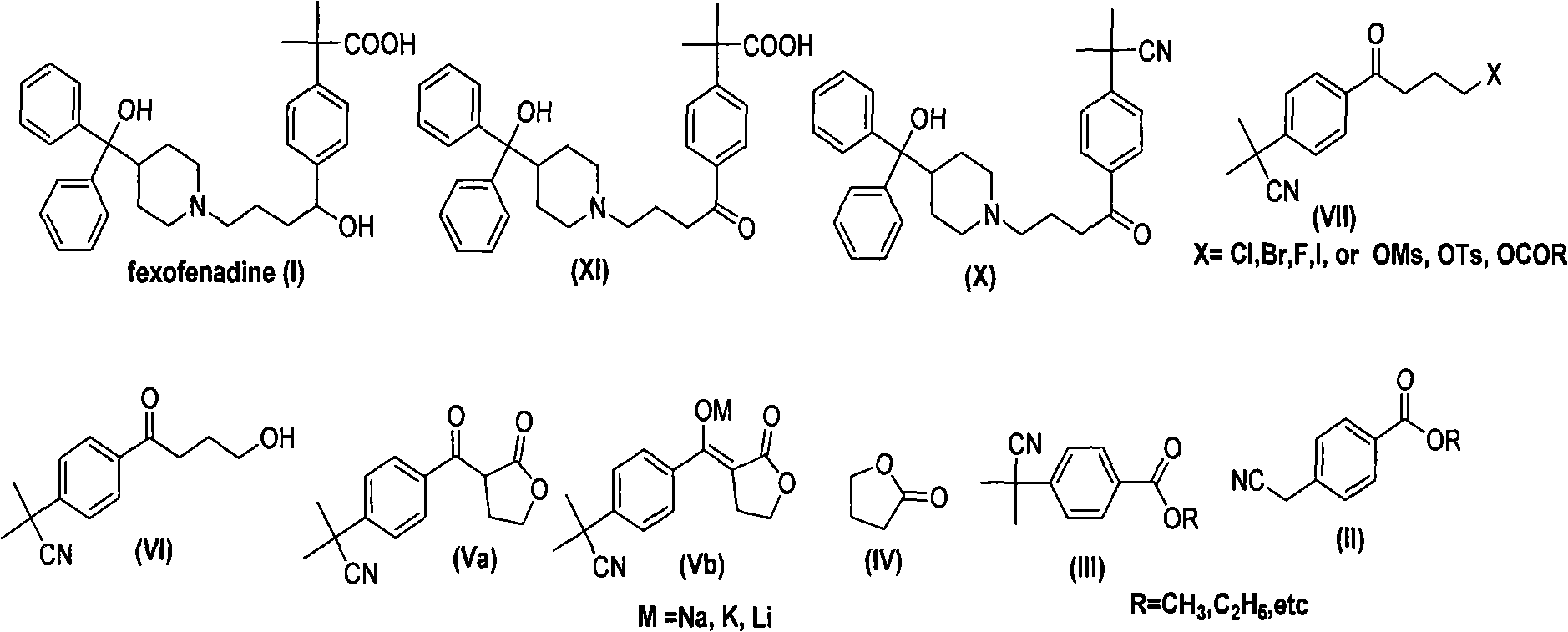
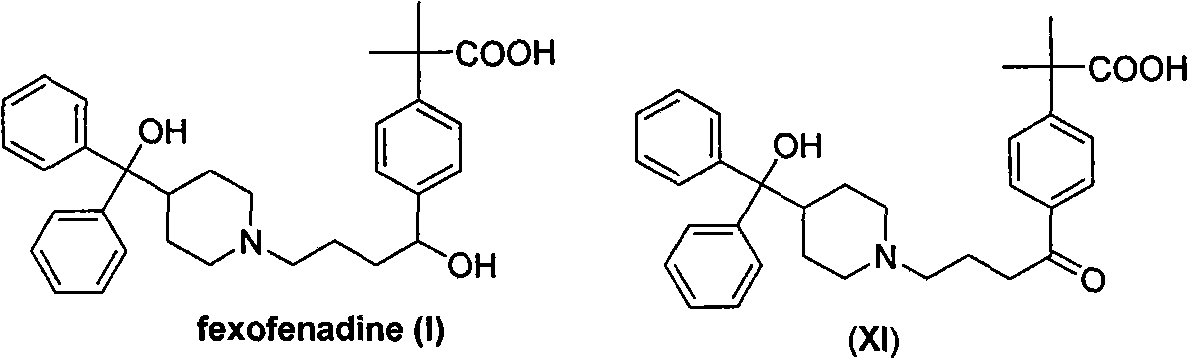
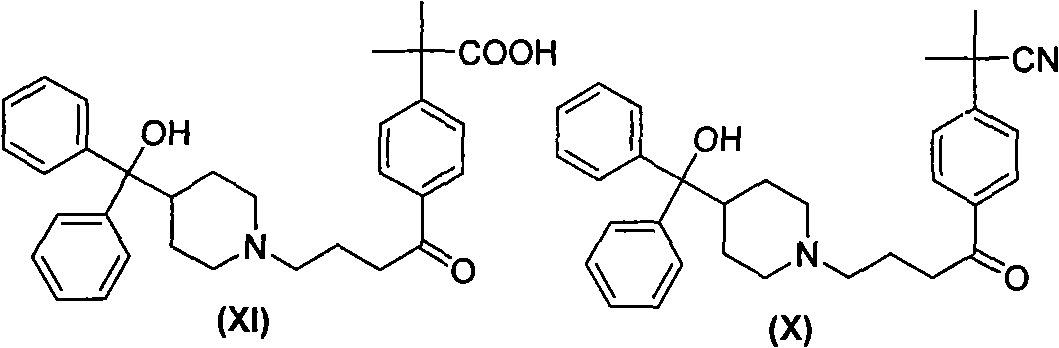
Synthesizing route and preparation method of high-purity fexofenadine and intermediate thereof
A fexofenadine, high-purity technology, used in the field of chemical pharmacy, can solve problems such as poor reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] At 20°C, add 30 g (0.75 mol) of sodium hydroxide and 140 ml of water into 2 g of tetra-n-butylammonium bromide and 21 g (0.12 mol) of methyl p-cyanomethylbenzoate, drop Add 53 g of dimethyl sulfate (0.42 mol), and drop it in about 1 hour. The reaction was continued for 4 hours. Add 100 milliliters of toluene and stir for 20 minutes, let stand, separate layers, extract the water liquid twice with 30×2 milliliters of toluene to combine the organic liquid, wash with water until neutral and recover the toluene under reduced pressure, and recover the toluene under reduced pressure (the recovered toluene continues to be applied to (2) and the resulting solid was recrystallized in isopropanol with a yield of 85%.
Embodiment 2
[0064] At 20°C, add 2 grams of tetra-n-butylammonium bromide and 23 g (0.12 mol) of ethyl p-cyanomethylbenzoate into 2 grams of tetra-n-butylammonium bromide and 23 g (0.12 mol) of ethyl p-cyanomethylbenzoate, drop Add 53 g of dimethyl sulfate (0.42 mol) in DMF, and drop it