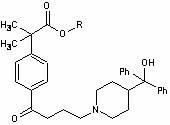
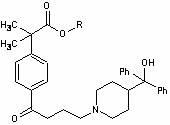
Preparation method of fexofenadine intermediate
A technology of fexofenadine and intermediates, which is applied in the field of medicine and fine chemical industry, can solve the problems of short synthesis route, difficulty in separating and purifying product isomers, high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. Preparation of methyl α,α-dimethylphenylacetate (II).
[0038] Add 480kg of methanol into a 1000L reactor, add 100kg of α,α-dimethylphenylacetic acid under stirring, and stir to dissolve. Pass ice brine to cool down, and cool to -5°C to 0°C. Slowly add 15kg of concentrated sulfuric acid dropwise, after the addition is complete, stir for 30 minutes. Slowly raise the temperature and reflux for 4-5 hours, then distill off excess methanol. Cool down, dissolve the residue with 320kg of dichloromethane, wash with 160kg of 10% aqueous sodium bicarbonate solution twice (80kg each), wash twice with 160kg of water (80kg each), and dry with 5kg of anhydrous sodium sulfate 6 hours. Suction filtration, concentration and recovery of dichloromethane to obtain 94kg of oily product α,α-dimethylphenylacetic acid methyl ester (II).
Embodiment 2、4
[0039] Example 2, Preparation of 4-[4-chloro-1-butyryl]-α,α-dimethylphenylacetic acid methyl ester (Ⅲ).
[0040] Add 950kg of dried dichloromethane (dried with anhydrous calcium chloride or molecular sieves) into a 2000L reactor, add 172kg of anhydrous aluminum trichloride under stirring, and stir for 3 hours. Cool to -5°C ~ 0°C. Control the temperature at -10°C to -5°C, and slowly add 90kg of 4-chlorobutyryl chloride dropwise. After the dropwise addition was completed, the mixture was incubated and stirred for 3 hours.
[0041] Control the temperature of the system at -10 to 0°C, and slowly add 94kg of methyl α,α-dimethylphenylacetate (II). After the feeding is completed, keep stirring for 2 hours, then raise the temperature to 20-25°C and react for 24 hours, and the raw material point basically disappears as monitored by TLC.
[0042]Mix 380kg of water and 170kg of concentrated hydrochloric acid to prepare dilute hydrochloric acid, pass cooling brine to cool down to 0-5°C...
Embodiment 3
[0045] Example 3. Preparation of 4-[4-chloro-1-butyryl]-α,α-dimethylphenylacetic acid (IV).
[0046] Add 140kg of 4-[4-chloro-1-butyryl]-α,α-dimethylphenylacetic acid methyl ester (Ⅲ), 350kg of concentrated hydrochloric acid, 180kg of water and 700kg of glacial acetic acid into a 1000L reactor, and heat to reflux for about 40- 45 hours.
[0047] The reaction was terminated, and the glacial acetic acid was evaporated under reduced pressure. After most of the glacial acetic acid was evaporated, 150kg of water was added.
[0048] The reaction solution was extracted 3 times with dichloromethane (200kg for the first time, 160kg for the second time, and 160kg for the third time). Combine the extracted organic phases, wash 3 times with drinking water, each 150kg. The organic phase was stirred and dried with 8 kg of anhydrous sodium sulfate, filtered with suction, and dichloromethane was recovered from the filtrate, and the oily product 4-[4-chloro-1-butyryl]-α,α-dimethylphenylaceti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com