Pharmaceutical composition, preparation method and application thereof
A composition and drug technology, applied in the field of medicine, to achieve the effects of good safety, improved pharmacokinetic properties, and rapid onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This embodiment provides a preferred prescription and coating layer screening example of the typical preparation of the present invention sustained-release pellet capsule:
[0046]The formulation screening work was carried out through the in vitro release characteristics of the drug. The stability and release of the trial product were investigated, and the following formulation process was developed. The dosage form of sustained-release pellets and capsules was developed, which is a multi-unit sustained-release drug. According to the characteristics of pellets, the fluidized bed coating technology was used for sample development.
[0047] (1) Prescription:
[0048] Ingredients Prescription (g)
[0049] Fexofenadine Hydrochloride 60
[0050] Pseudoephedrine hydrochloride 120
[0051] Ethyl cellulose 180
[0052] Hypromellose 60
[0053] Make 1000 capsules.
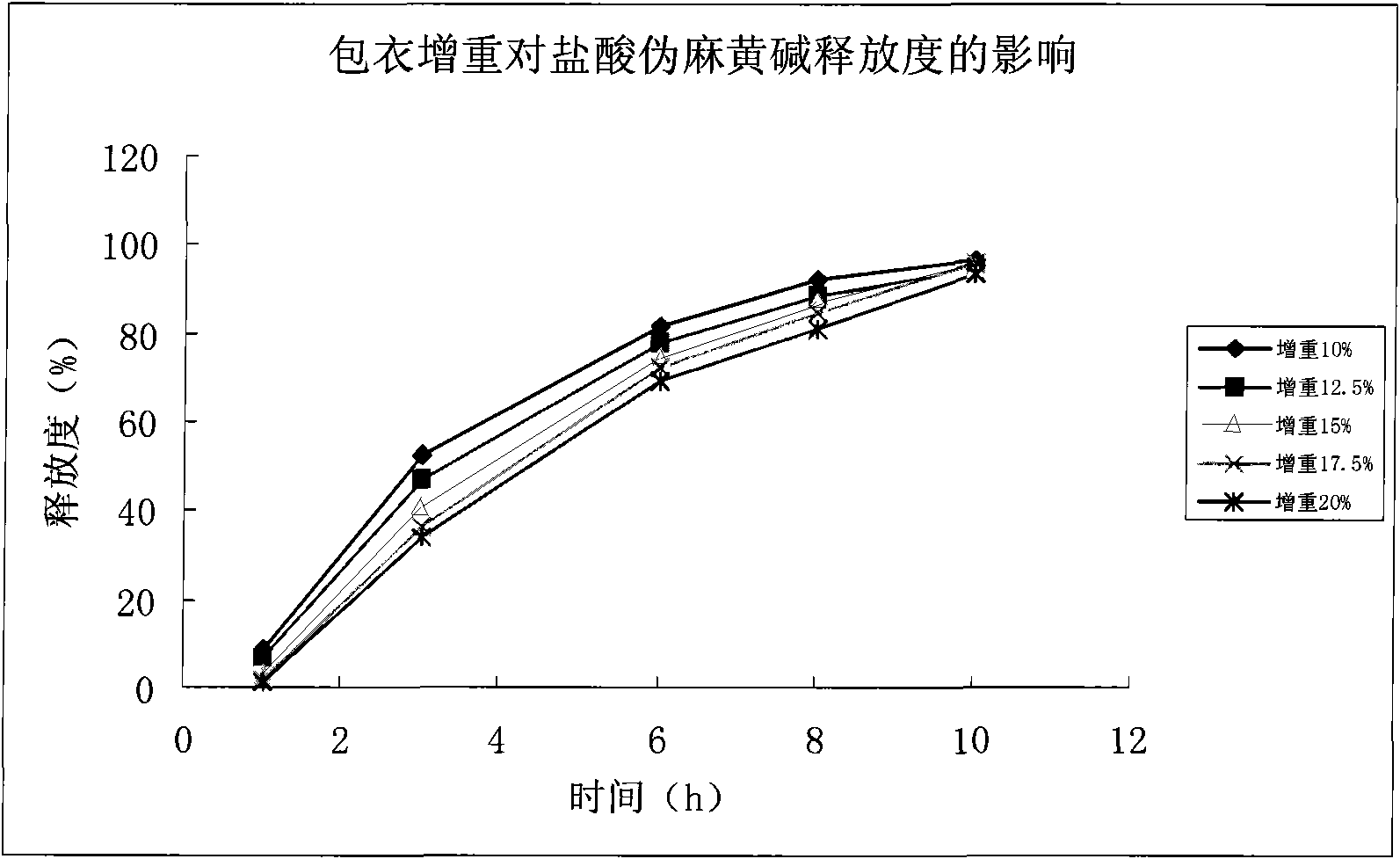
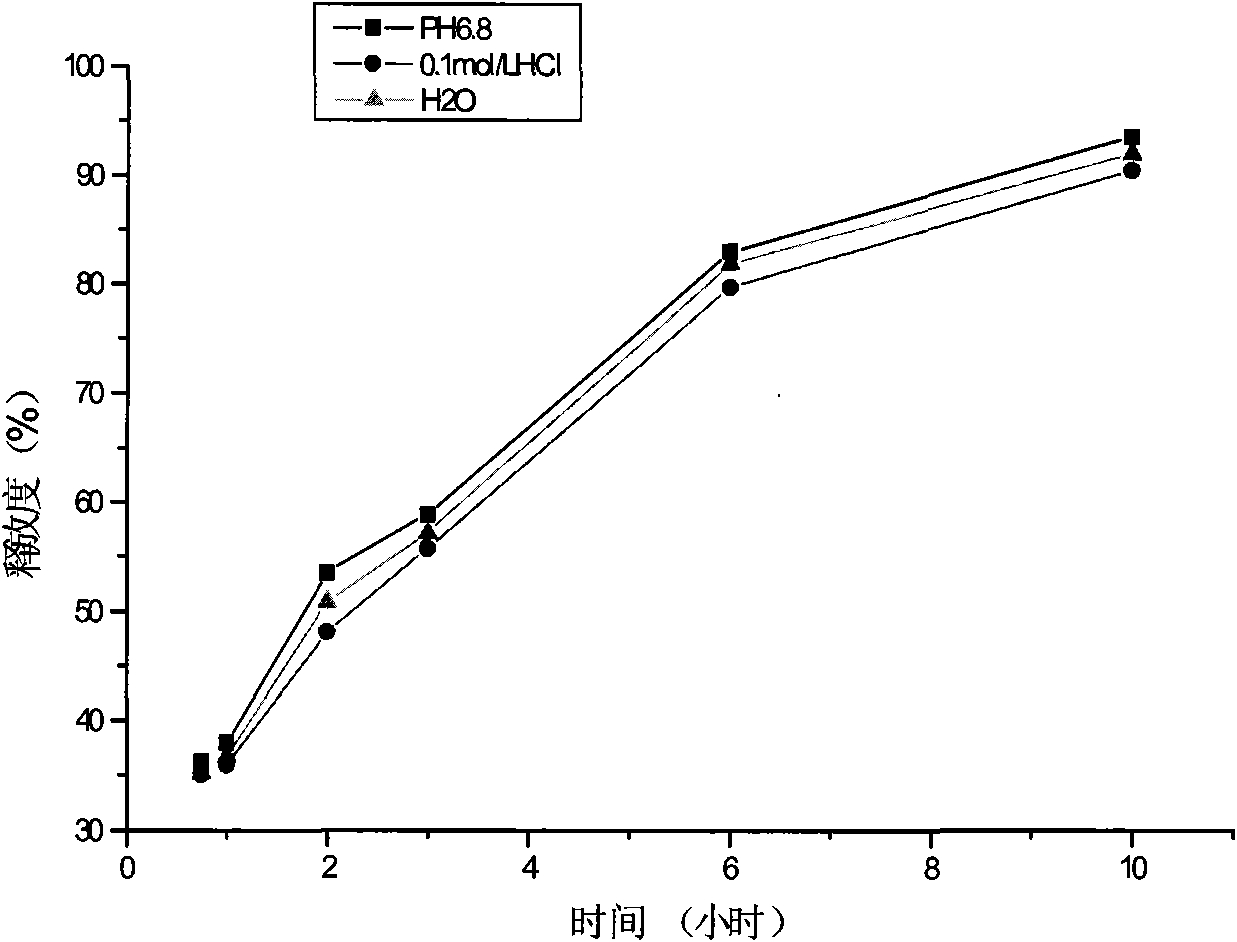
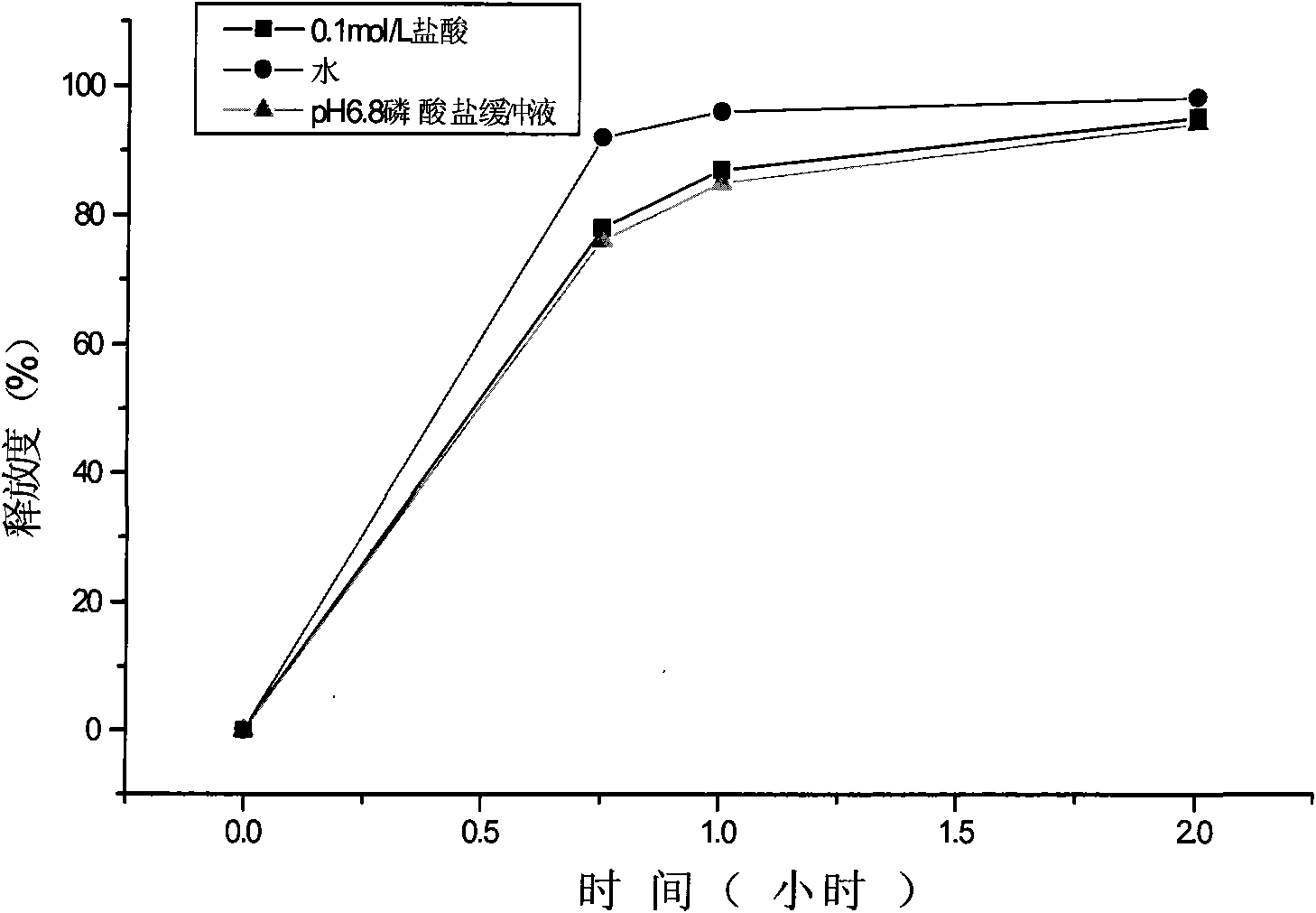
[0054] (2) Determination of the release rate of pseudoephedrine hydrochloride and the screening of the coat...
Embodiment 2
[0065] This example provides a method for preparing a typical formulation of the invention.
[0066] (1) Pseudoephedrine hydrochloride drug layer:
[0067] 1. Raw material ratio
[0068] table 3
[0069] raw material name
weight ratio
Pseudoephedrine hydrochloride
500
1
[0070] 2. Preparation of pseudoephedrine hydrochloride drug layer
[0071] Dissolve hypromellose in water, add pseudoephedrine hydrochloride (PSD), and stir until dissolved. spare.
[0072] 3. Fluidized bed (fluidized bed) feeding operation
[0073] Put 1000g of blank sugar pills into the coating machine, adjust the air volume and sleeve position to a proper fluidization state, spray the above suspension, adjust the spray speed to make the bed temperature 32-35°C.
[0074] (2) Isolation layer:
[0075] 1. Raw material ratio
[0076] Table 4
[0077] raw material name
weight ratio
1
talcum powder
...
Embodiment 3
[0108] This embodiment provides the sustained-release pellet capsules of embodiment 1 or 2 and loratadine pseudoephedrine sustained-release tablets to carry out multi-center randomized double-blind double-dummy positive drug parallel controlled trials and results for evaluating Feso pseudoephedrine sustained-release capsules Efficacy and safety in the treatment of allergic rhinitis and colds.
[0109] The comparison results in the group before and after the two groups of drug treatment are as follows:
[0110] 3.1. After the two groups of subjects received different test drugs, the total score of the disease decreased significantly compared with the baseline level on the 7th day and the 14th day of drug use (p<0.0001).
[0111] FAS analysis results:
[0112] On the 7th day of medication: the total effective rate of the test group was 92.92%, and the marked rate was 45.13%; the total effective rate of the control group was 89.19%, and the marked rate was 38.74%.
[0113] On t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com