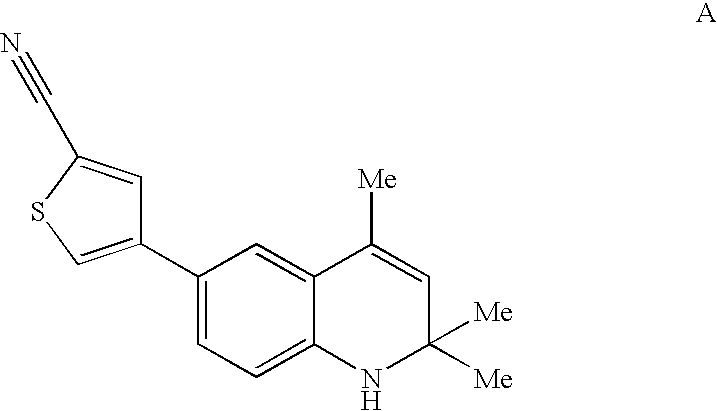
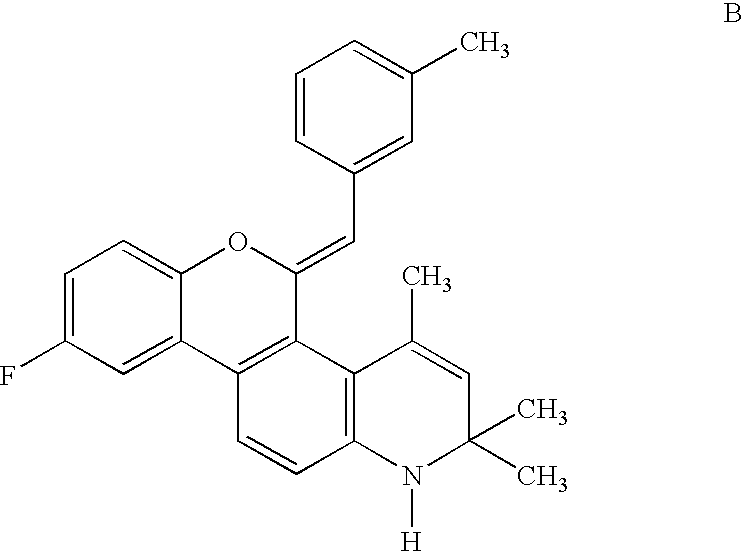
Patents
Literature
55 results about "Contragestazol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Contragestazol (DL111-IT) inhibits proliferation of human androgen-independent prostate cancer cell line PC3 in vitro and in vivo
Thio-oxindole derivatives
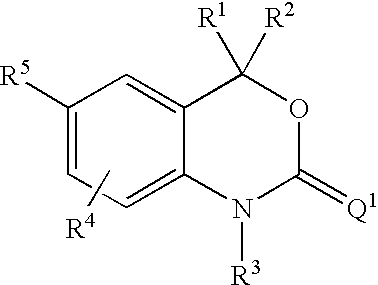
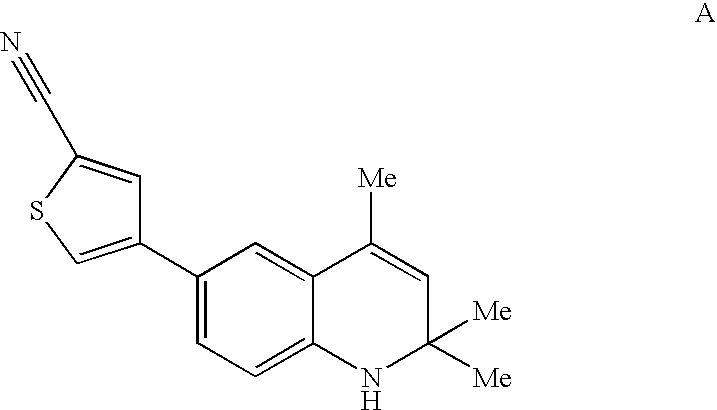
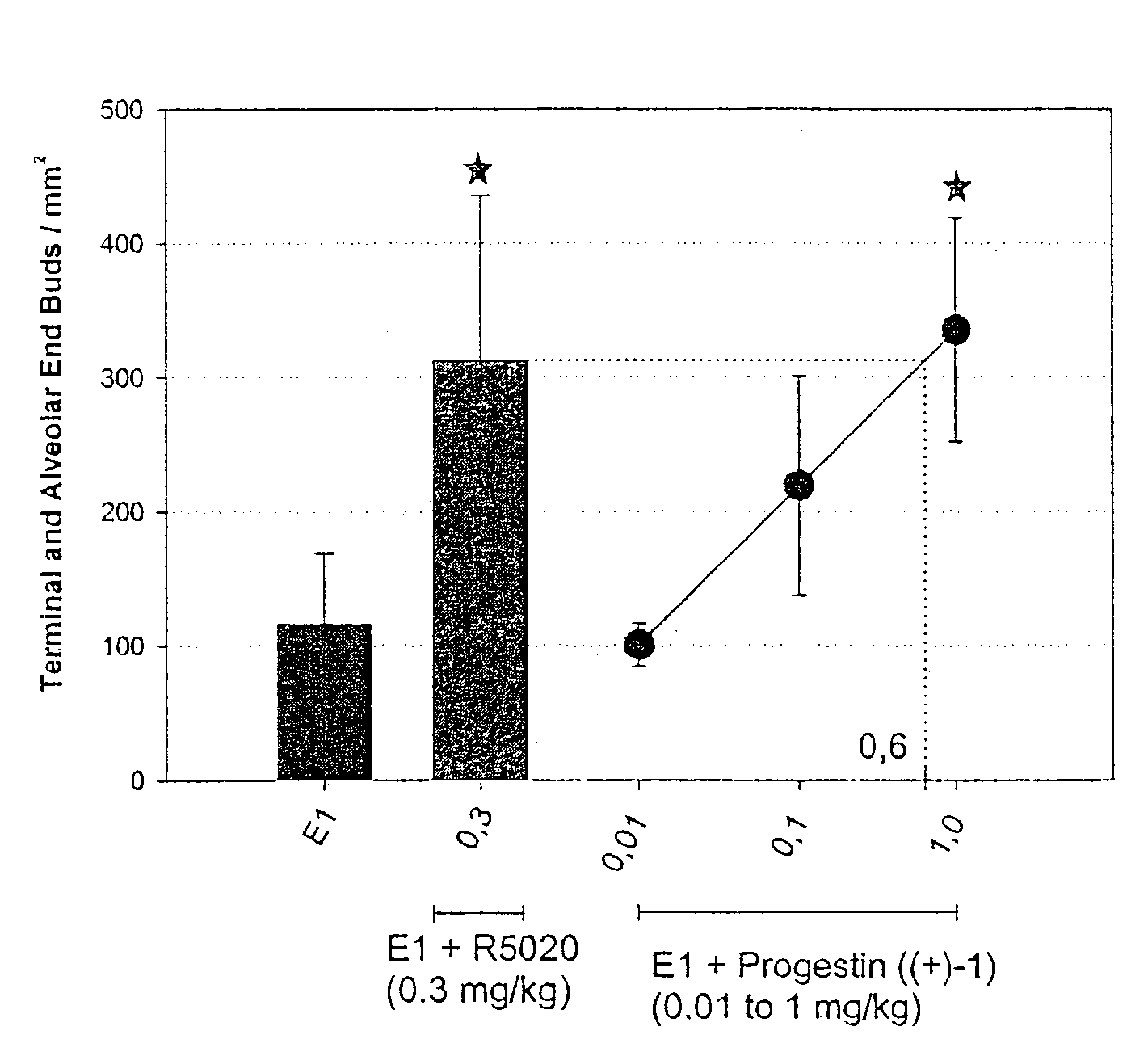
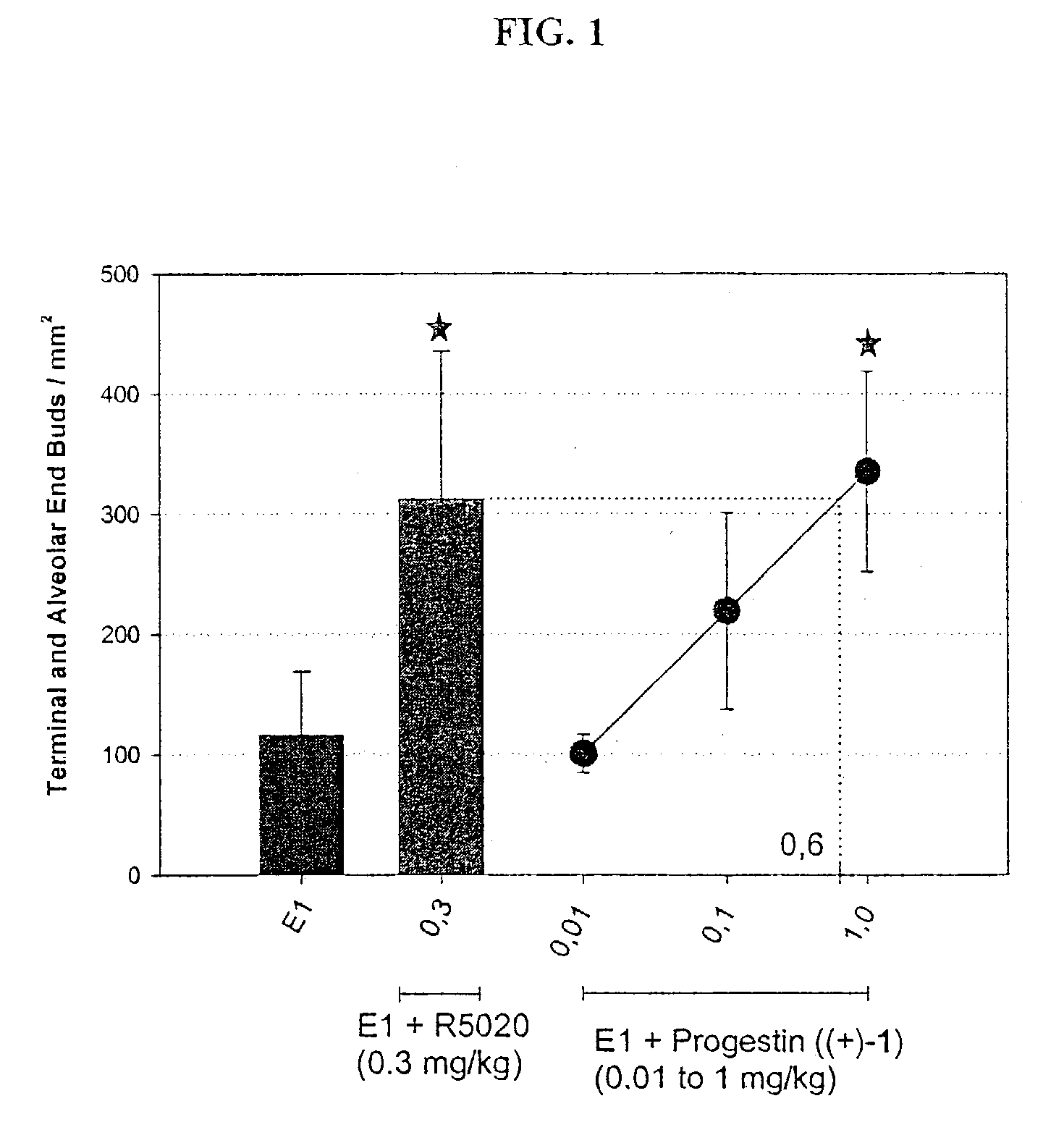
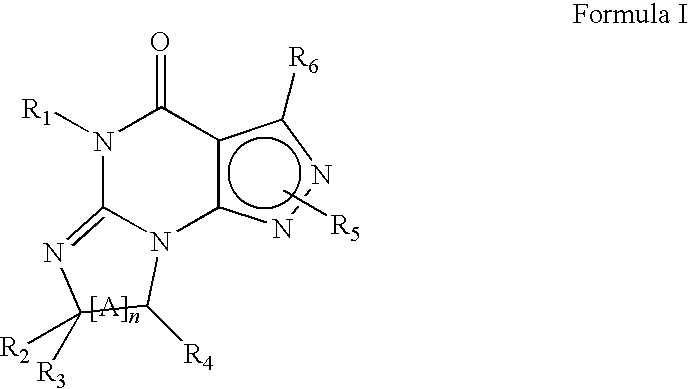
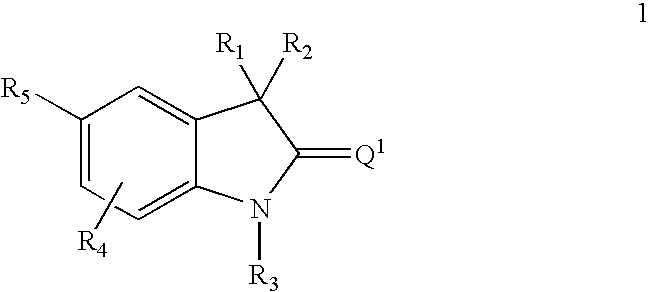
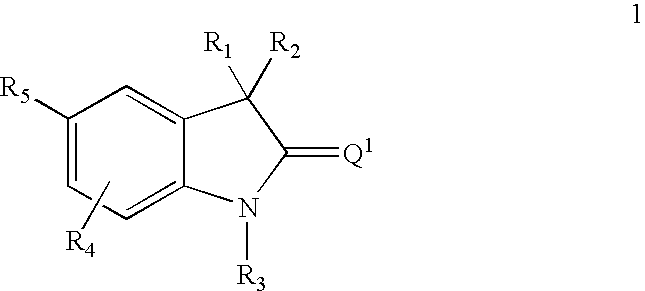
This invention relates to methods of co-administering compounds of formula 1 which are agonists of the progesterone receptor which have the general structure: wherein: R1, R2, R3, R4, R5 and Q1 are as defined herein, or a pharmaceutically acceptable salt thereof, with estrogen, an estrone, or an estrogen receptor agonist for contraception, hormone replacement therapy, or treating progesterone-related carcinomas and adenocarcinomas.
Owner:WYETH LLC
Non-steroidal progesting
InactiveUS7388006B2Suitable for useBiocideOrganic chemistryPR - Progesterone receptorOral contraceptive drug
