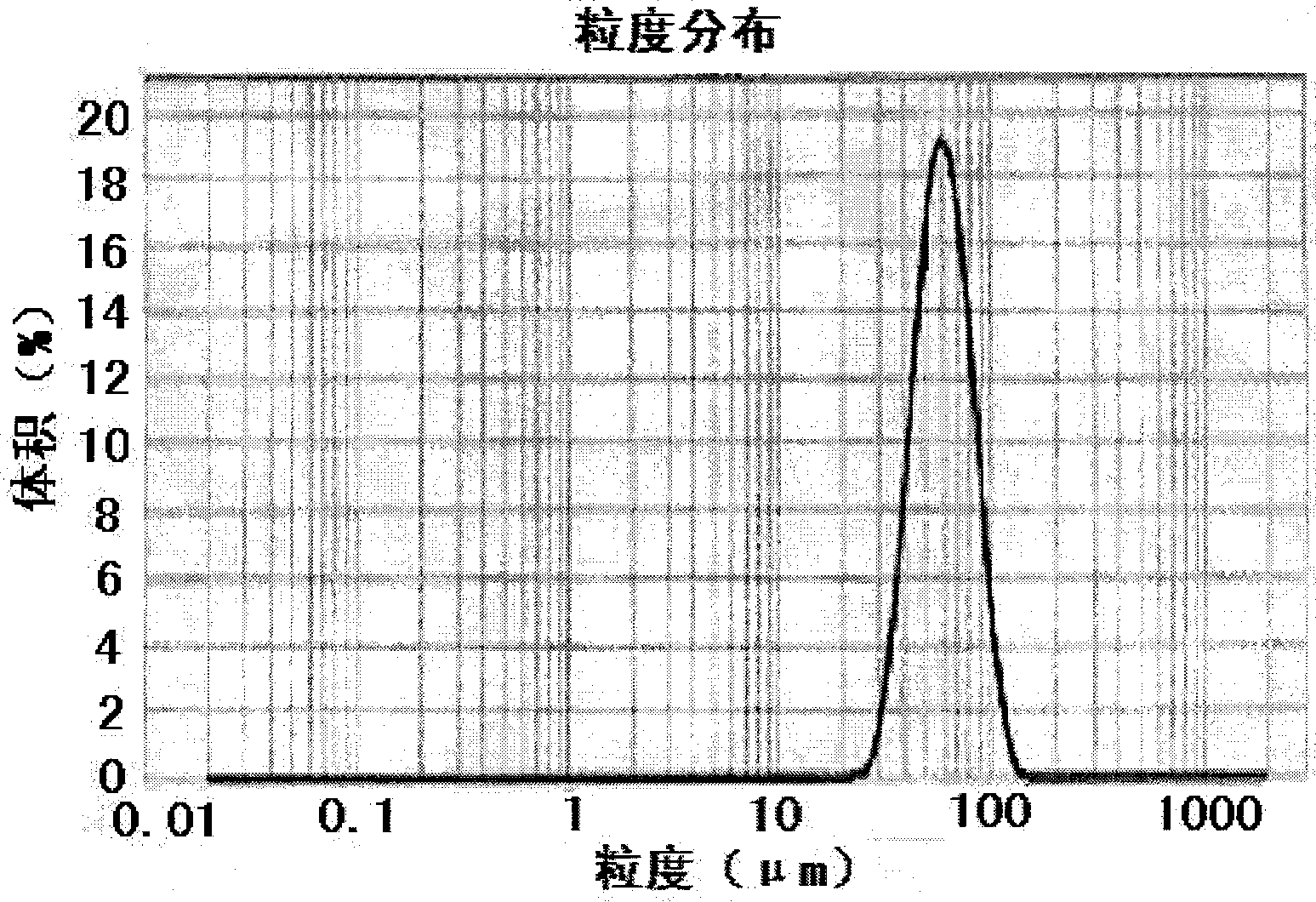
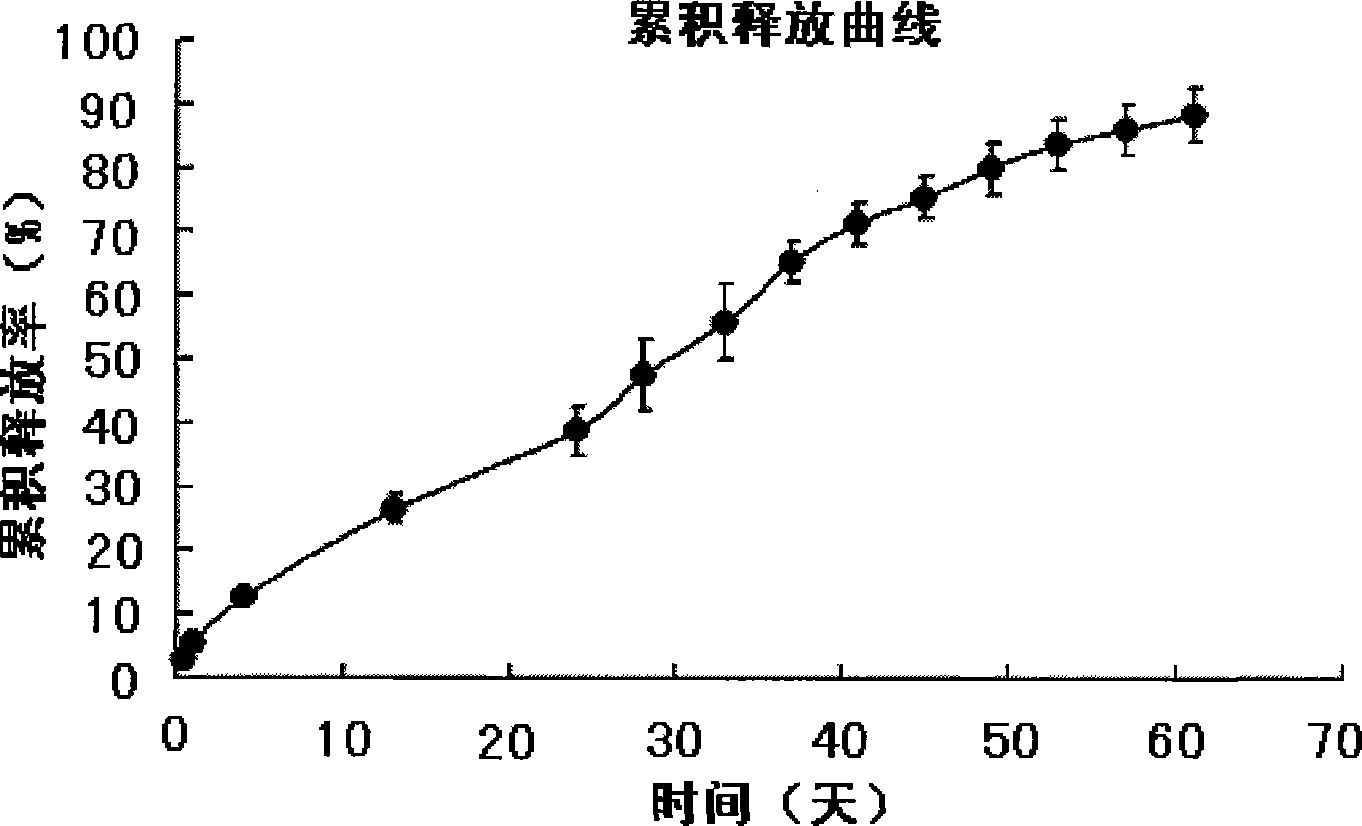
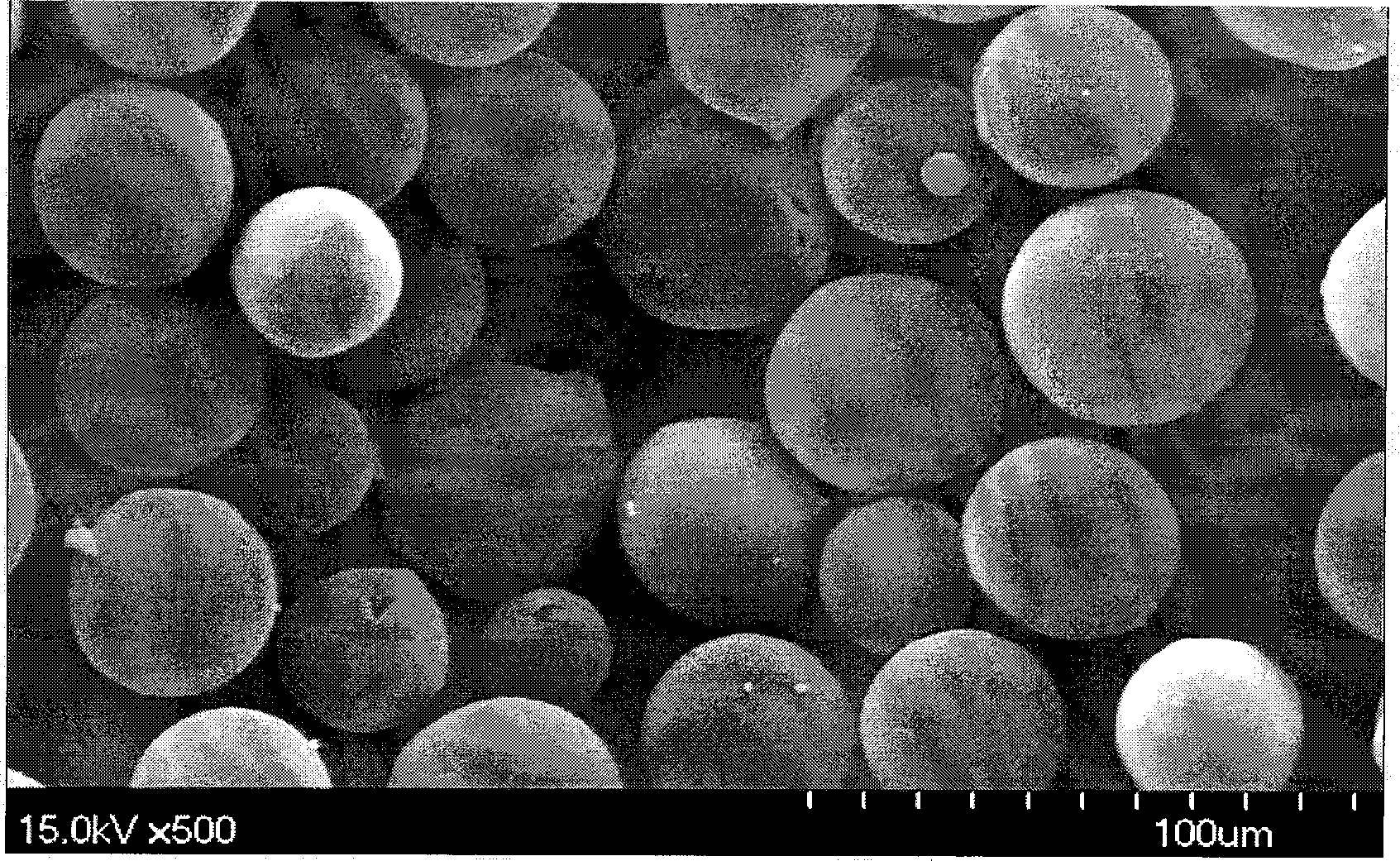Asiatic acid injectable sustained-release microballoons and preparation method thereof
A technology of asiatic acid and slow-release microspheres, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of low blood drug concentration and poor oral absorption, and achieve Good biocompatibility, low manufacturing cost, and stable preparation technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation steps of asiatic acid injectable slow-release microspheres of the present invention are as follows:
[0018] 1. Take 2% glacial acetic acid solution as solvent, add chitosan, fully swell, stir to dissolve, filter, discard insoluble matter, and make into 5% concentration (W / V).
[0019] 2. Asiatic acid (particle diameter<10 μ m, chromatographic purity greater than 90%) is added in the above-mentioned 5% chitosan solution and mixes uniformly as the water phase (the addition of Asiatic acid is 0.5-2 times of chitosan (W / W)); under the condition of mechanical stirring (800-1200r / min), slowly add the water phase to the oil phase containing emulsifier, and keep stirring for 5-10min to form a water-in-oil type (W / O) Emulsion.
