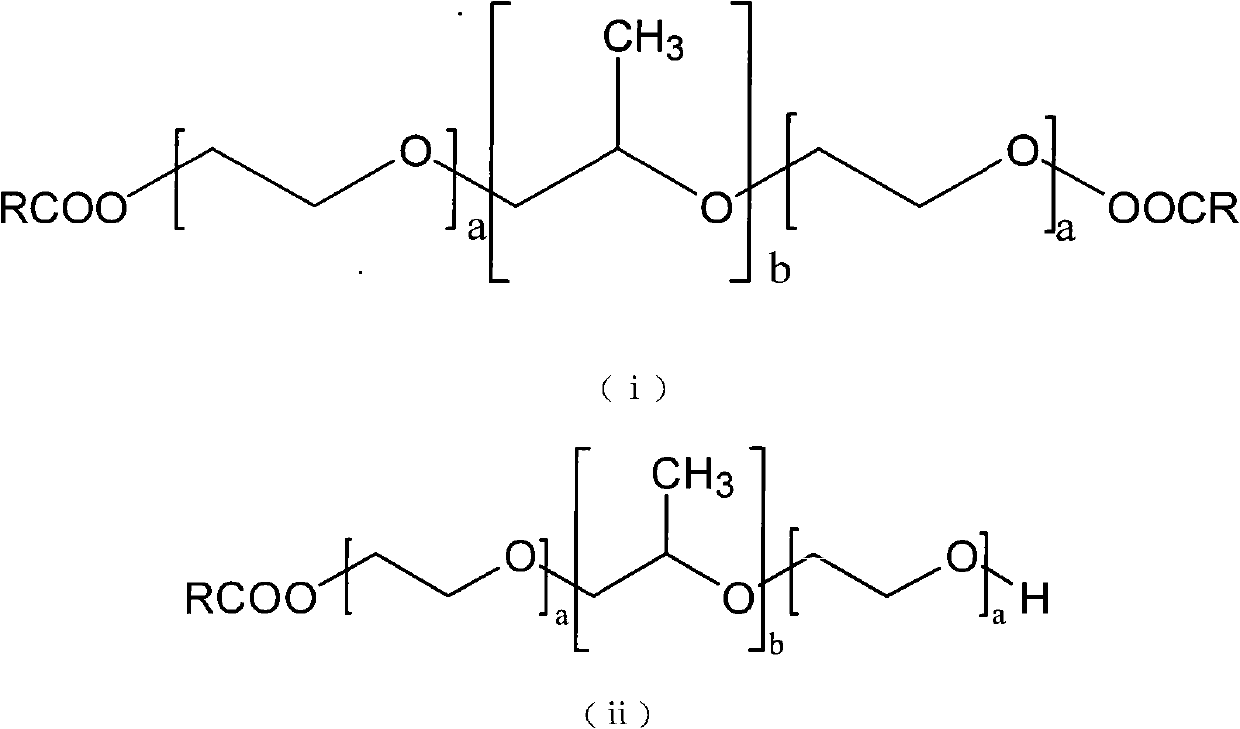
Poloxamer-carboxylic acid drug conjugate and preparation method and application thereof
A technology of carboxylic acid drugs and poloxamers, which is applied in pharmaceutical formulations, medical preparations of non-active ingredients, powder delivery, etc., can solve the problems of fast degradation, lack of drug release effect, and difficult chemical reactions of polymers And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
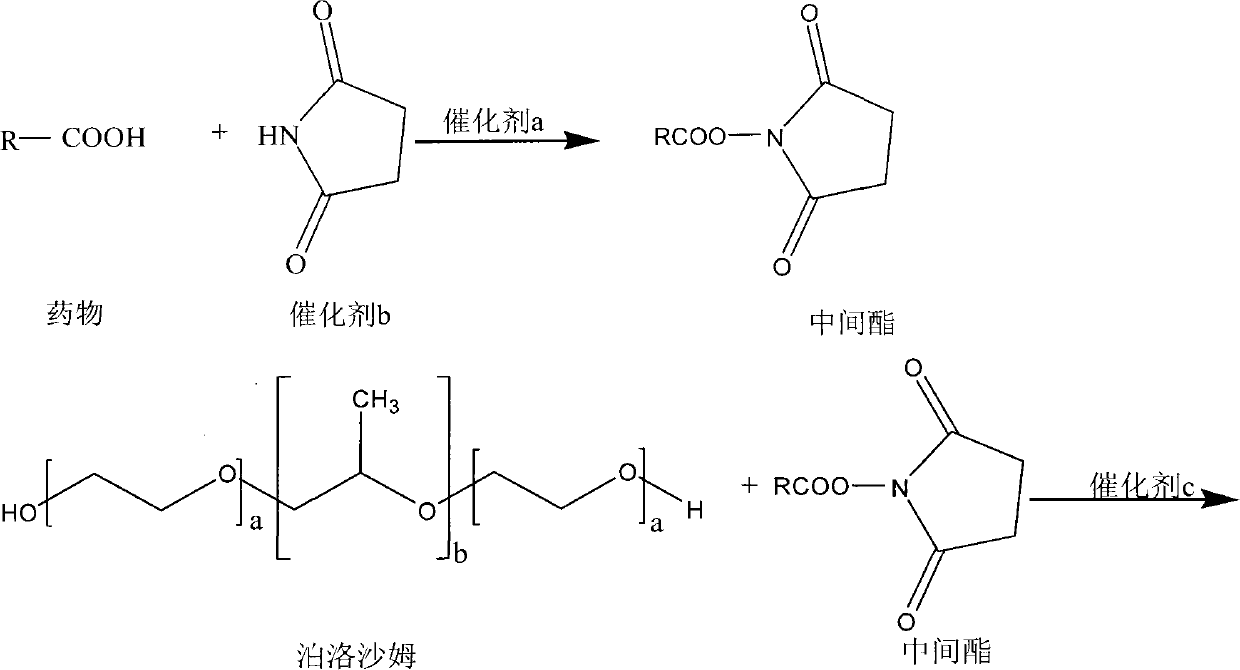
[0022] 1. Preparation of active intermediate ester
[0023] Dissolving carboxylic acid drugs in a suitable organic solvent, adding catalyst a and catalyst b, and performing condensation reaction under temperature control to obtain an active intermediate ester.