The present invention relates to non-steroidal progestins of the general formula (I)whereinR1 and R2 are independently of each other —H or —F,R3 is —CH3 or —CF3, andAr isor a pharmaceutically acceptable derivative or analogue thereof. These progestins are suitable for selectively modulating progesterone receptor mediated effects in different target tissues, particularly in uterine tissue versus breast tissue. Therefore, the progestins of the present invention, optionally in combination with estrogens, may be used for contraception (in particular in estrogen-free oral contraceptives), hormone replacement therapy and the treatment of gynecological disorders. The present invention furthermore relates to methods for selectively modulating progesterone receptor mediated effects in different target tissues or organs.
Owner:BAYER SCHERING PHARMA AG
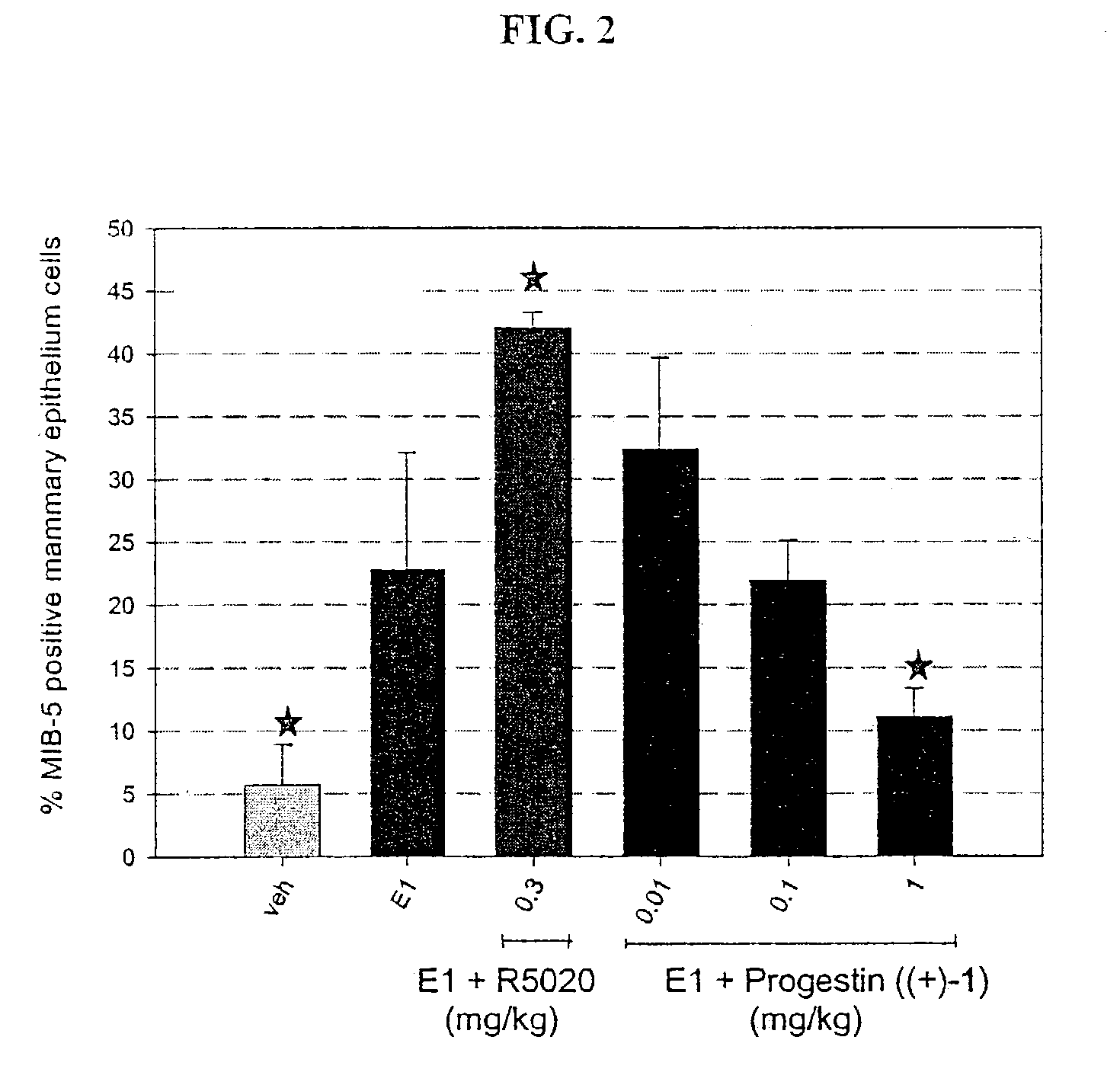
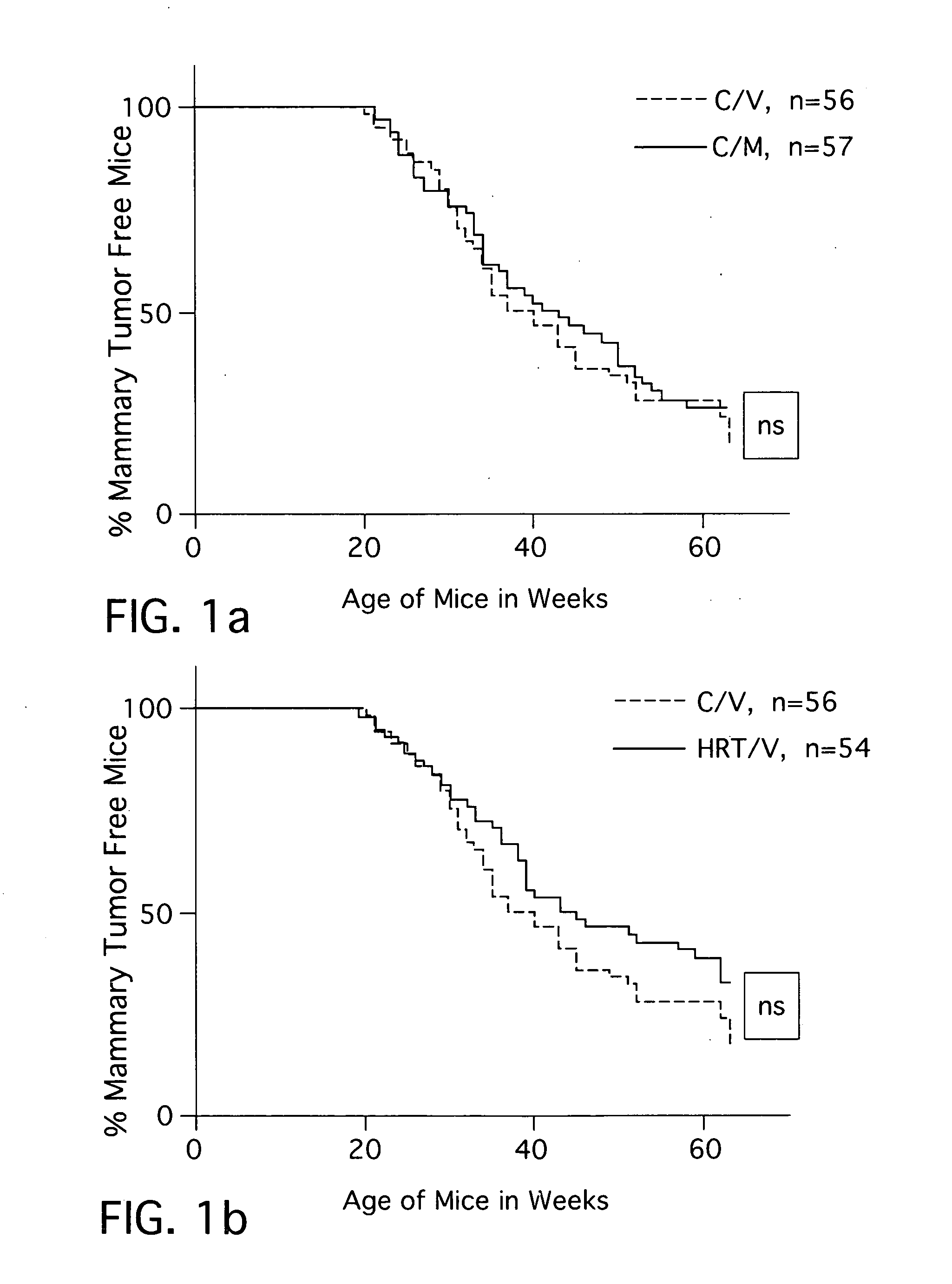
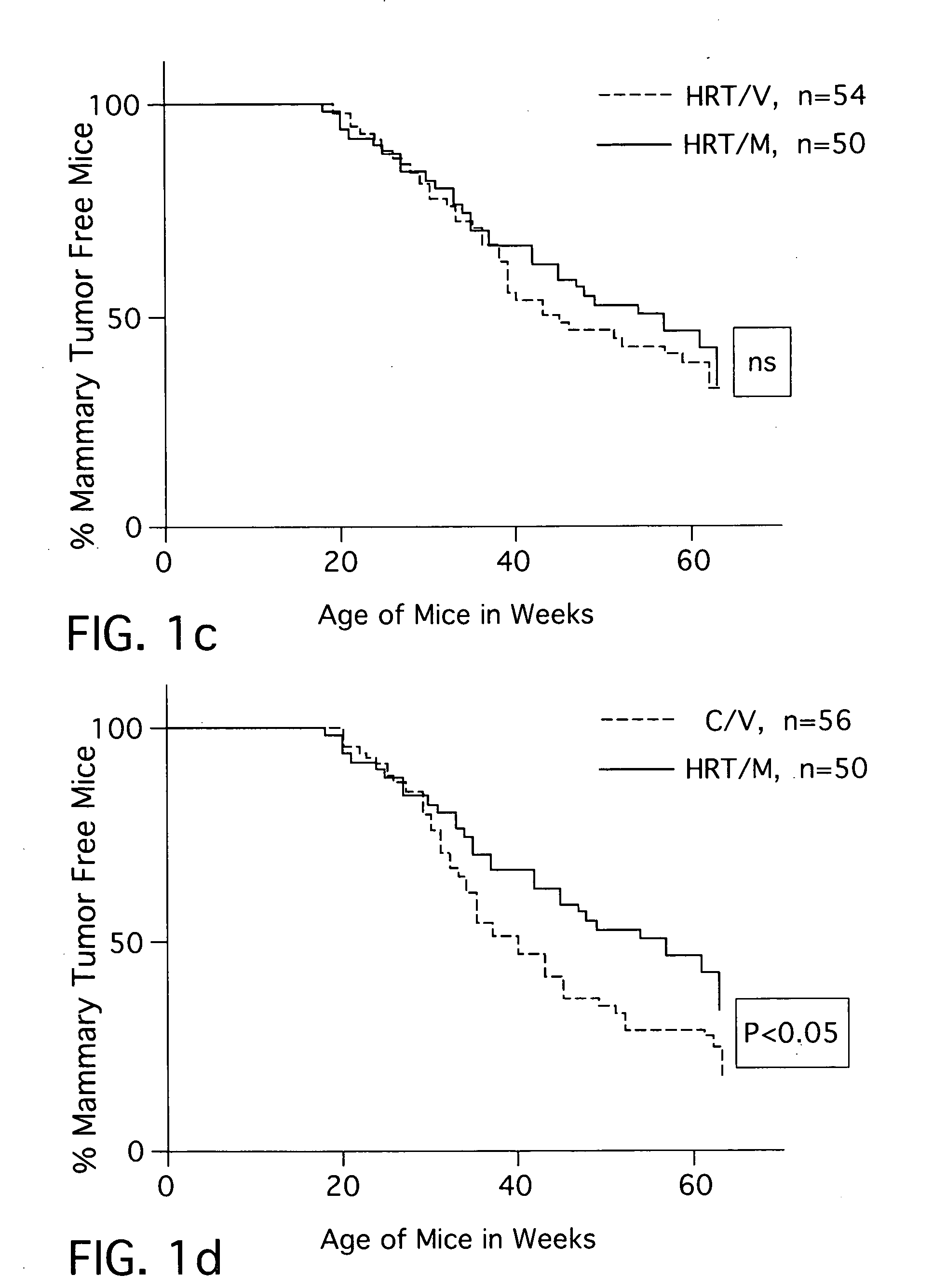
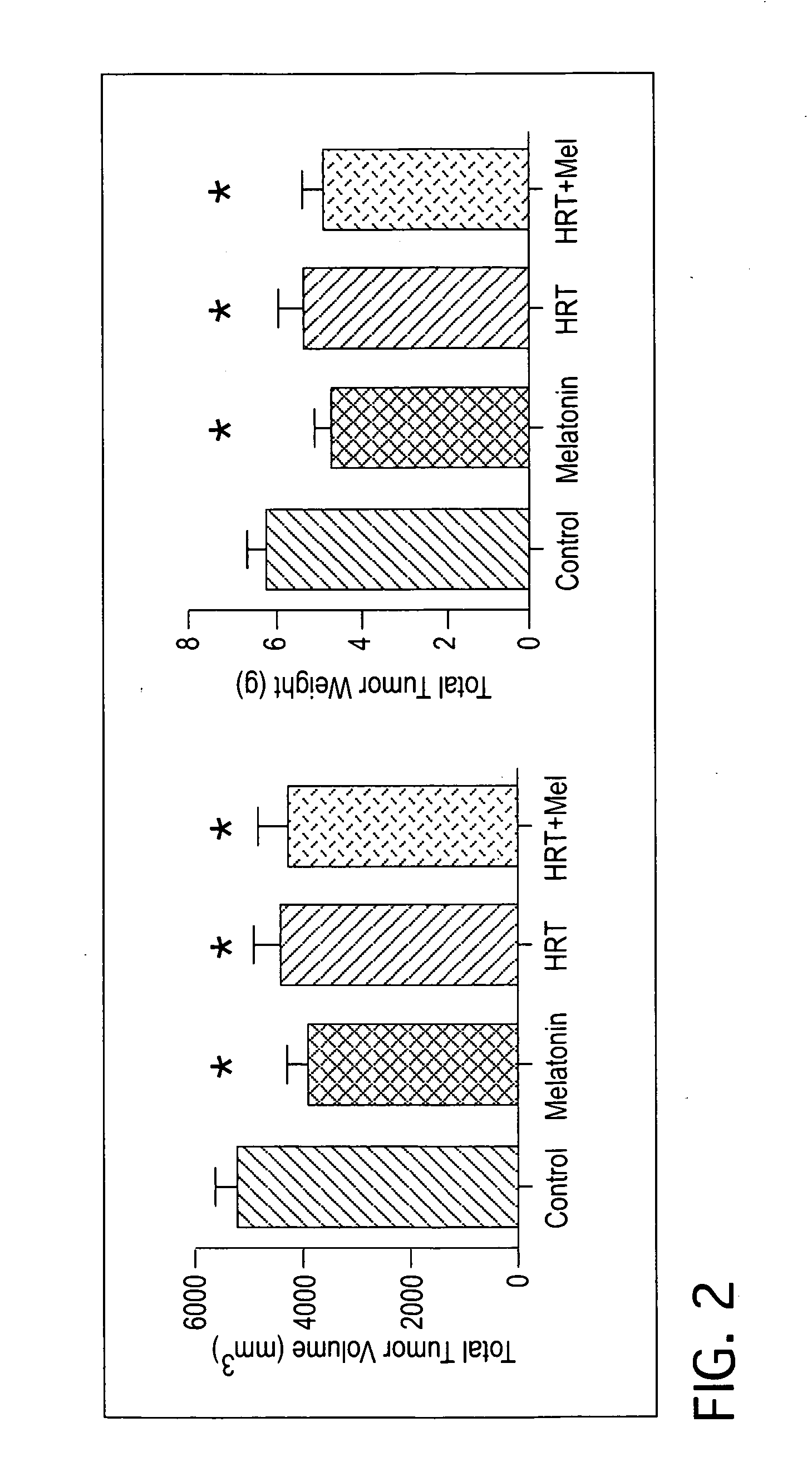
Combination hormone replacement therapy (HRT) and melatonin to prevent and treat mammary cancer
ActiveUS20110028439A1Reduce mammary cancer incidenceTreat symptomsBiocideOrganic active ingredientsReceptorHormone replacement
A combination hormone and melatonin therapy is provided to reduce the risk of developing, or to reduce the severity of, breast cancer by administering at least one estrogen hormone and optionally at least one progesterone-receptor-binding compound or composition and melatonin together, preferably at normal bed time.
Owner:DUQUESNE UNIVERSITY
Novel uses
InactiveUS20100323997A1Enhancement of signaling pathwayAmeliorate any conditionOrganic active ingredientsBiocideSexual functioningContragestazol
The present invention relates to a new use for compounds that inhibit phosphodiesterase 1 (PDE1), e.g., that inhibit PDE1-mediated suppression of the dopamine D1 receptor and / or progesterone signaling pathways, including, e.g., methods of treatment or prophylaxis for conditions which may be ameliorated by enhancing the progesterone signaling response, particularly female sexual dysfunction.
Owner:INTRA CELLULAR THERAPIES INC
Thio-oxindole derivatives
This invention relates to compounds which are agonists of the progesterone receptor which have the general structure: wherein:R1, R2, R3, R4, R5 and Q1 are as defined herein, or a pharmaceutically acceptable salt thereof, as well as methods of using these compounds to induce contraception or treat progesterone-related carcinomas and adenocarcinomas.
