Method for preparing oxaliplatin sustained release suppository
An oxaliplatin and sustained-release technology, applied in suppository delivery, medical preparations of non-active ingredients, pharmaceutical formulas, etc., can solve problems such as major side effects, reduce toxicity and side effects, prolong action time, and improve slow control release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
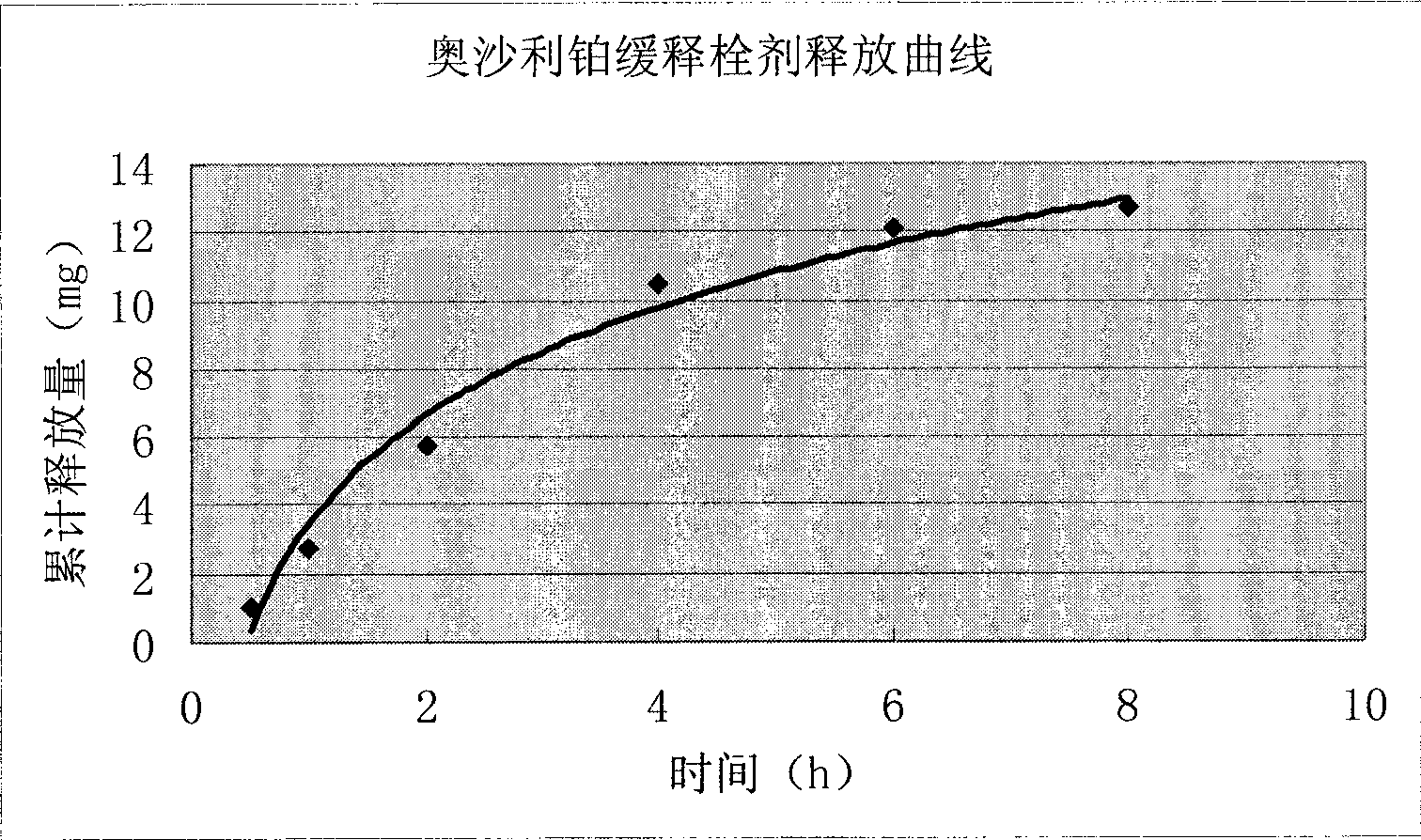
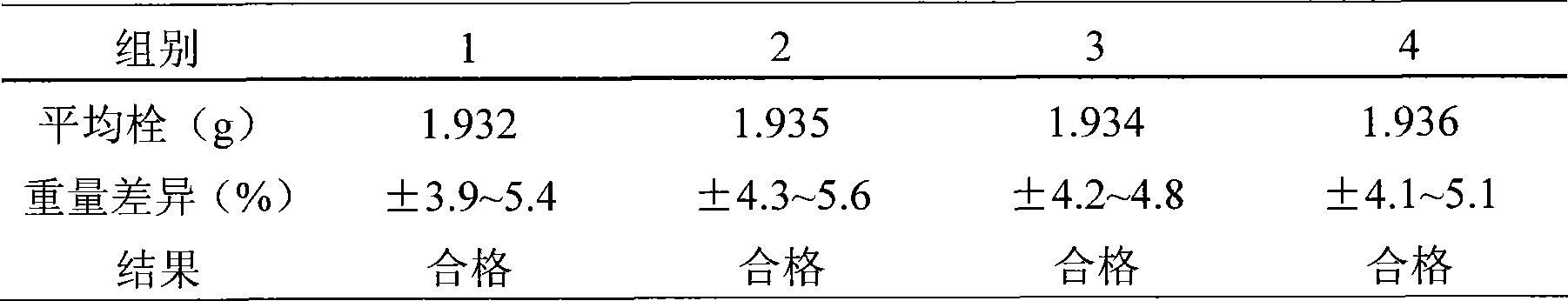
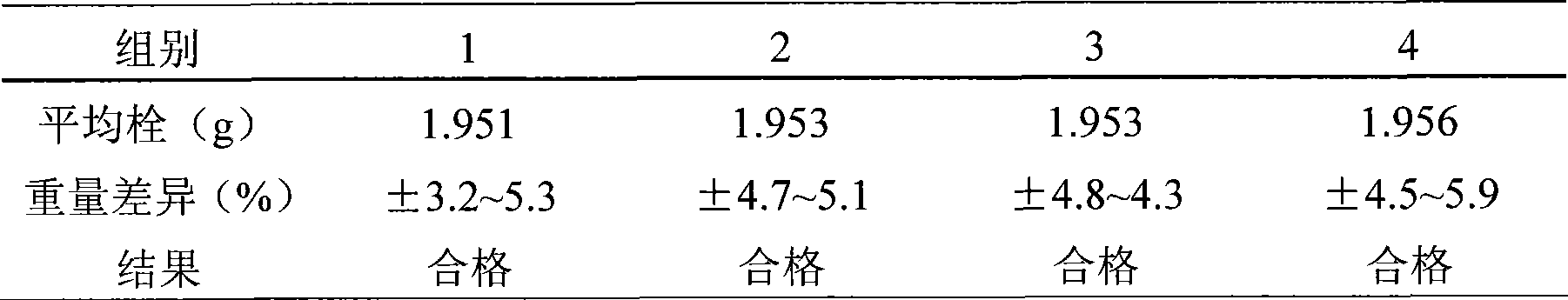
Image
Examples
Embodiment 1
[0022] The preparation method of oxaliplatin sustained-release suppository, it comprises the steps:
[0023] 1. Preparation of Oxaliplatin Microspheres
[0024] Measure 8.33mL of 36% (mass percentage) acetic acid, dilute to 100mL to obtain a 3% (mass percentage) acetic acid solution, weigh 0.300g of oxaliplatin and dissolve it in the above acetic acid solution, and ultrasonically dissolve it to obtain 3.00 3% (mass percentage) oxaliplatin acetate solution in mg / mL. Accurately measure 10 mL of oxaliplatin acetic acid solution, weigh 0.3 g of chitosan and dissolve it in the above solution, and ultrasonically dissolve it. Get a beaker, add 30mL liquid paraffin to it, stir on a magnetic stirrer, and add about 2.4ml of Span-80 while stirring, and then add the above-mentioned oxaliplatin acetate solution containing chitosan after mixing evenly. After emulsification for 1.5 h, 0.5 ml of glutaraldehyde was added, and the pH value was adjusted to 9 with 20% (g / mL) ammonia water. 1.5...
Embodiment 2
[0050] The preparation method of oxaliplatin sustained-release suppository, it comprises the steps:
[0051] 1. Preparation of Oxaliplatin Microspheres