in about 1 hour. The reaction was continued for 4 hours, DMF was evaporated under reduced pressure, 100 ml of toluene was added and stirred for 20 minutes, allowed to stand, separated into layers, the aqueous liquid was extracted twice with 30×2 ml toluene to combine the organic liquid, washed with water until neutral and the toluene was recovered under reduced pressure, Toluene was recovered under reduced pressure (the recovered toluene was continuously used here), and the obtained solid was recrystallized in isopropanol with a yield of 88%.
Embodiment 3
[0066] At 5°C, add 2 grams of tetra-n-butylammonium bromide and 21 g (0.12 mol) of methyl p-cyanomethylbenzoate to 2 grams of tetra-n-butylammonium bromide and 21 g (0.12 mol) of methyl p-cyanomethylbenzoate, drop Add 53 g of dimethyl sulfate (0.42 mol), and drop it in about 1 hour. The reaction was continued for 4 hours. Add 100 ml of toluene and stir for 20 minutes (the original temperature is still maintained at this time), stand still, separate layers, extract the water liquid twice with 30×2 ml toluene to combine the organic liquid, wash with water until neutral and recover the toluene under reduced pressure, and recover under reduced pressure Toluene (recovered toluene continues to be used here), and the obtained solid is recrystallized in isopropanol with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com