Owner:WYETH LLC
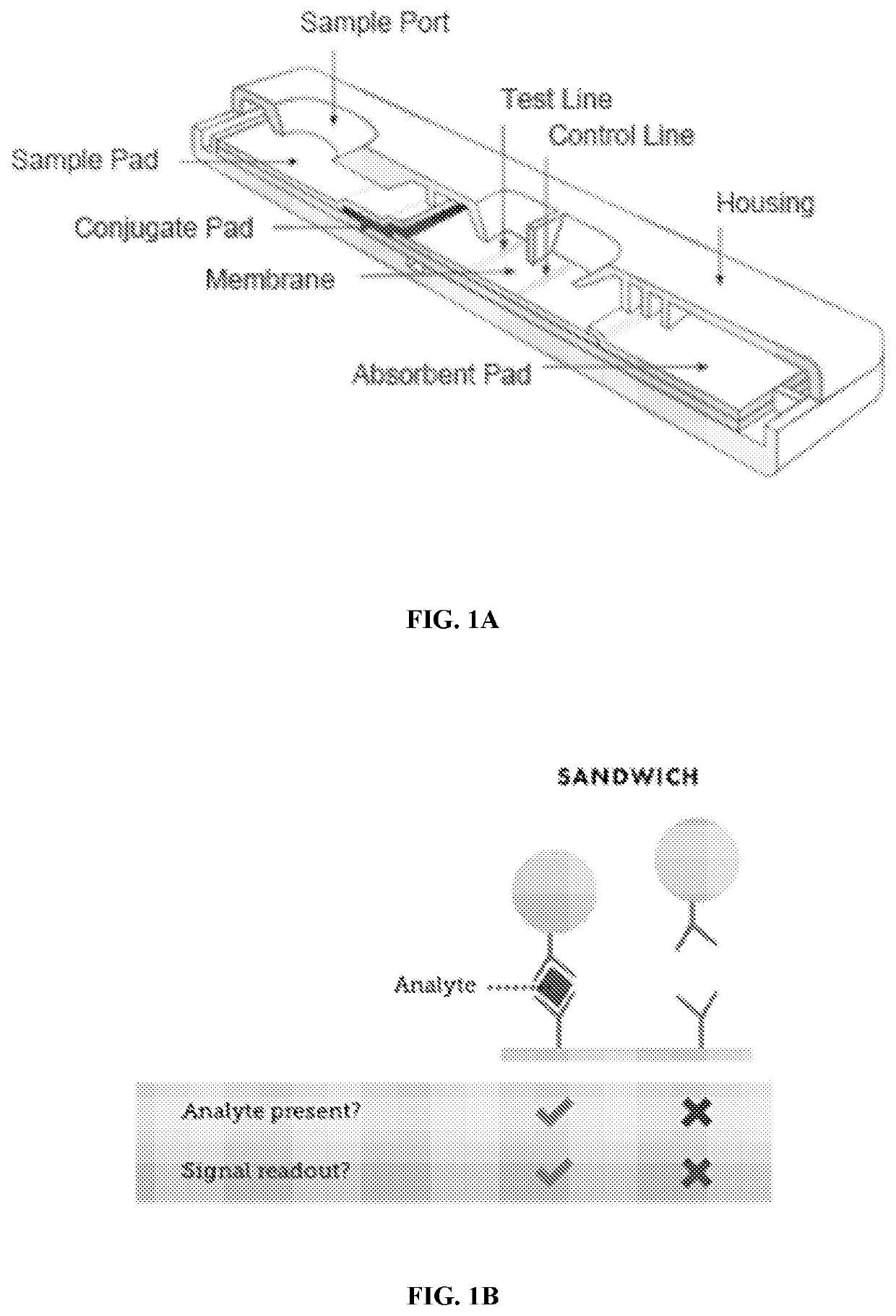
Pregnancy test device and method
InactiveUS20150094227A1Avoid false negative resultsImprove the level ofLibrary screeningBiological material analysisPregnancy testsMetabolite
Disclosed is a test device to detect pregnancy in a human female subject, the test device comprising:an assay means to measure the absolute or relative amount of hCG in a sample from the subject;an assay means to measure the absolute or relative amount of FSH in a sample from the subject;and an assay means to measure the absolute or relative amount of one or more progesterone metabolites in a sample from the subject.
Owner:SPD SWISS PRECISION DIAGNOSTICS
Therapy of Prostate Cancer With Ctla-4 Antibodies and Hormonal Therapy
InactiveUS20080279865A1Peptide/protein ingredientsAntibody ingredientsHistrelinAntiendomysial antibodies
The invention relates to methods for treating prostate cancer comprising administration of an anti-CTLA4 antibody, or an antigen-binding portion thereof, particularly a human antibody to human CTLA4, e.g., antibody 3.1.1, 4.1.1, 4.8.1, 4.10.2, 4.13.1, 4.14.3, 6.1.1, ticilimumab (also known as 11.2.1), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also known as MDX-010 and 10D1), in combination with hormonal therapy. Hormonal therapy agents include, inter alia, an anti-androgen (e.g., megestrol, cyproterone, flutamide, nilutamide, and bicalutamide), a GnRH antagonist (e.g., abarelix and histrelin), and a LH-RH agonist (e.g., leuprolide, goserelin, and buserelin). The invention relates to neoadjuvant therapy, adjuvant therapy, therapy for rising PSA, first-line therapy, second-line therapy, and third-line therapy of prostate cancer, whether localized or metastasized.
Owner:PFIZER PFIZER PRODS
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20160193357A1Receive treatment wellGood effectOrganic active ingredientsHeavy metal active ingredientsBreast cancer metastasisAntiendomysial antibodies
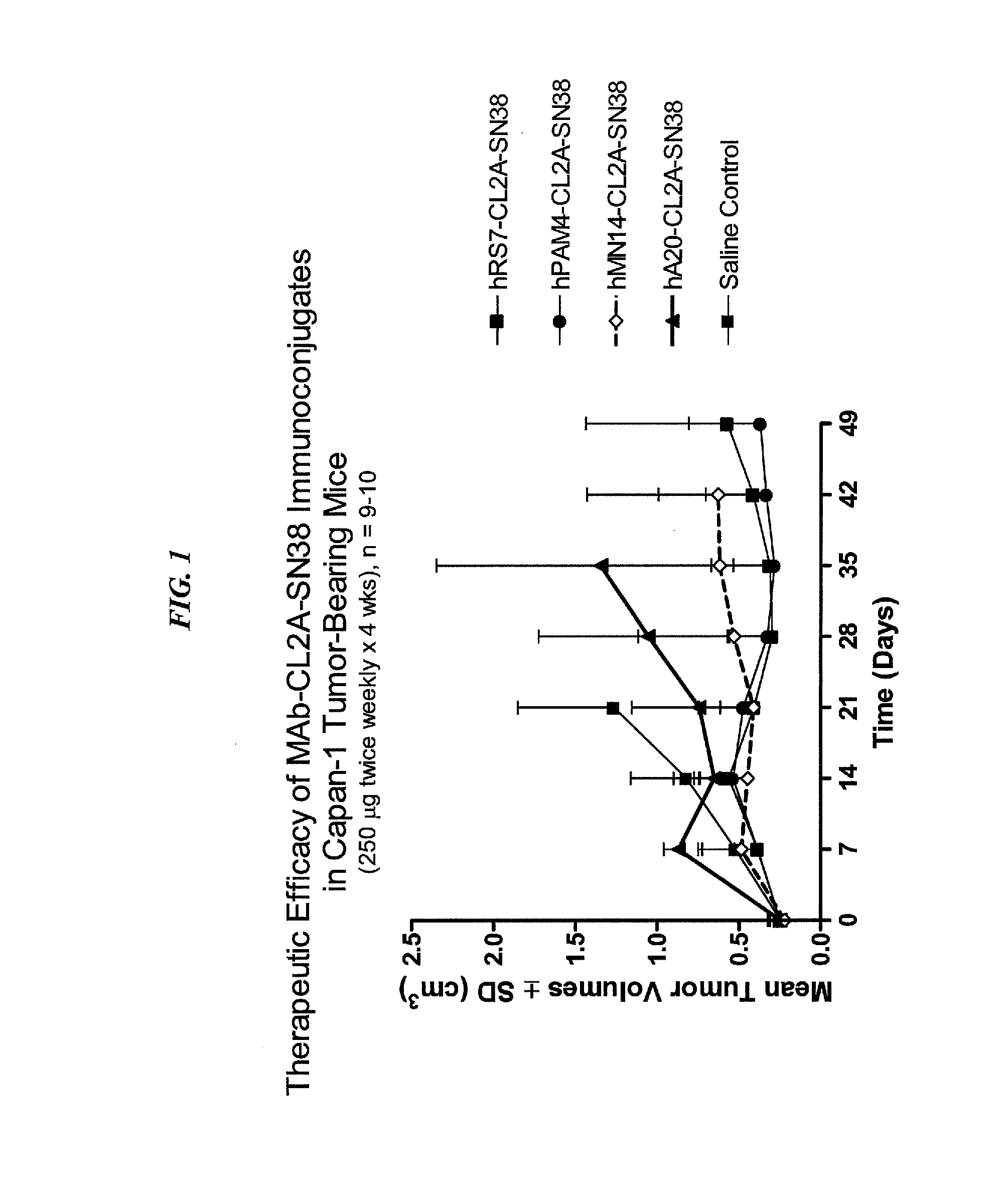
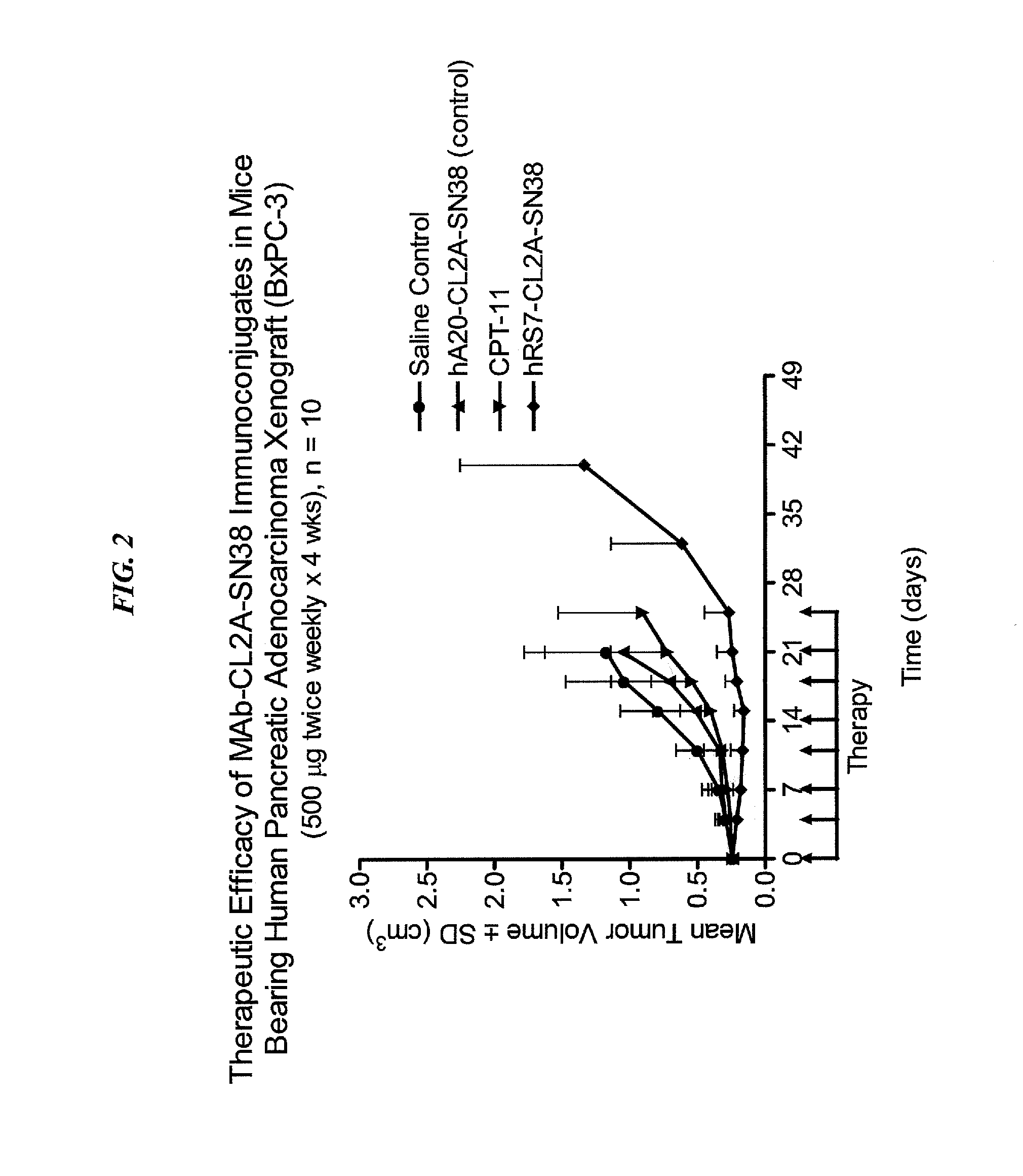
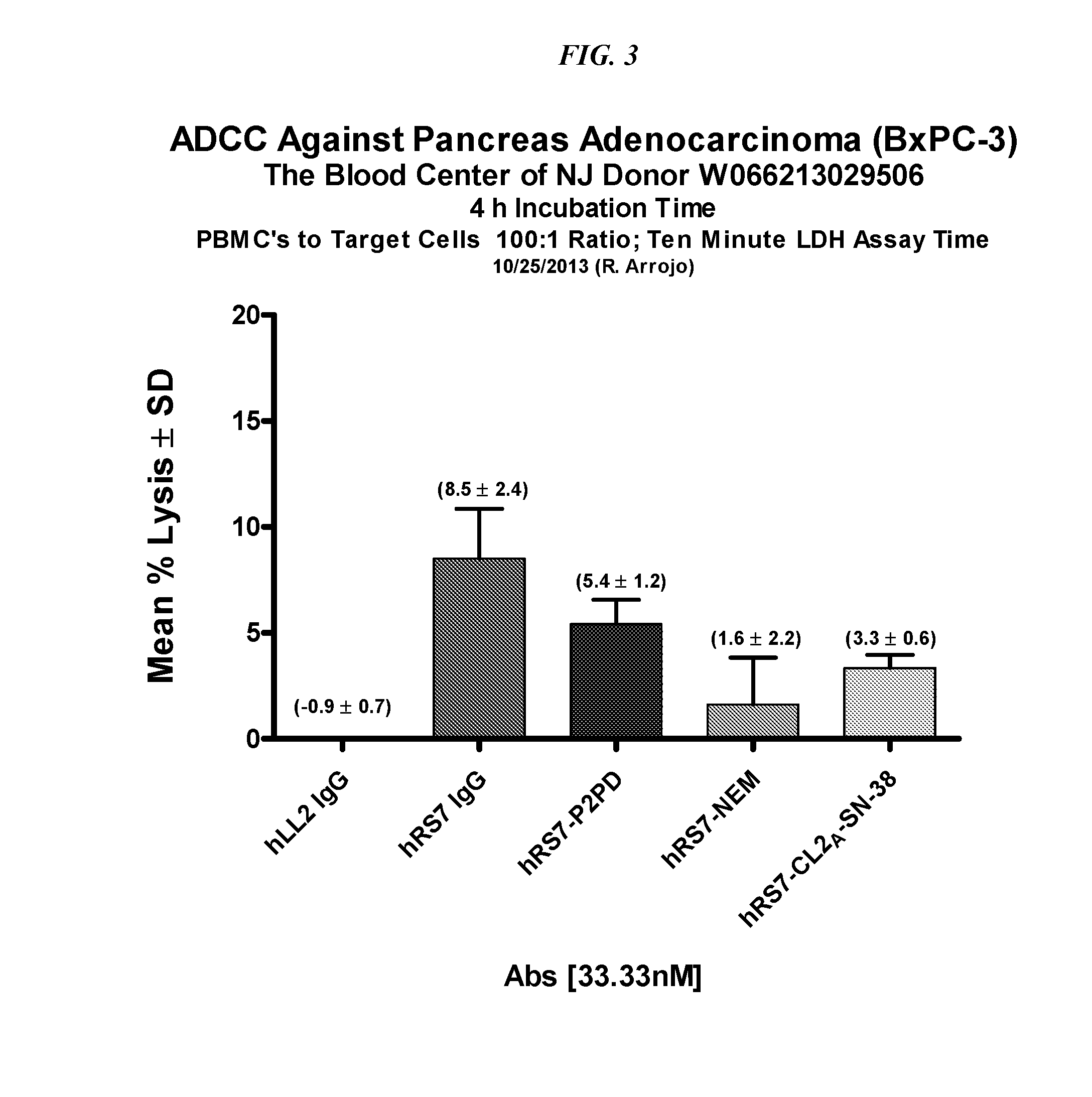
