Modified release ibuprofen dosage form
A solid dosage form and preparation technology, applied in the field of ibuprofen improved release dosage form, can solve problems such as delay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In a specific embodiment, the formulation comprises ibuprofen, hydroxypropylmethylcellulose (HPMC K15M and HPMC K100LV), glycine and sodium carbonate, in a compressed monolithic, wherein HPMC K15M is present At a concentration of 18% by weight of ibuprofen, HPMC K100LV was present at a concentration of 17% by weight of ibuprofen, glycine was present at a concentration of 2.5% by weight of ibuprofen, and sodium carbonate was present at a concentration of 17% by weight of ibuprofen. The specific preparations are as follows:
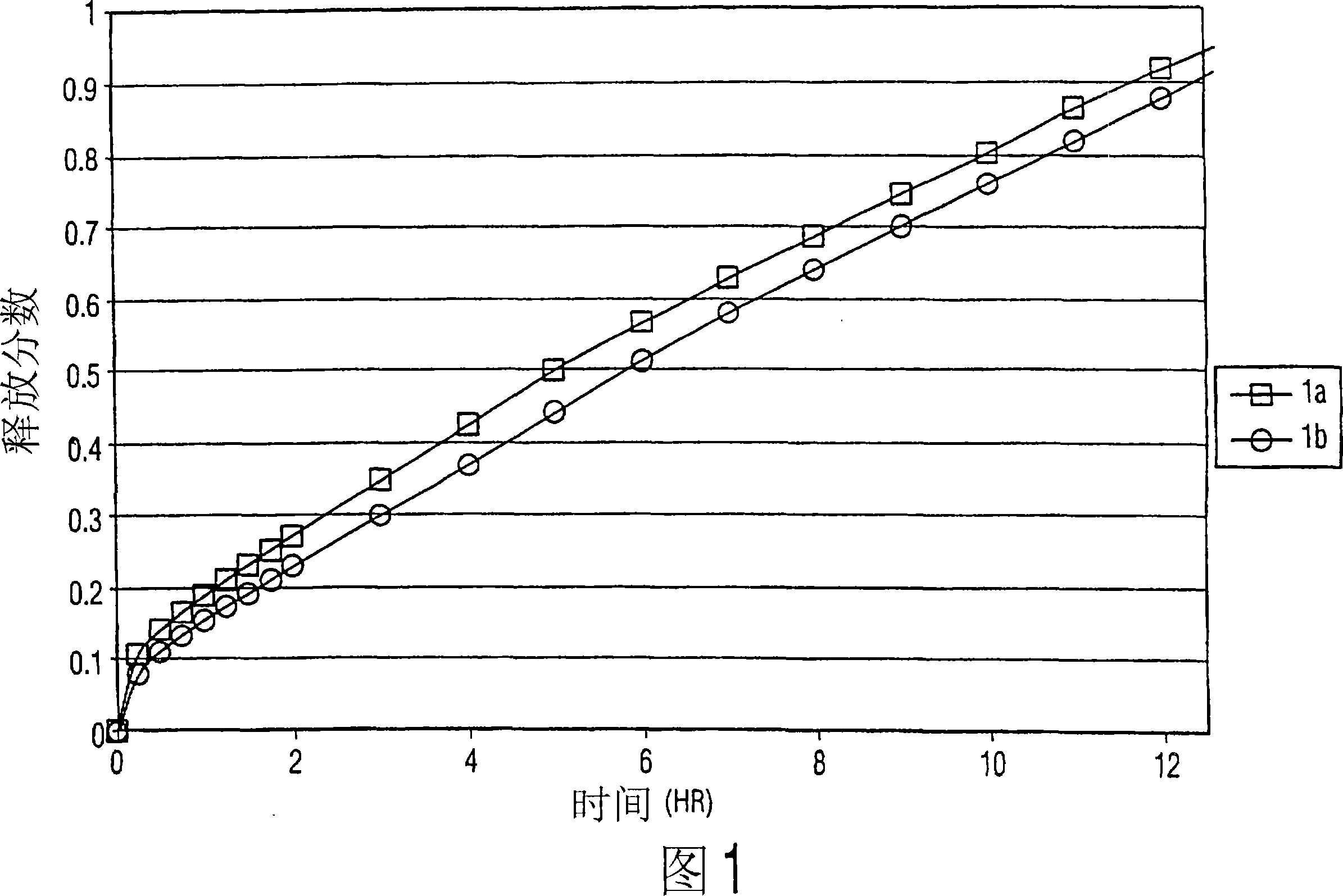
[0042] Example 1a
mg
ibuprofen level 90
600
HPMC K15M
110
HPMC K100LV
100
MCC PH102
100
Na 2 CO 3 , anhydrous
150
15
Silica, Syloid 244
20
10
Total: A
1105
Example 1b
mg
ibuprofen level 90
600
HPMC K15M
125
HPMC K100LV
100
MCC ...
