Modified release ibuprofen dosage form
a technology of ibuprofen and modified release, which is applied in the direction of biocide, drug composition, inorganic non-active ingredients, etc., can solve the problems of tablets sticking, not maintaining the therapeutic level of one treatment, and substantial delay between administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
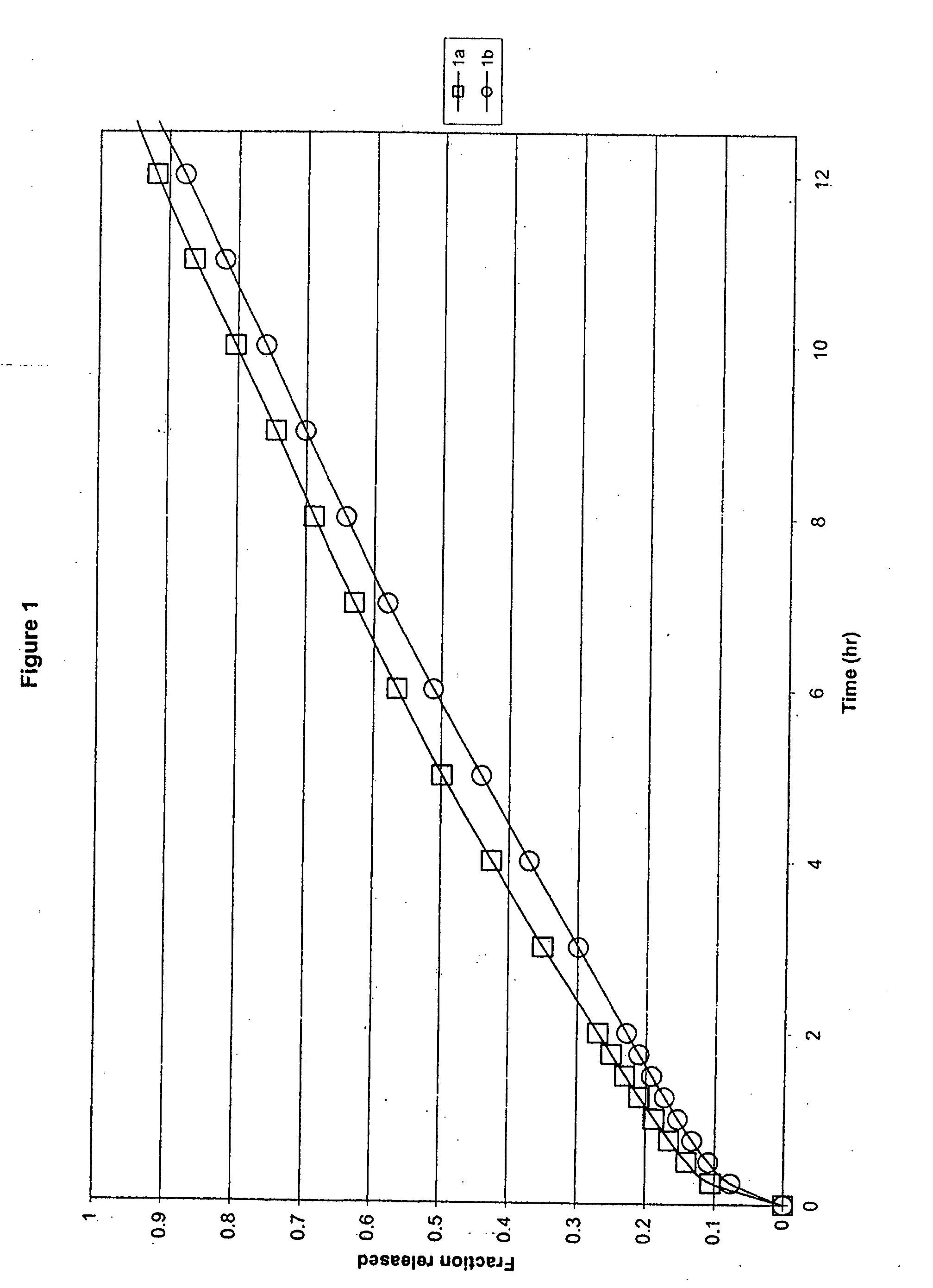
[0043] In one embodiment, the formulation comprised ibuprofen, hydroxypropyl methylcellulose (HPMC K15M and HPMC K100LV), glycine and sodium carbonate, in which HPMC K15M was present at a concentration of 18% by weight of ibuprofen, Ex. 1a, and at a concentration of 21% by weight of ibuprofen, Ex. 1b, HPMC K100LV was present at a concentration of 17% by weight of ibuprofen, glycine was present at a concentration of 2.5% by weight of ibuprofen, and sodium carbonate was present at a concentration of 17% by weight of ibuprofen within a monolithic compressed tablet. The specific formulations are as follows:
Ex. 1amgEx. 1bmgIbuprofen 90 grade600Ibuprofen 90 grade600HPMC K15M110HPMC K15M125HPMC K100LV100HPMC K100LV100MCC PH102100MCC PH102100Na2CO3, anhydrous150Na2CO3, anhydrous150Glycine15Glycine15Silica, Syloid 24420Silica, Syloid 24420Mg Stearate10Mg Stearate10Total:1105Total:1120
[0044] All ingredients were passed through a 30-mesh screen and blended with the remaining formulation comp...