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. In preferred embodiments, the antibody may be an hRS7 antibody. The methods and compostions are of use to treat Trop-2 expressing cancers in human patients, preferably in patients who are resistant to or relapsed from at least one prior anti-cancer therapy, more preferably in patients who are resistant to or relapsed from treatment with irinotecan. The immunoconjugate may be administered at a dosage of 3 mg / kg to 18 mg / kg, preferably 8 to 12 mg / kg, more preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size and reduce or eliminate metastases. Preferred tumors to treat with the subject immunoconjugates include triple-negative breast cancer, HER+, ER+, progesterone+ breast cancer, metastatic non-small-cell lung cancer, a metastatic small-cell lung cancer and metastatic pancreatic cancer.
Owner:IMMUNOMEDICS INC
Prognostic biomarkers to predict overall survival and metastatic disease in patients with triple negative breast cancer
The present invention relates to a method for prognosing cancer in a subject with triple negative (TN) breast cancer, whose tumors lack expression of the estrogen receptor (ER), the progesterone receptor (PR) and normal (not amplified) levels of the human epidermal growth factor receptor 2 (HER2). Methods and biomarkers are disclosed that are useful for predicting the overall survival (OS) potential of cancer in a subject with triple negative breast cancer or for predicting metastatic disease in a subject with triple negative breast cancer. For example, the method comprises detecting in a sample from a subject one or more biomarkers selected from the group consisting of ANK3, CD24, EIF1, KLF6, KRAS, KRT1, MAP2K4, SDC4, SLC2A3, STK3, TFAP2C, and WRN. An increase or decrease in one or more biomarkers as compared to a standard is prognostic of OS of TN breast cancer. Likewise, in another example, the method comprises detecting in a sample from a subject one or more biomarkers selected from the group consisting of ANG, DICER1, EIF1, and MSH6. An increase or decrease in one or more biomarkers as compared to a standard is prognostic of metastasis of TN breast cancer.