[0020] 3. Under stirring at 200r / min, control the temperature at 25-30°C, add the crosslinking agent dropwise to the emulsion in an amount of 1-2ml, and cure for 1-6h. The solid microspheres are collected, washed and dried to obtain ...
Embodiment 1
[0024] Weigh 1 g of chitosan (molecular weight 200 kDa), add 20 ml of 2% glacial acetic acid solution to fully swell, stir to dissolve, filter, discard insoluble matter, and make into 5% concentration (W / V). Add 448 mg of asiatic acid (particle size<10 μm) into 4 ml of the above 5% chitosan solution, mix well as the water phase. Under the condition of mechanical stirring (900r / min), slowly add dropwise in the liquid paraffin (80ml) that contains 1.6g Span-80, keep stirring for 5min, make to form water-in-oil type (W / O) emulsion; Under stirring, control the temperature at 25-30°C, add 1.5ml of 25% glutaraldehyde solution, solidify for 1 hour, filter, wash with petroleum ether, distilled water, petroleum ether / isopropanol (1:1) and petroleum ether, and dry in vacuum , to obtain asiatic acid sustained-release microspheres. The drug-loading capacity of the microspheres is 15.6%, and the cumulative release in 24 hours (release on the first day): 9.6%.
Embodiment 2
[0026] Weigh 1 g of chitosan (molecular weight 126 kDa), add 20 ml of 2% glacial acetic acid solution to fully swell, stir to dissolve, filter, discard insoluble matter, and make into 5% concentration (W / V). Add 224mg of Asiatic acid (particle size<10 μm) into the above 4ml 5% chitosan solution, and mix well as the water phase. Under the condition of mechanical stirring (1000r / min), slowly add it dropwise into the liquid paraffin (80ml) containing 1.6g Span-80, keep stirring for 10min to form water-in-oil (W / O) emulsion; at 200r / min Under stirring, control the temperature at 25-30°C, add 2ml of 25% glutaraldehyde solution, solidify for 3 hours, filter, wash with petroleum ether, distilled water, petroleum ether / isopropanol (1:1) and petroleum ether, and dry in vacuum. The asiatic acid sustained-release microspheres were obtained. The drug loading amount of the microspheres is 7.8%, and the cumulative release in 24 hours (release amount on the first day): 10.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com