example 2
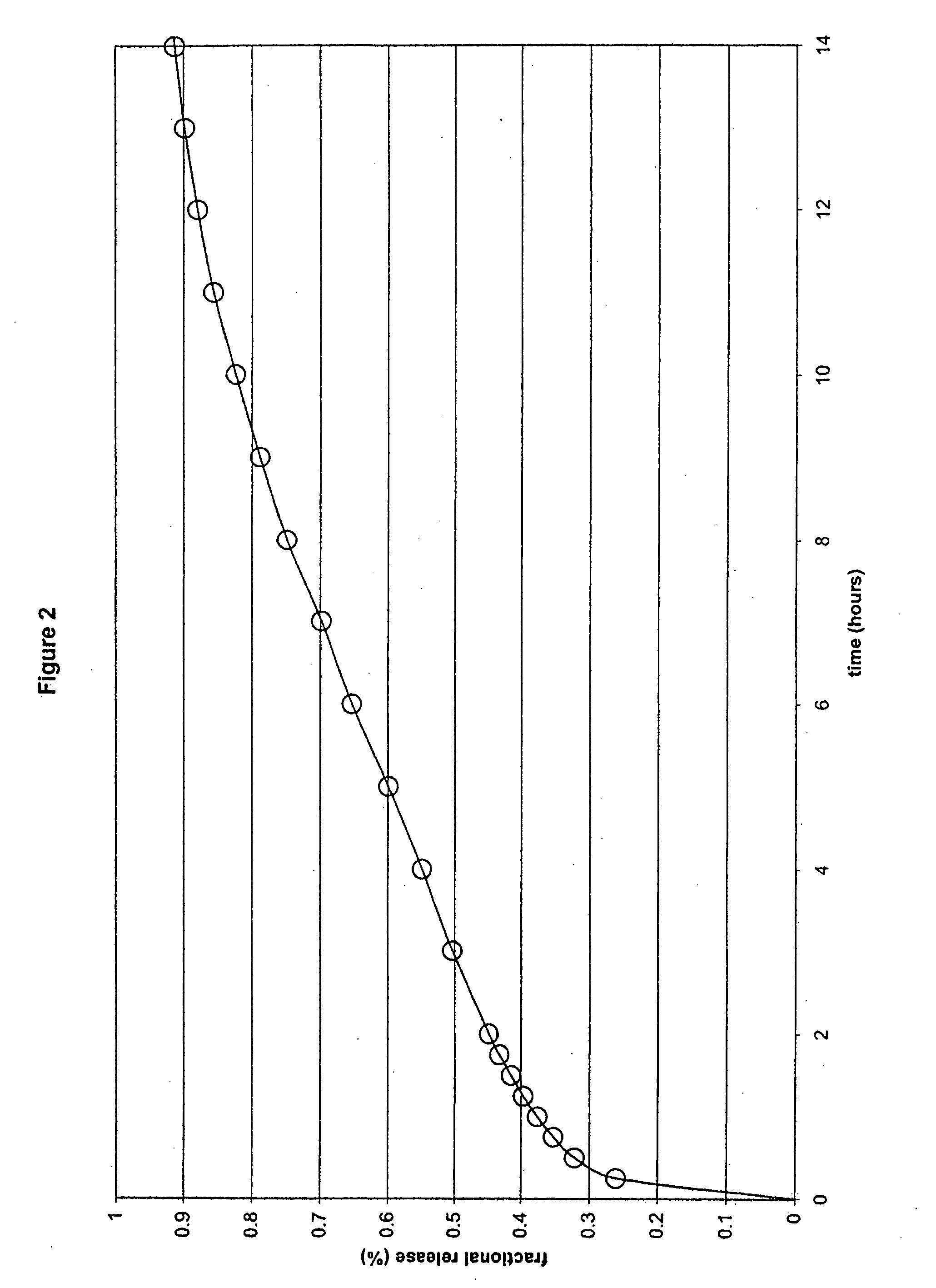
[0046] In another embodiment, the formulation comprised ibuprofen, hydroxypropyl methylcellulose (HPMC K100M and HPMC K100LV), sodium carbonate, flow agents and tableting aids, in which HPMC K100M was present at a concentration of 17% by weight of ibuprofen, HPMC K100LV was present at a concentration of 17% by weight of ibuprofen, and sodium carbonate was present at a concentration of 25% by weight of ibuprofen within a compressed monolithic tablet. The specific formula is as follows:
Ex. 2mgIbuprofen600HPMC K100M100HPMC K100LV100Na2CO3, anhydrous150MCC PH102150Silica, Syloid 24420Mg Stearate10Total:1130
[0047] The formulation components were mixed in a V-blender. The resulting powder was compressed into tablets using conventional technologies. In this Example a combination of a medium to high viscosity HPMC and a low viscosity HPMC were used.