Owner:VM INST OF RES
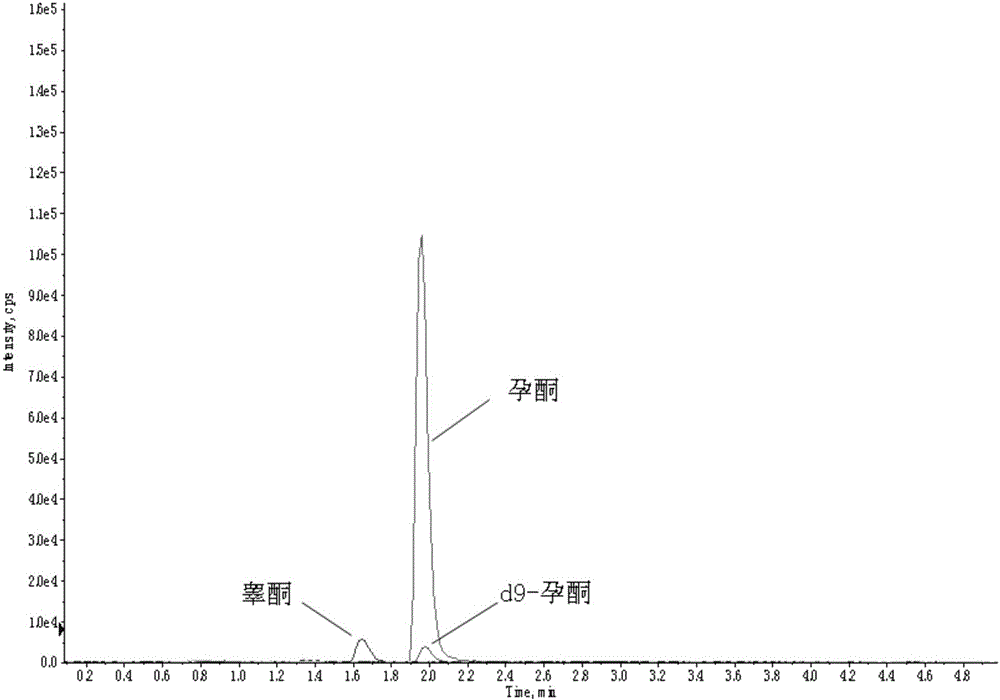
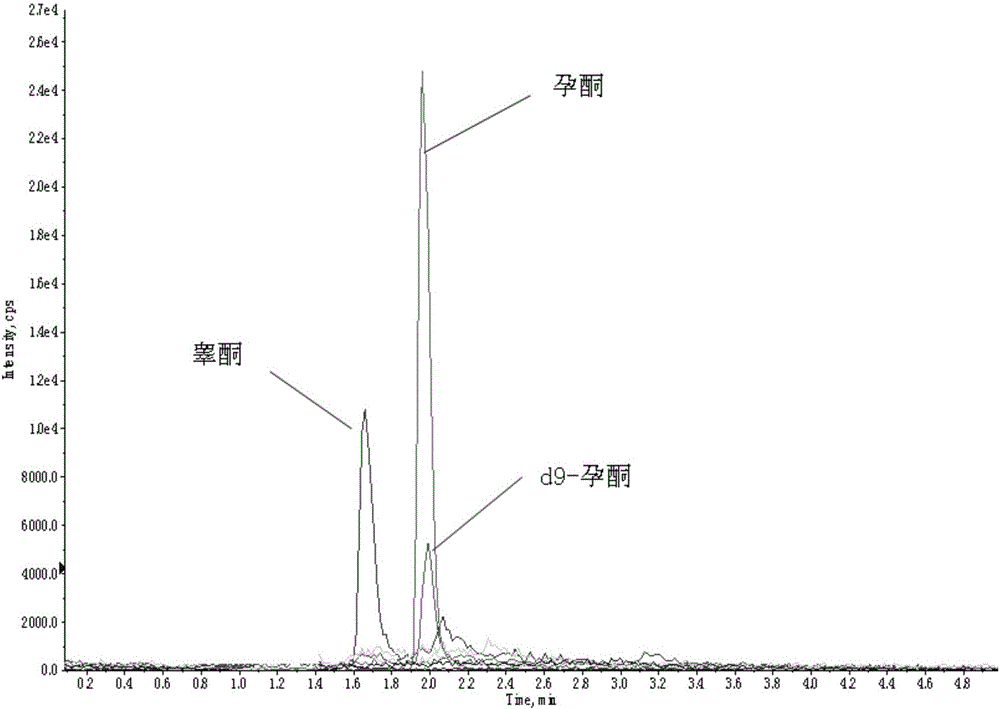
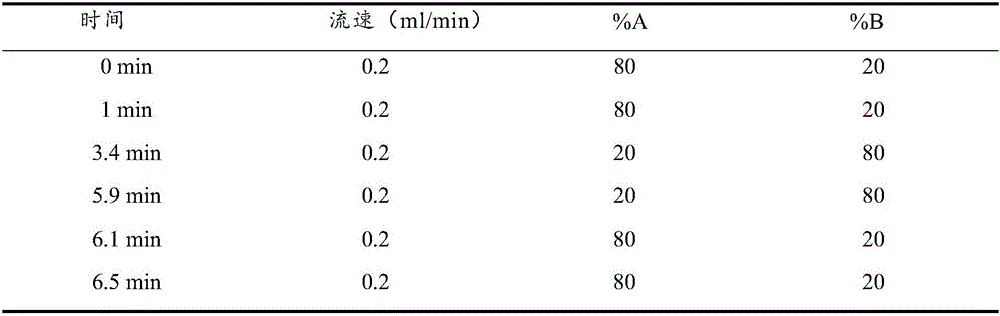
Method and kit for detecting progesterone and testosterone in saliva through high performance liquid chromatography-tandem mass spectrometry technique
The invention provides a method for detecting progesterone and testosterone in saliva through a high performance liquid chromatography-tandem mass spectrometry technique.The method comprises the steps that the progesterone and the testosterone in pretreated saliva are detected by adopting the high performance liquid chromatography-tandem mass spectrometry technique, the progesterone and the testosterone are separated from impurities through high performance liquid chromatography, quantification is performed through an internal standard method, an isotope of the progesterone is taken as an internal standard, a calibration curve is built by taking the concentration ratio of a standard product to the internal standard as an X axis and taking the peak area ratio of the standard product to the internal standard as a Y axis, and the content of the progesterone and the testosterone is calculated.The method is high in sensitivity and specificity, good in accuracy and simple in pretreatment process; the analysis time is short, analysis of the progesterone and the testosterone can be completed in 6.5 min, and the precision degree and the standard recovery rate meet the detection requirement.
Owner:北京洛奇医学检验实验室股份有限公司
Improved Neurobasal B27 culture medium, preparing method and application
The invention discloses an improved Neurobasal B27 culture medium. One liter of a Neurobasal culture solution comprises 0.09-0.11 g of biotin, 18-22 micrograms of corticosterone, 1-3 g of L-carnitine, 0.9-1.1 g of linoleic acid, 0.9-1.1 g of ethanol amine, 0.9-1.1 g of linolenic acid, 13-17 g of D(+)-galactose, 6-7 micrograms of progesterone, 15-17 g of putrescine dihydrochloride, 0.08-0.12 g of retinyl acetate, 2.2-2.8 g of thyroid-hormone-removing bovine serum albumin, 0.8-1.5 g of DL-alpha-tocopherol, 2-3 g of catalase, 0.9-1.2 g of DL-alpha-tocopheryl acetate, 0.8-1.3 g of reduced glutathione, 42-52 micrograms of lipoic acid, 2-3 g of superoxide dismutase, 4-6 g of transferring, 3-5 g of human insulin and 0.12-0.16 mg of sodium selenite. The invention further discloses a preparing method and application of the improved Neurobasal B27 culture medium. The improved Neurobasal B27 culture medium can be used for researching the influence of thyroid hormones on the process that embryonic stem cells are differentiated into nerve cells.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Pregnancy test device & method
ActiveUS20180088136A1Avoid false negative resultsImprove the level ofBiological material analysisBiological testingObstetricsPregnancy test
Disclosed is a test device to detect pregnancy In a human female subject, the test device comprising: an assay means to measure the absolute or relative amount of hCG m a sample from the subject; an assay means to measure the absolute or relative amount of FSH in a sample from the subject; and m assay means to measure the absolute or relative amount of one or mere progesterone metabolites I>> a sample from the subject.
Owner:SPD SWISS PRECISION DIAGNOSTICS
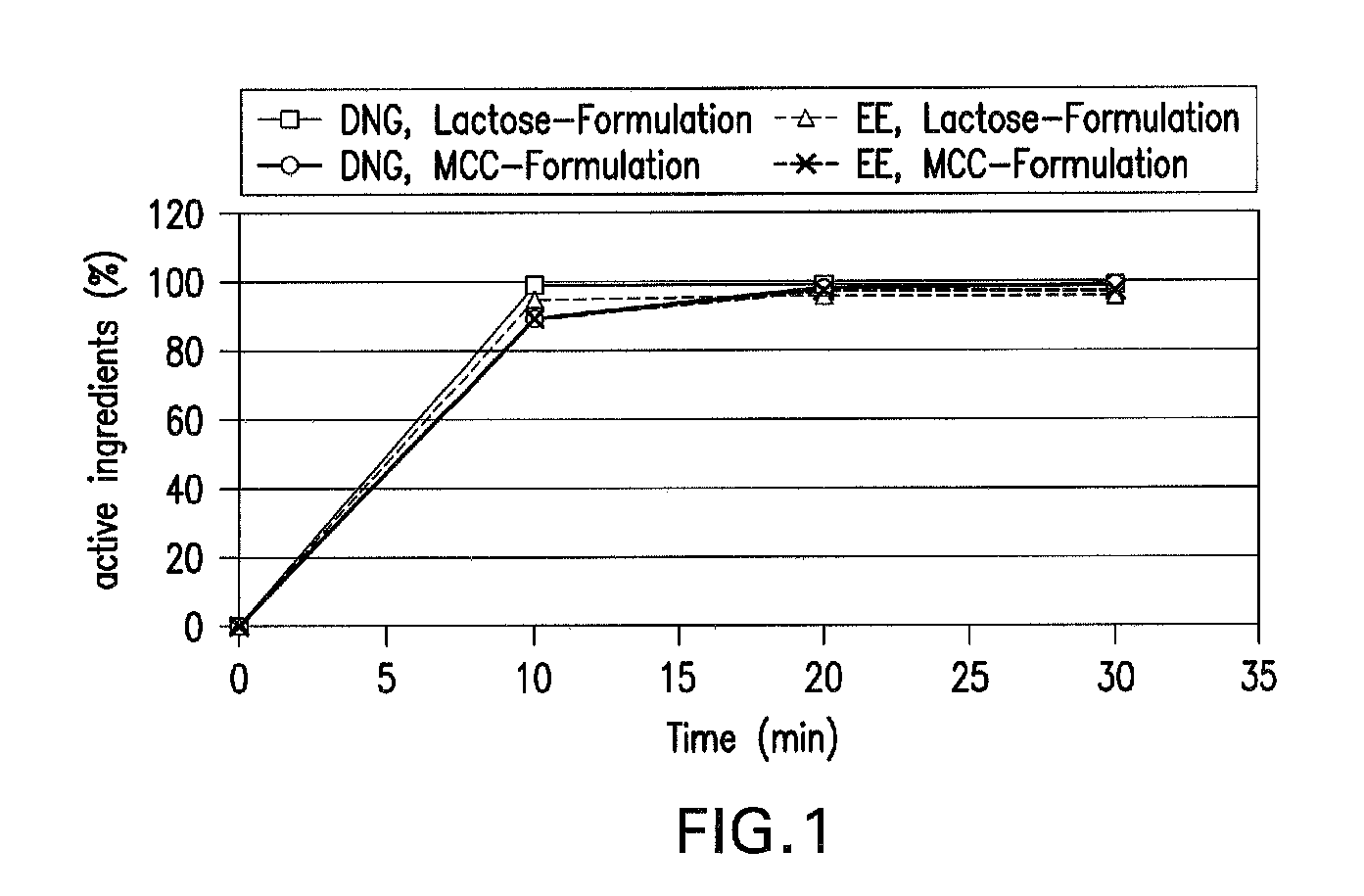
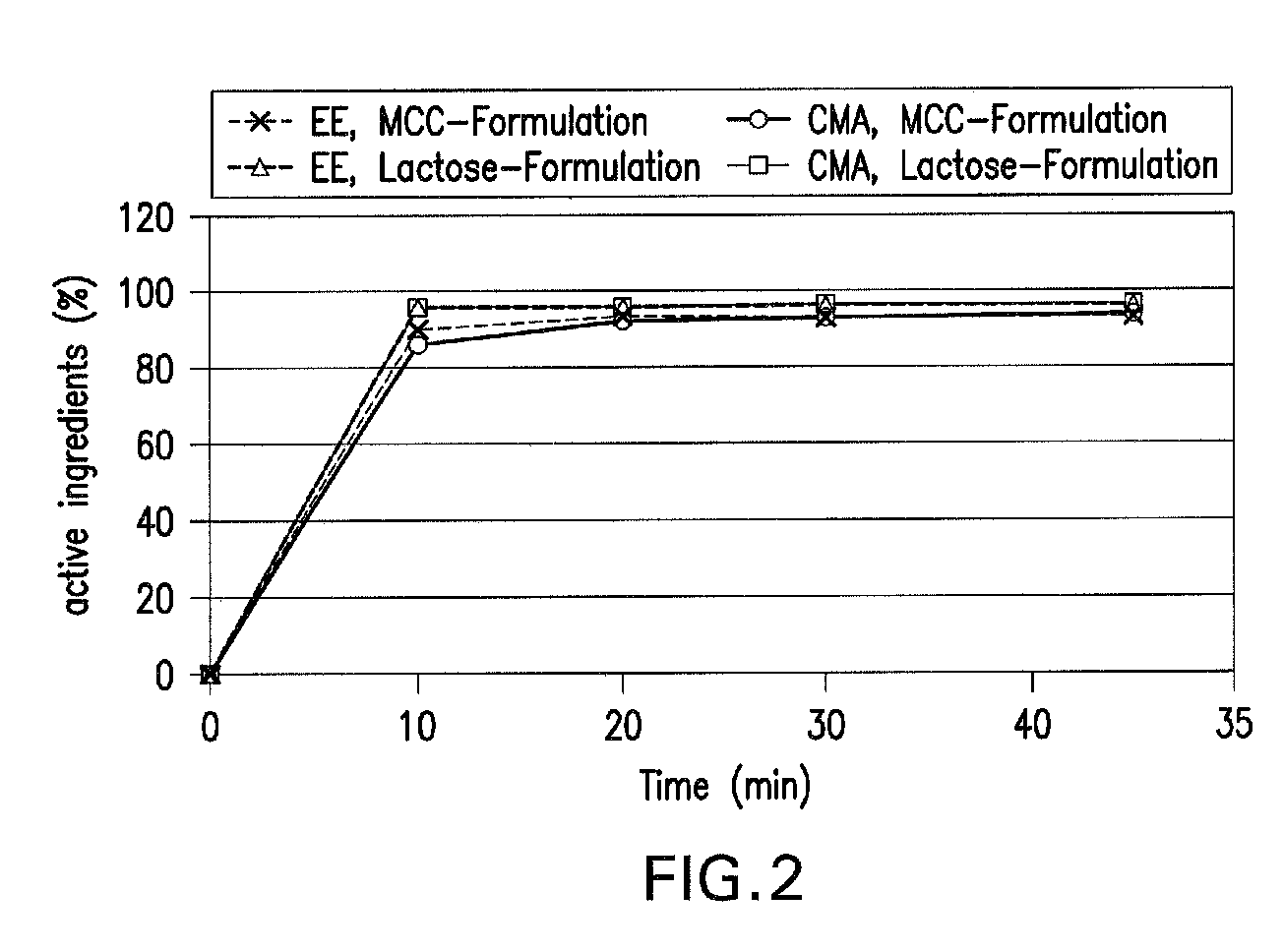
Oral contraceptive containing a gestagen and an estrogen combined with pharmaceutically acceptable auxiliary agents and/or excipients, but not containing lactose, and method of making same
InactiveUS20090117183A1Easy to solveLow costBiocideOrganic active ingredientsPhysiologyAdditive ingredient