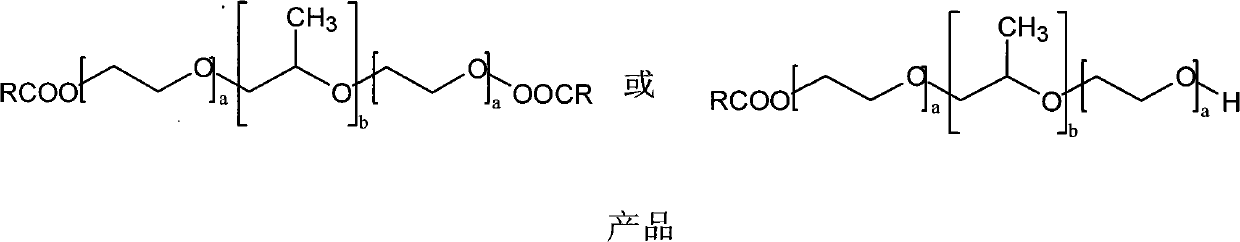
[0024] 2. Preparation of poloxamer-carboxylic acid drug conjugates
[0025] a. Dissolve a certain amount of poloxamer and catalyst c in an appropriate solvent, slowly add the organic solution of the above-mentioned intermediate ester into the poloxamer solution dropwise at 0-4°C, and control the temperature until the reaction is complete.
[0026] b. The above reaction solution is dialyzed in an appropriate organic solvent, the dialysate is constantly replaced, and the drug concentration in the external dialysate is detected at the same time, until the free drug is completely separated by dialysis, add 5 to 10 times the volume of water to the product in the dialysis bag, Continue dialysis in distilled water to remove ...
Embodiment 1
[0044] Example 1: Synthesis of Poloxamer-Methotrexate
[0045] Take 10mmol of methotrexate, 15mmol of dicyclohexylcarbodiimide (DCC), and 15mmol of hydroxysuccinimide (NHS), dissolve them in 30ml of N,N-dimethylformamide, protect them from light and nitrogen, React in an ice bath for 30 minutes, then rise to room temperature and react for 24 hours. After the reaction, centrifuge to remove the precipitate to obtain an organic solution of the intermediate ester; dissolve 10 mmol poloxamer and 15 mmol 4-lutidine (DMAP) in 20 ml N, N -In dimethylformamide, slowly drop the intermediate ester solution into the above solution, and react at 50°C for 24 hours; after the reaction, transfer the reaction solution to a dialysis bag, and dialyze in N,N-dimethylformamide, Remove free drugs and catalysts, and change the dialysate continuously until the presence of drugs is no longer detected in the dialysate; dilute the dialysate in the dialysis bag by 5 times, add it to another dialysis bag ...
Embodiment 2
[0046] Embodiment 2: the synthesis of poloxamer-all-trans retinoic acid
[0047] Take 10mmol all-trans retinoic acid, 20mmol dicyclohexylcarbodiimide (DCC), 20mmol hydroxysuccinimide (NHS), dissolve in 30ml N,N-dimethylformamide, protect from light and nitrogen , react in ice bath for 30min, then rise to room temperature and react for 24h. After the reaction is over, centrifuge to remove the precipitate to obtain an organic solution of intermediate ester; dissolve 5mmol poloxamer and 20mmol 4-lutidine (DMAP) in 20ml dichloro In the methane solution, the intermediate ester solution was slowly added dropwise to the above solution, and reacted at room temperature for 24 hours; after the reaction, the reaction solution was transferred to a dialysis bag, and dialyzed in N,N-dimethylformamide to remove free drugs and catalysts , and constantly change the dialysate until the presence of drugs is no longer detected in the dialysate; dilute the dialysate in the dialysis bag by 5 times,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com