[0048] As shown in FIG. 2, the results of this Example demonstrate an in vitro release profile comprising a burst effect, followed by the sus...
example 3
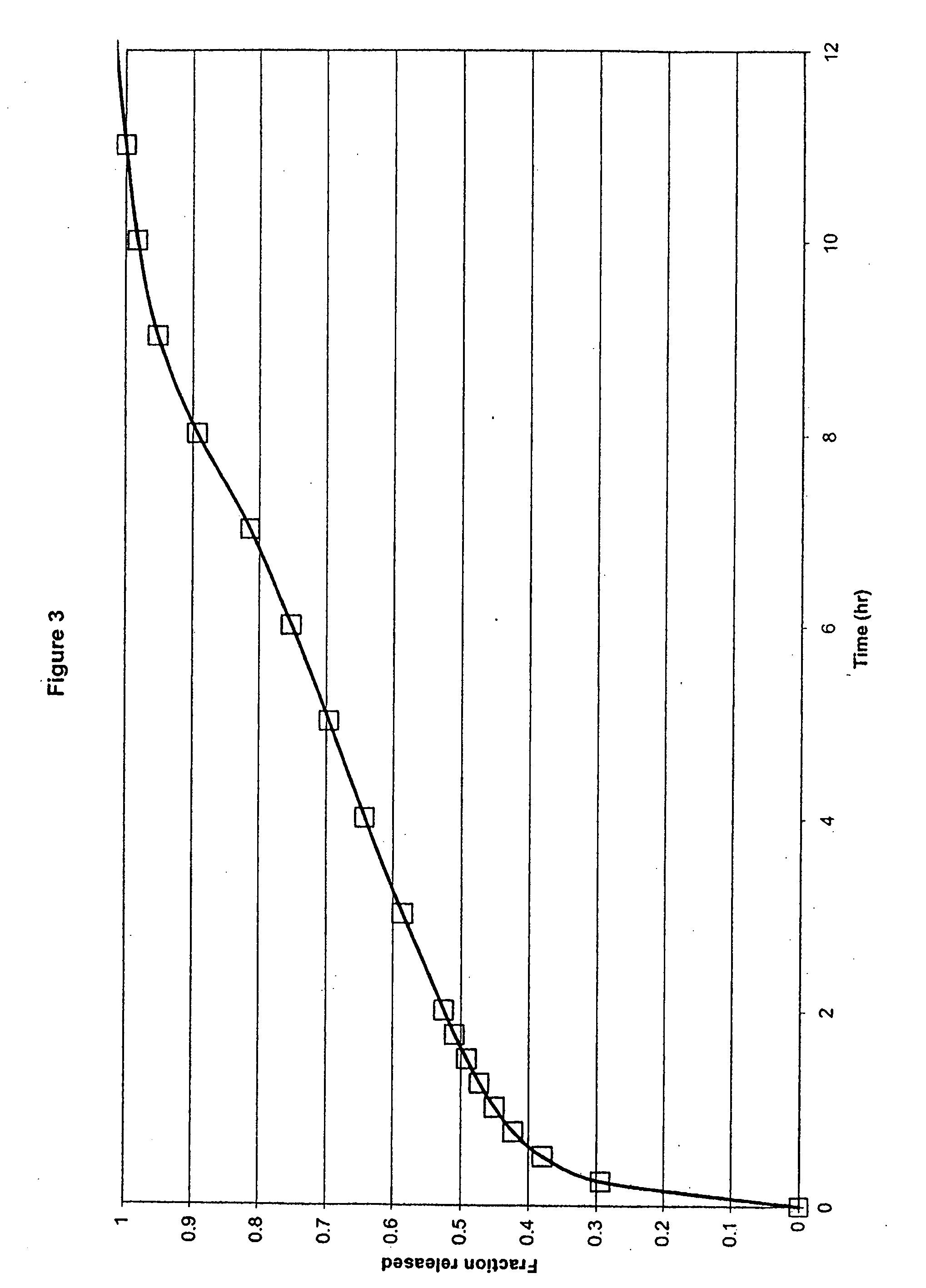
[0049] In another embodiment, the formulation comprised ibuprofen, hydroxypropyl methylcellulose (HPMC K15M and HPMC K100LV), sodium carbonate, glycine, flow agents and tableting aids, in which HPMC K15M was present at a concentration of 17% by weight of ibuprofen, HPMC K100LV was present at a concentration of 17% by weight of ibuprofen and sodium carbonate was present at a concentration of 25% by weight of ibuprofen within a compressed monolithic tablet.
Ex. 3mgIbuprofen600HPMC K15M100HPMC K100LV100MCC PH102100Na2CO3, anhydrous150Glycine15Silica, Syloid 24420Mg Stearate10Total:1095
[0050] The formulation components were mixed in a V-blender. The resulting powder was compressed into tablets using conventional compression technology. In this Example a combination of a medium to high viscosity HPMC and a low viscosity HPMC was used.
[0051] As shown in FIG. 3, the results of this Example demonstrate an in vitro release profile comprising a burst effect providing release of 20% of ibupr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com