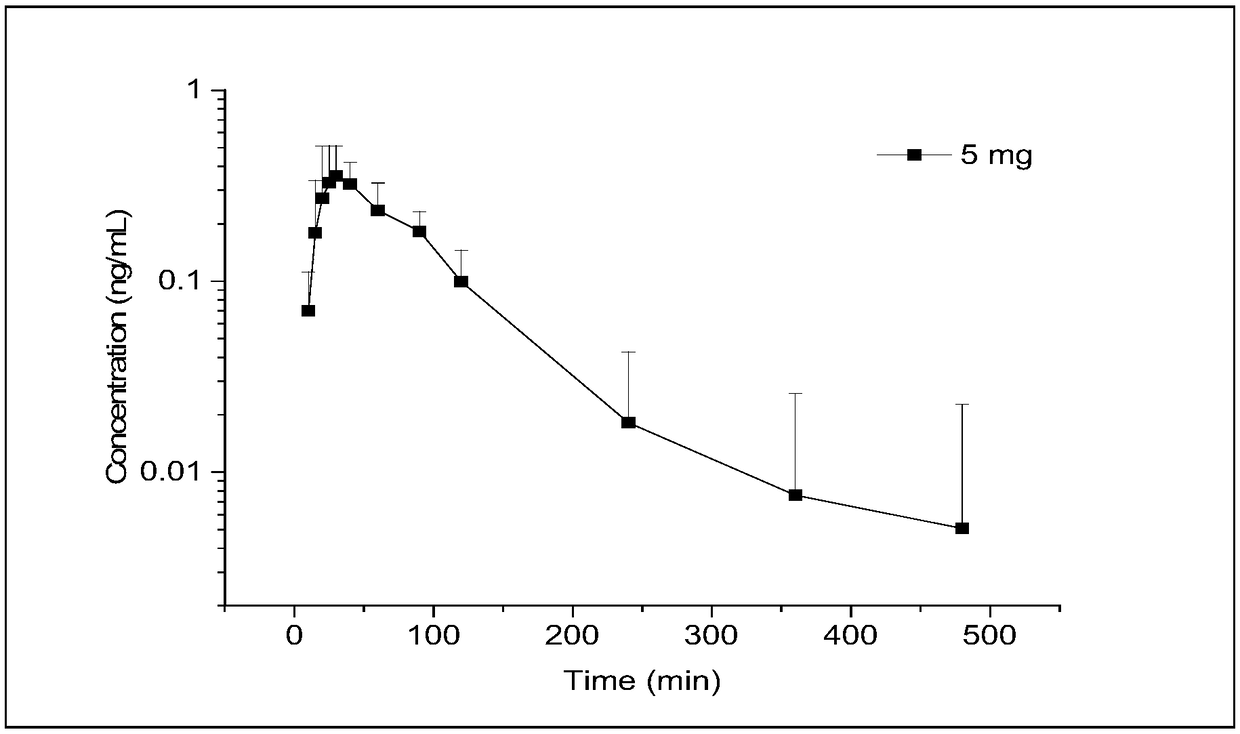
Method for measuring concentration of phenylephrine in plasma by LC-MS/MS, and pretreatment method of sample
A technology for phenylephrine and samples, which is applied in the field of medical testing, can solve the problems of inability to accurately measure the concentration of phenylephrine, incomplete separation of target compounds and impurities, and high quantitative limit of detection methods, so as to achieve rapid and effective detection and reduce detection The effect of limiting and improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Collection of plasma samples to be tested: 12 subjects were fasted overnight for at least 10 hours the day before the administration, and at 0 min, the subjects took renin Namin tablets (batch number: 20170401, each containing hydrochloric acid to Oxynephrine 5mg), each subject was given one tablet, and the subject’s forearm vein was embedded with an indwelling needle. 1 mL of blood was collected at different time points at 40, 60, 90, 120, 240, 360, and 480 min, and placed in a 2 Put in vacuum blood collection tubes with EDTA anticoagulant, mix well, let stand, centrifuge, take 200 μL of the upper layer of plasma, cover the tube tightly, and store in an ultra-low temperature freezer at -60°C to -90°C.
[0068] The method for measuring the concentration of phenylephrine in plasma by LC-MS / MS described in this embodiment specifically includes the following steps:
[0069] (a) Sample pretreatment
[0070] (1) Preparation of standard curve samples: Accurately weigh pheny...
Embodiment 2
[0085] Collection of plasma samples to be tested: 12 subjects were fasted overnight for at least 10 hours the day before the administration, and at 0 min, the subjects took renin Namin tablets (batch number: 20170301, each containing hydrochloric acid to Oxynephrine 10mg), each subject took one tablet, and the subjects were respectively before administration (0min) and after administration 10, 15, 20, 25, 30, 40, 60, 90, 120, 240, 1 mL of blood was collected at different time points at 360 and 480 min, and placed in a 2 Put in vacuum blood collection tubes with EDTA anticoagulant, mix well, let stand, centrifuge, take 200 μL of the upper layer of plasma, cover the tube tightly, and store in an ultra-low temperature freezer at -60°C to -90°C.
[0086] The method for measuring the concentration of phenylephrine in plasma by LC-MS / MS described in this embodiment specifically includes the following steps:
[0087] (a) Sample pretreatment
[0088] (1) Preparation of standard curv...
Embodiment 3
[0101] Collection of plasma samples to be tested: 12 subjects were fasted overnight for at least 10 hours the day before the administration, and at 0 min, the subjects took renin Namin tablets (batch number: 20170402, each containing hydrochloric acid to Oxynephrine 20mg), each subject took one tablet, and the subjects were respectively before administration (0min) and after administration 10, 15, 20, 25, 30, 40, 60, 90, 120, 240, 1 mL of blood was collected at different time points at 360 and 480 min, and placed in a 2 Put in vacuum blood collection tubes with EDTA anticoagulant, mix well, let stand, centrifuge, take 200 μL of the upper layer of plasma, cover the tube tightly, and store in an ultra-low temperature freezer at -60°C to -90°C.
[0102] The method for measuring the concentration of phenylephrine in plasma by LC-MS / MS described in this embodiment specifically includes the following steps:
[0103] (a) Sample pretreatment
[0104] (1) Preparation of standard curv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com