Embodiment 2
[0046] In another specific embodiment, the formulation comprises ibuprofen, hydroxypropyl methylcellulose (HPMC K100M and HPMC K100LV), sodium carbonate, a flow agent and a tableting aid, in compressed A single tablet in which HPMC K100M is present at a concentration of 17% by weight of ibuprofen, HPMC K100LV is present at a concentration of 17% by weight of ibuprofen, and sodium carbonate is present at a concentration of 17% by weight of ibuprofen 25% of. The specific preparations are as follows:
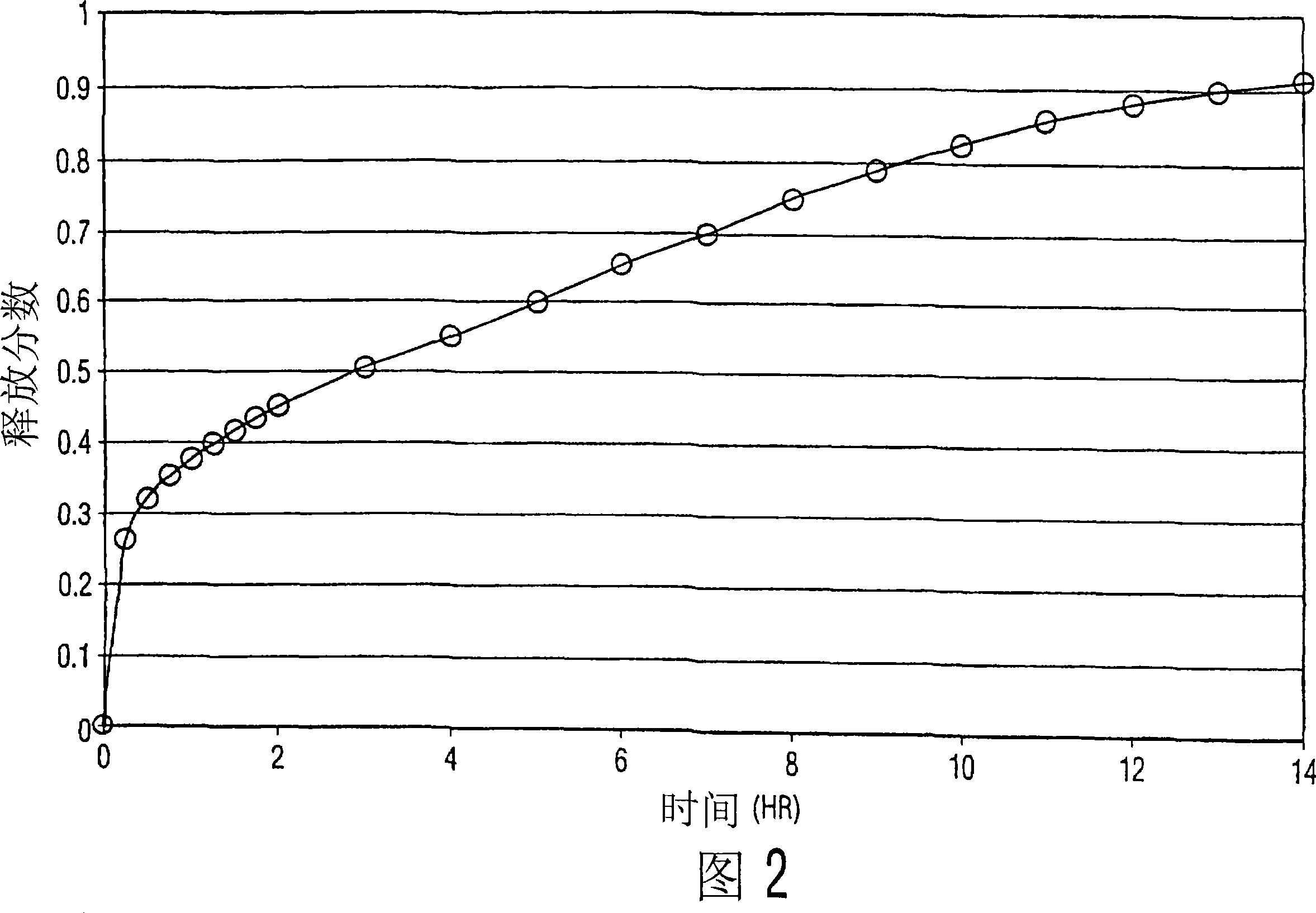
[0047] Example 2
mg
ibuprofen
600
HPMC K100M
100
HPMC K100LV
100
Na 2 CO 3 , anhydrous
150
MCC PH 102
150
Silica, Syloid 244
20
10
Total:
1130
[0048] The formulation components were mixed in a V-blender. The resulting powder is compressed into tablets using conventional techniques. In this example, a combination of medium to high v...