The method produces a lactose-free oral contraceptive composition containing a combination of a gestagen and an estrogen together with one or more pharmaceutically acceptable auxiliary agents and / or excipients. The contraceptive composition is a tablet, powder, or capsule that contains the gestagen and estrogen, filler material such as microcrystalline cellulose and a binder such as hydroxypropylcellulose, but no lactose. Preferably the gestagen is dienogest, chlormadinone acetate, or levonorgestrel and the estrogen is ethinylestradiol, 17β-estradiol, or estradiol valerate. A method is provided for improving the prophylaxis of lactose intolerance in women taking oral contraceptives. The oral contraceptive preparations for a standard 28-day cycle or for long-term use contain at least 21 daily dose units of the gestagen and the estrogen in a low-dosage but without lactose and at most 7 daily dose units containing no active ingredient or a placebo.
Owner:BAYER SCHERING PHARMA AG
Isoflavonoid analogs and their metal complexes as anti-cancer agents
InactiveUS20070122843A1Lipophilic natureCellular internalization is improvedBiocideOrganic compound librariesAnticarcinogenPharmacophore
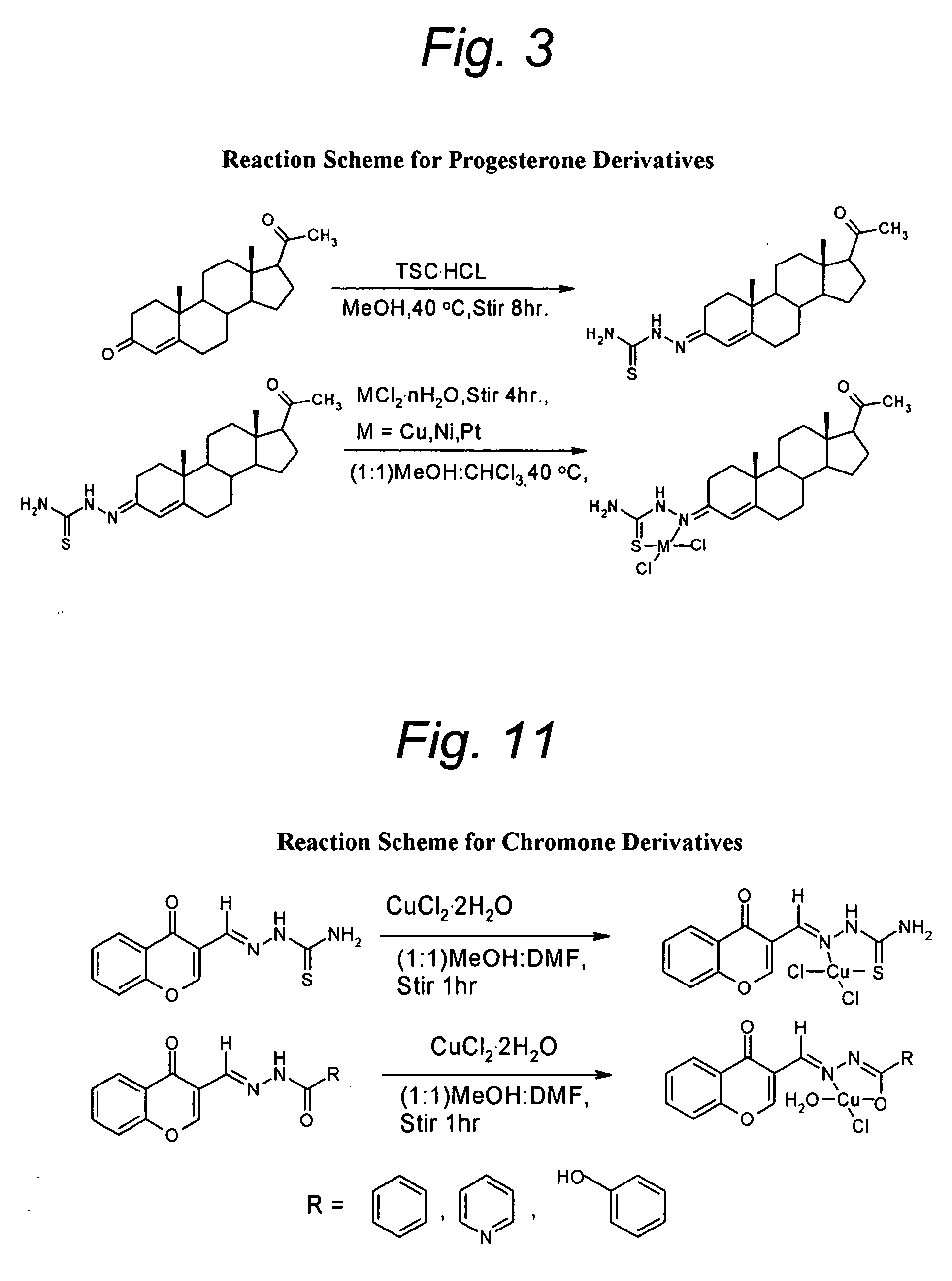
A pharmacologic agent for treating and / or preventing cancer, among other diseases and conditions, and particularly breast, prostate, and pancreatic cancer, in humans and animals. The novel pharmacologic agent is an isoflavonoid or isoflavonoid mimetic covalently attached to a cytotoxic pharmacophore that, preferably has the ability to conjugate with a metal salt to form a more potent metal complex, particularly a Cu(II) complex. The isoflavonoid or isoflavonoid mimetic may be non-fragmented steroidal hormone, such as progesterone which is structurally related to the isoflavone genistein, or a small molecule hormone mimetic, such as chromone. An illustrative non-fragmented steroidal embodiment is 17-acetyl-10,13-dimethyl-1,2,6,7,8,9,11,12,13,14,15,16,17-tetradecahydrocyclopenta[a]phenantnren-3-thiosemicarbazone and its Cu(II) complex. Effective chromone analogs include the thiosemicarbazone and hydrazone analogs of 4-oxo-4H-chromene-3-carboxaldehyde and their Cu(II) complexes.
Owner:SARKAR FAZLUL +1
Systems and methods for tracking progesterone
InactiveUS20200078781A1Conveniently and effectively be evaluated instrumentallyEasy assessment processAnalysis material containersObstetricsProgesterone metabolism
Embodiments of the invention comprise a test strip configured to detect one or more metabolites of progesterone in urine, and optionally further detect one or more additional metabolites or hormones in urine within the same test strip. Embodiments of the invention are methods of utilizing such a test strip in association with avoidance of pregnancy, detection of menopause, and in association with fertility planning purposes. Embodiments of the invention are associated with the incorporation of a test strip within a testing kit, optionally further comprising a digital reader.
Owner:MFB FERTILITY INC
Thio-oxindole derivatives
This invention relates to compounds which are agonists of the progesterone receptor which have the general structure: wherein: R1, R2, R3, R4, R5 and Q1 are as defined herein, or a pharmaceutically acceptable salt thereof, as well as methods of using these compounds to induce contraception or treat progesterone-related carcinomas and adenocarcinomas.
Owner:WYETH LLC
Methods, devices, and systems for detecting analyte levels
The present disclosure describes a method of quantifying analyte levels using gold nanoparticles. The present disclosure also describes a device to quantify analyte levels using gold nanoparticles and image processing of pixels isolated from a single vector. The methods, devices, and systems of the disclosure can be used to quantify analytes such as luteinizing hormone, progesterone, estradiol, or testosterone.
