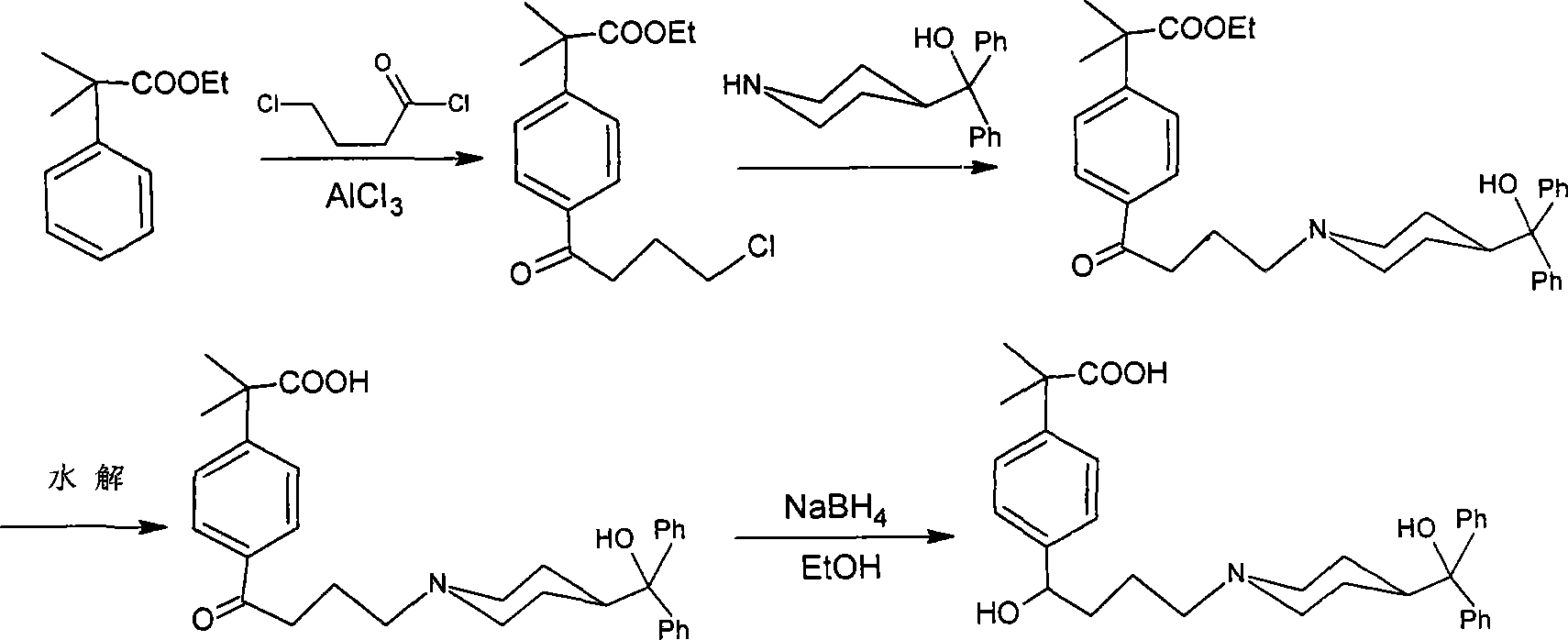
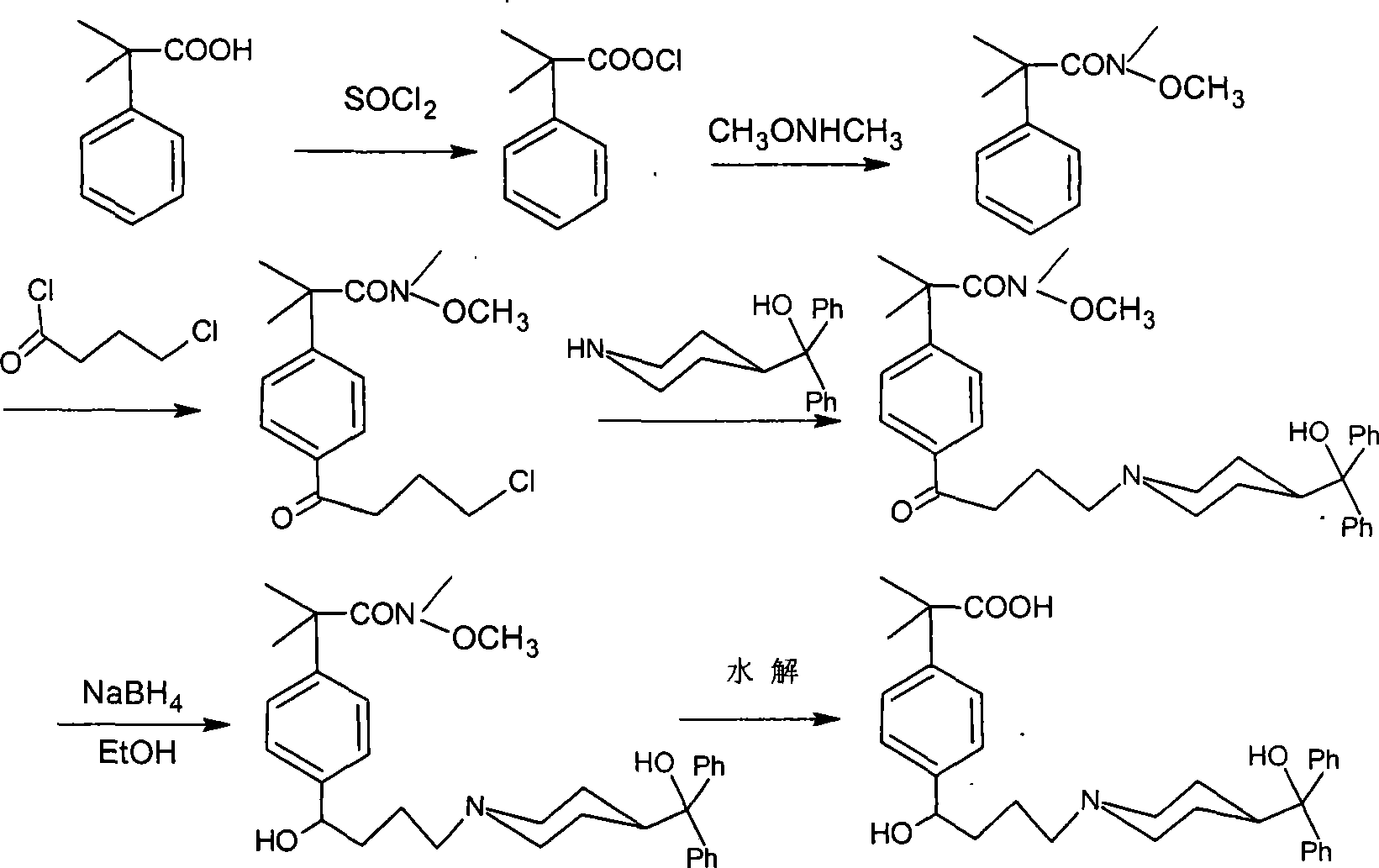
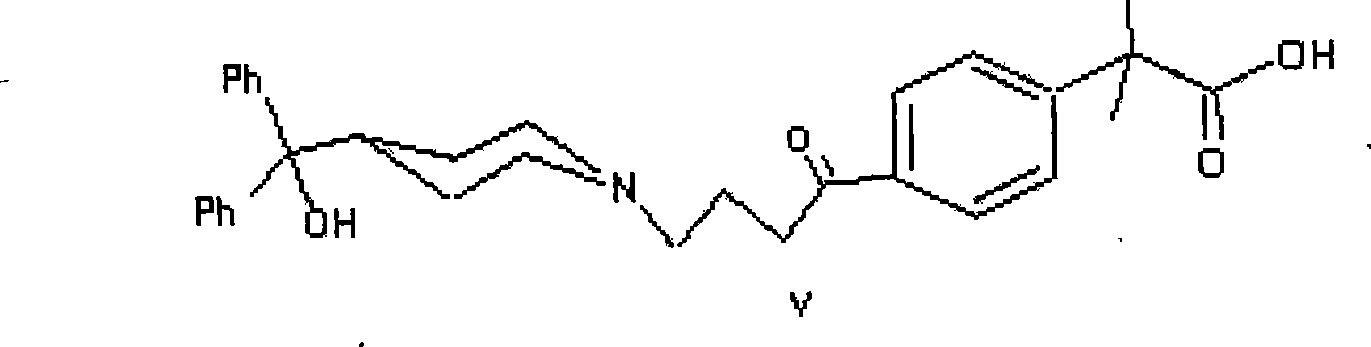Method for synthesizing intermediate of fexofenadine
A synthetic method and intermediate technology, which is applied in the field of synthesis of fexofenadine intermediates, can solve the problems of lower yield, higher cost, longer overall route, etc., and achieve the effect of solving the problem of product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthesis of embodiment one intermediate 2-phenyl-2-methyl propanol acetate (i)
[0039] After adding methacryl acetate (228 g, 2.0 mol) and 1200 ml of benzene into the three-necked flask, the temperature was lowered to 5°C. And keeping the temperature below 5°C, add anhydrous aluminum trichloride (399g, 3.0mol), keep the reaction at this temperature for 1 hour, pour into the ice-water mixture, and add 100ml of hydrochloric acid, separate the organic phase, and wash the organic phase with water. phase to neutral, dried over sodium sulfate, and after recovering benzene, distilled under reduced pressure to obtain the intermediate 2-phenyl-2-methacryl alcohol acetate, whose content was detected by GC.
Embodiment 2
[0040] Example two Synthesis of 2-(4-(4-chloro-1-oxo-butyl))phenyl-2-methylpropanol acetate (ii)