Embodiment 3
[0051] In another specific embodiment, the formulation comprises ibuprofen, hydroxypropyl methylcellulose (HPMC K15M and HPMC K100LV), sodium carbonate, a glidant and a tableting aid, in a compressed single tablet, Wherein the existing concentration of HPMC K100M is 17% by weight of ibuprofen, the existing concentration of HPMC K100LV is 17% by weight of ibuprofen, and the existing concentration of sodium carbonate is 25% by weight of ibuprofen.
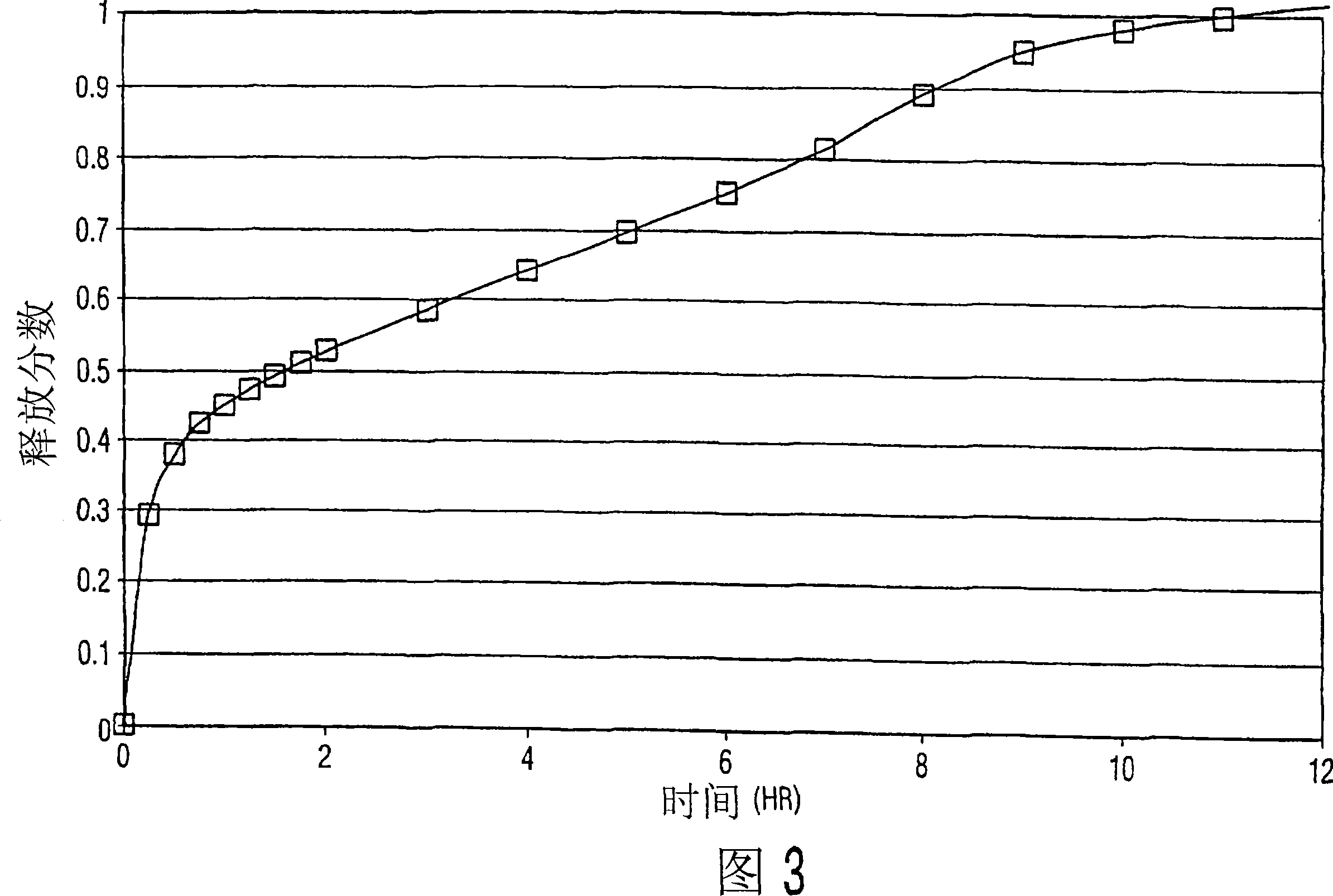
[0052] Example 3
mg
ibuprofen
600
HPMC K15M
100
HPMC K100LV
100
MCC PH102
100
Na 2 CO 3 , anhydrous
150
15
Silica, Syloid 244
20
10
Total:
1095
[0053] The formulation components were mixed in a V-blender. The resulting powder is compressed into tablets using conventional tableting techniques. In this example, a combination of medium to high viscosity HPMC and low v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com