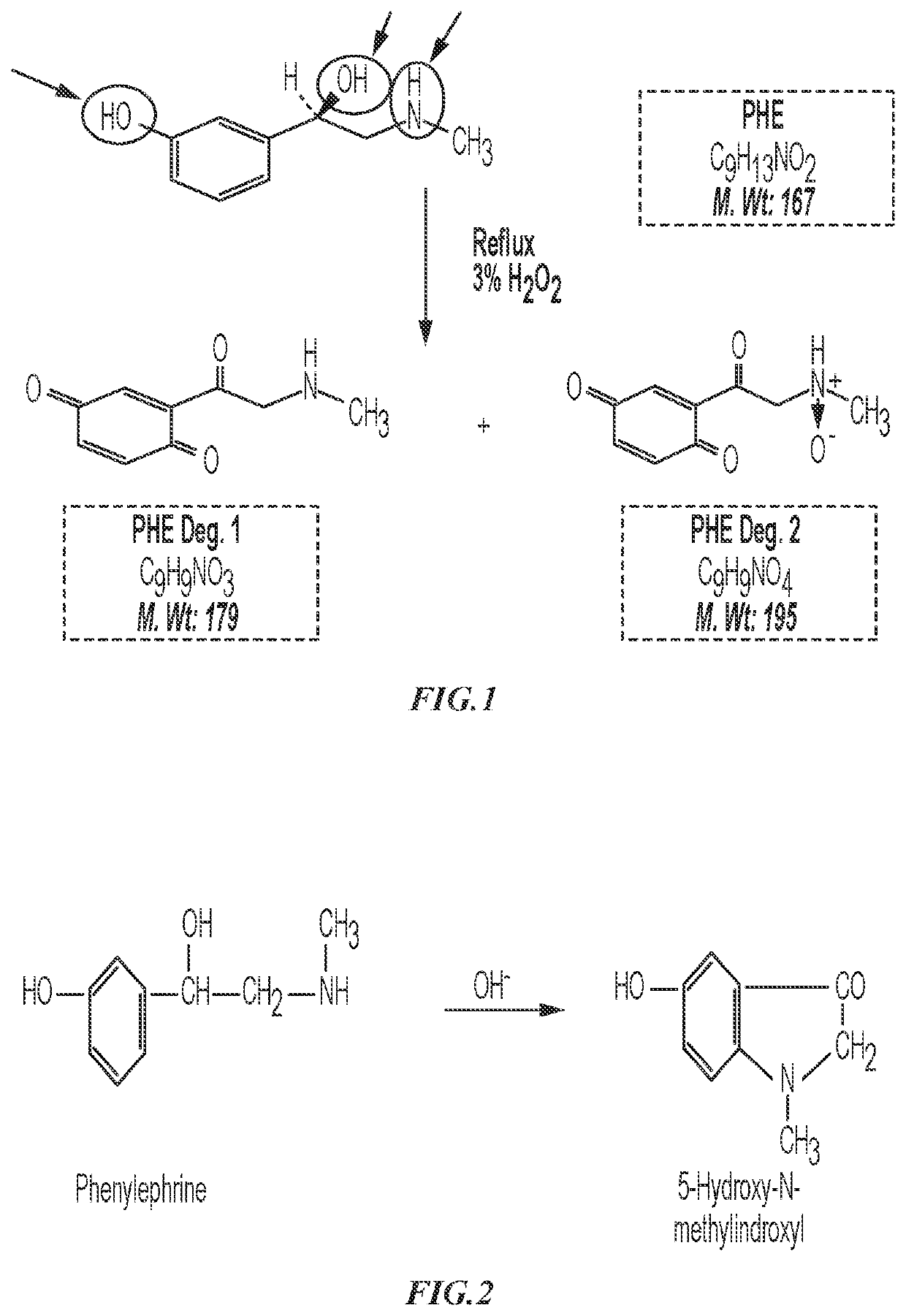
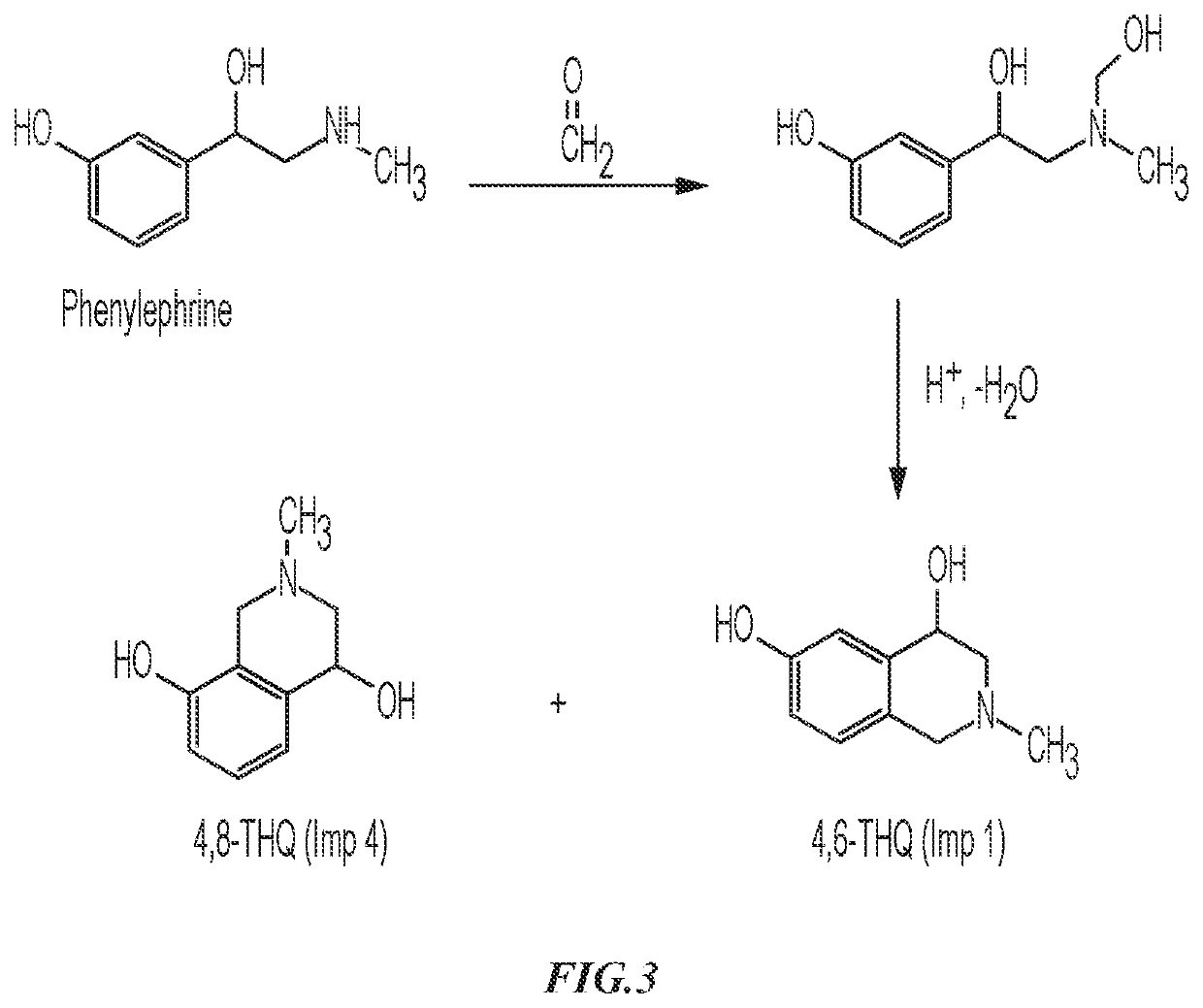
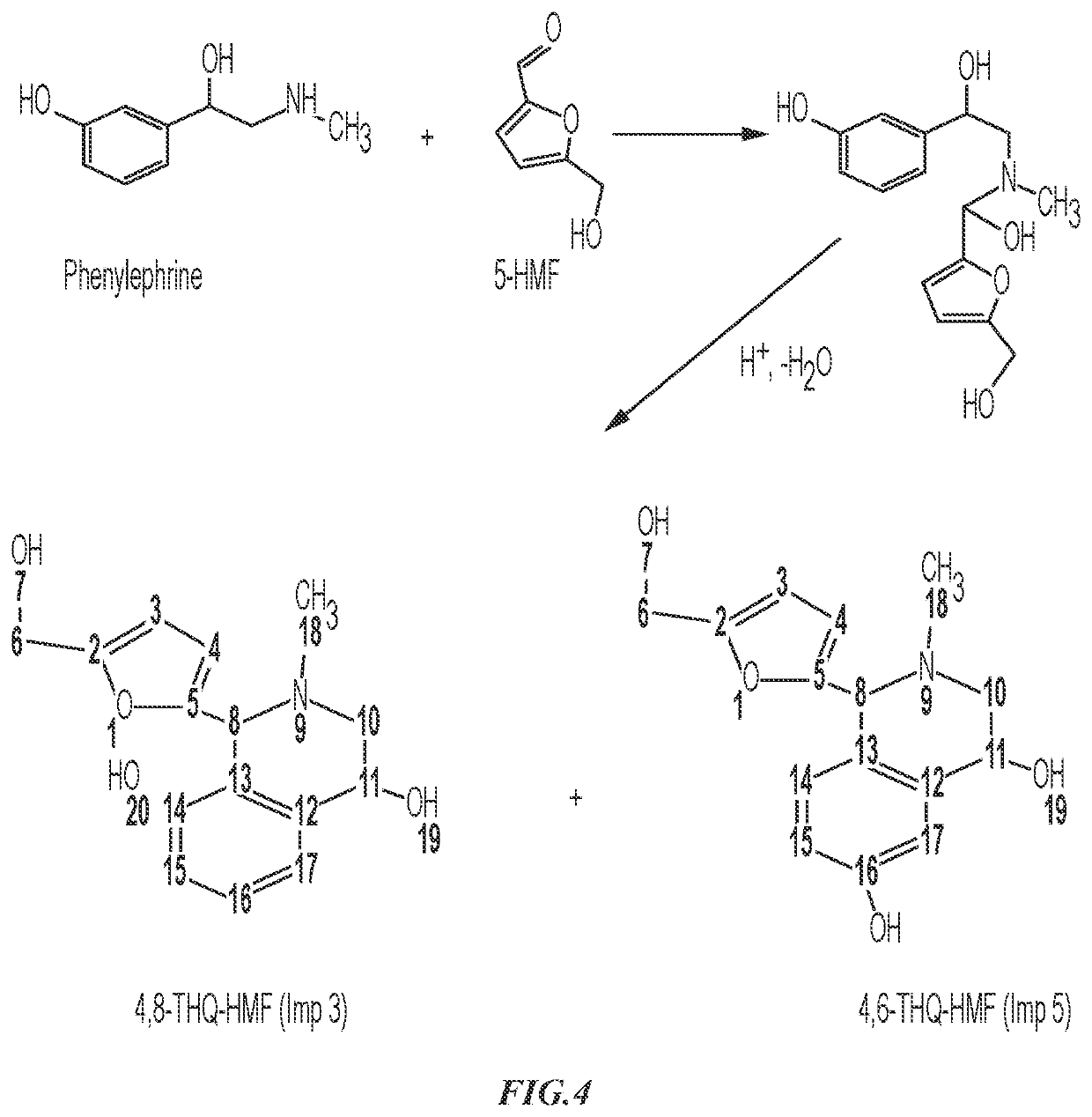
Phenylephrine Hydrochloride Compositions and Containers
a technology of phenylephrine and compositions, applied in the field of pharmaceutical compositions and methods, can solve problems such as microbial contamination or dilution errors, extended stability at low and ready-to-use concentrations, and reflex bradycardia, so as to prolong the shelf life of products and enhance or preserve the chemical stability of phenylephrin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0054]The following examples are provided for illustrative purposes only and should not be interpreted as limiting the present invention. Unless noted otherwise, all quantities indicated in % are weight percent following the general analytical protocol as indicated below.
[0055]Analytical protocol: phenylephrine hydrochloride (PEP) assay is performed using an isocratic reversed-phase HPLC-UV method. Biphenyl-modified core-shell silica particles are used as a stationary phase for chromatographic analysis using Kinetex® 2.6 μm Biphenyl LC Column, 4.6×50 mm, 2.6 μm. The mobile phase is a methanol-water mixture containing ˜0.1% o-phosphoric acid (3:97 v / v Methanol). Quantitation of PEP is accomplished by comparing corresponding peak areas from the sample solution chromatogram and the Phenylephrine Hydrochloride Reference Standard (RS) solution.
[0056]Related substances: Determination of PEP related compounds is performed using a linear-gradient reversed-phase HPLC-UV method. Biphenyl-modi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com