[0041] Intermediate i (150g, 0.78mol) was dissolved in 500ml of dichloromethane, cooled to below 5°C, anhydrous aluminum trichloride (208g, 1.56mol) was added, and 4-chlorobutyryl chloride was added dropwise at this temperature, React at 0-5°C for 15h. The reaction solution was slowly poured into a mixture of ice and water, 150ml of hydrochloric acid was added, the organic phase was separated, the organic phase was washed with aqueous sodium bicarbonate solution and water respectively, dried, and the solvent was evaporated to dryness to obtain intermediate ii as an oil (221.1g, 95.6% ). It was directly used in the next reaction without purification.
[0042] 1 H NMR (300MHz, CDCl 3 ( 2H each, d).
Embodiment 3
[0043] Synthesis of Example Three 2-(4-(4-chloro-1-oxo-butyl))phenyl-2-methyl propanol (iii)
[0044] Add 2-(4-(4-chloro-1-oxo-butyl))phenyl-2-methylpropenol acetate, 600ml of 25% hydrochloric acid, and 1500ml of ethanol into the reaction flask, and reflux for 3 hours. After ethanol was evaporated to dryness, dichloromethane was added to the residue, and the organic phase was washed with aqueous sodium bicarbonate and water, dried, and the solvent was evaporated to give intermediate iii as an oil (211 g, 95%). It was directly used in the next reaction without purification.
[0045] 1 H NMR (300MHz, CDCl 3 )δ1.35 (6H, s), 2.21 (2H, quent.) 3.15, (2H, t), 3.64 (2H, s), 3.66 (2H, 5), 7.48, 7.93 (2Heach, d).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com