[0052]Measure 5.56 mL of 36% (mass percentage) acetic acid, dilute to 100 mL to obtain a 2% (mass percentage) acetic acid solution, weigh 0.200 g of oxaliplatin and dissolve it in the above acetic acid solution, and ultrasonically dissolve it to obtain 2.00 mg / mL 2% (mass percentage) oxaliplatin acetate solution. Accurately measure 10 mL of oxaliplatin acetic acid solution, weigh 0.1 g of chitosan and dissolve it in the above solution, and ultrasonically dissolve it. Take a beaker, add 20mL of liquid paraffin to it, stir on a magnetic stirrer, and add about 1.6ml of Span-80 while stirring, and then add the above-mentioned oxaliplatin acetate solution containing chitosan after mixing evenly. After emulsification for 1.0 h, 0.3 ml of glutaraldehyde was added, and the pH value was adjusted to 10 with 20% (g / mL) ammonia water. ...
Embodiment 3
[0056] 1. Preparation of Oxaliplatin Microspheres
[0057] Measure 13.89 mL of 36% (mass percentage) acetic acid, dilute it to 100 mL to obtain a 5% (mass percentage) acetic acid solution, weigh 0.500 g of oxaliplatin and dissolve it in the above acetic acid solution, and ultrasonically dissolve it to obtain 5.00 mg / mL 5% (mass percentage) oxaliplatin acetate solution. Accurately measure 10 mL of oxaliplatin acetic acid solution, weigh 0.5 g of chitosan and dissolve it in the above solution, and ultrasonically dissolve it. Take a beaker, add 40mL of liquid paraffin to it, stir on a magnetic stirrer, and add about 3ml of Tween-80 while stirring, after mixing evenly, add the above-mentioned oxaliplatin acetate solution containing chitosan, and emulsify After 2 hours, 0.5 ml of glutaraldehyde was added, and the pH value was adjusted to 9.5 with 0.5 mol / L NaOH. 2.0 h after the start of crosslinking, the stirring was stopped. At this time, the microspheres have been formed, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com