Owner:OOVA INC
Pregnancy test device and method
ActiveUS10794920B2Avoid false negative resultsImprove the level ofBiological material analysisBiological testingObstetricsPregnancy test
Owner:SPD SWISS PRECISION DIAGNOSTICS
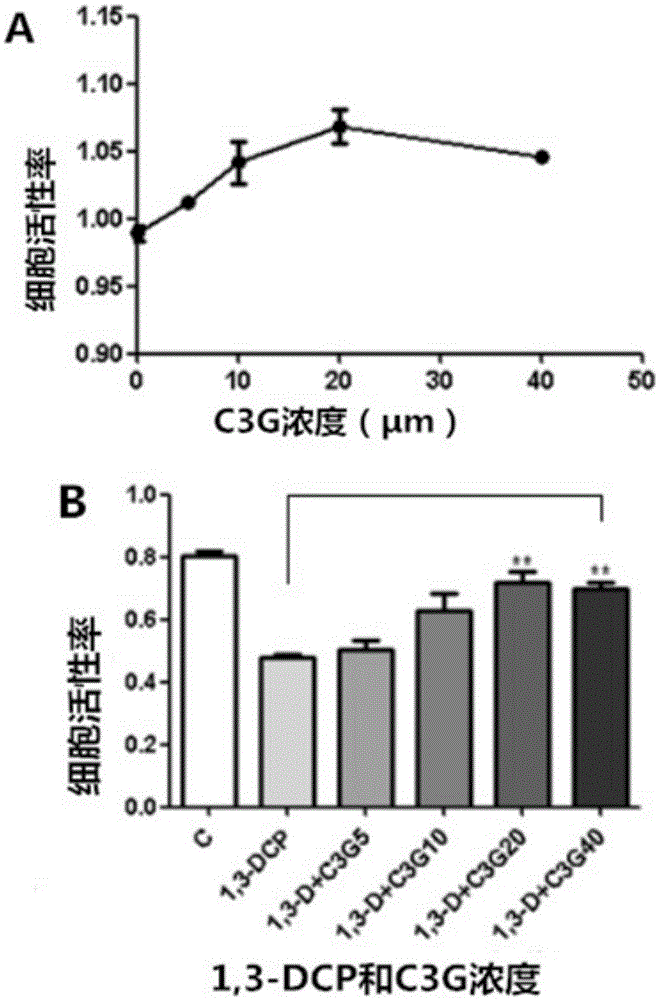
Interventional effect of cyanidin-3-O-glucoside on reproductive toxicity of 1,3-dichloro-2-propanol
ActiveCN105079017AUnderstand the functionOrganic active ingredientsAntinoxious agentsApoptosisGlucoside
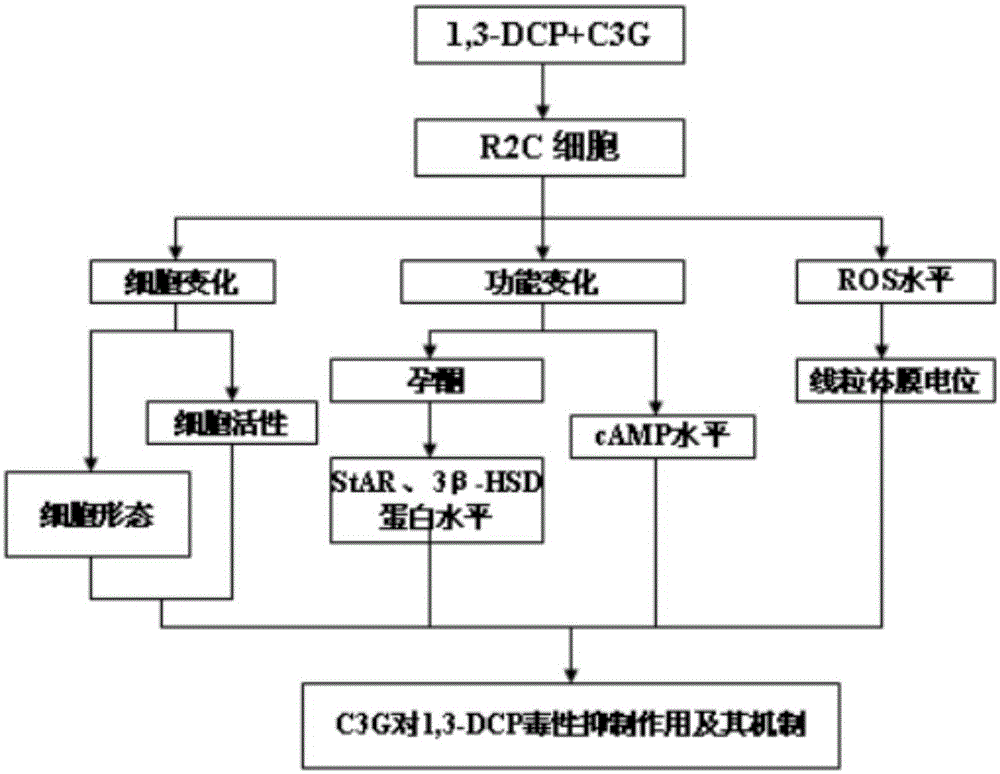
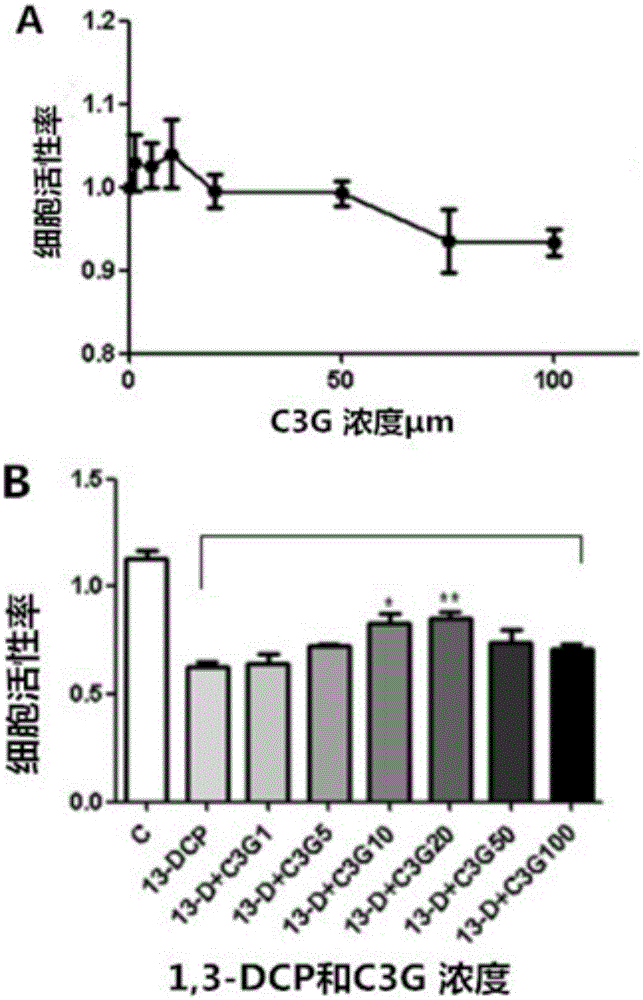
The invention discloses application of cyanidin-3-O-glucoside in preparing medicine for preventing and / or treating male genital diseases caused by 1,3-dichloro-2-propanol. Research results show that the C3G has a significant inhibitory effect on R2C cell activity decline, cell morphological damage, increase in cell apoptosis rate and the like exposed by 1,3-DCP, the C3G, by promoting the expression of StAR, 3beta-HSD and like key protein, intervenes in R2C cell progesterone synthesis exposed by the 1,3-DCP and simultaneously the C3G, by reducing ROS (reactive oxygen species) inside the R2C cell exposed by the 1,3-DCP and further inhibiting MMP (mitochondrial membrane potential) and the like, intervenes in R2C cell apoptosis and progesterone expression function injury caused by the 1,3-DCP. The research achievement of the invention provides an important basis for human to prevent and treat male genital diseases caused by 1,3-dichloro-2-propanol and has a great research significance.
Owner:JINAN UNIVERSITY
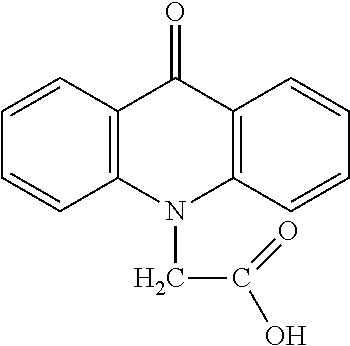
Method for Treatment of Primary Hormone Resistant Endometrial and Breast Cancers
InactiveUS20170202854A1Antineoplastic agentsHeterocyclic compound active ingredientsGynecologyAgonist
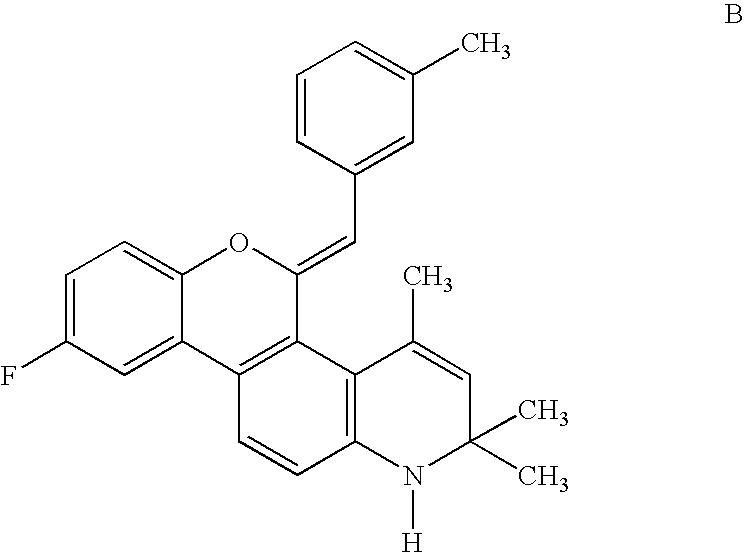
The invention provides a method for treatment of primary progesterone receptor-negative (PR−) endometrial cancer comprising administering (i) cridanimod or a salt or an ester thereof and (ii) a progesterone receptor (PR) agonist. The invention further provides a method for treatment of a primary estrogen receptor-negative (ER−) breast cancer, comprising administering (i) cridanimod or a salt or an ester thereof and (ii) a selective estrogen receptor modulator (SERM) or a selective estrogen receptor down-regulator (SERD). Also provided are compositions related to the above methods.
Owner:LIPOXEN TECHNOLOGIES LTD
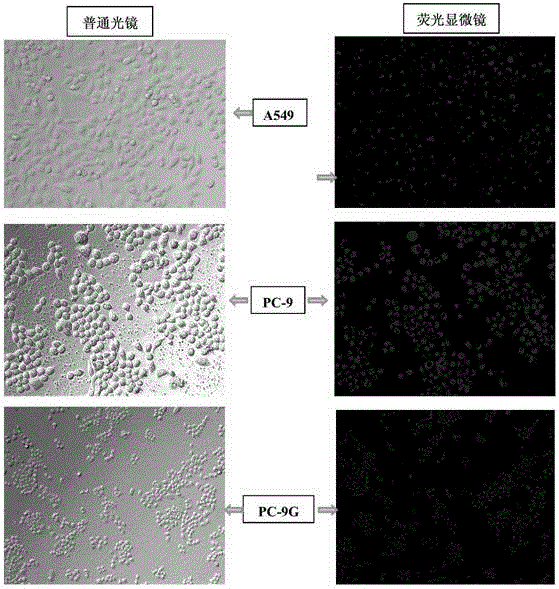
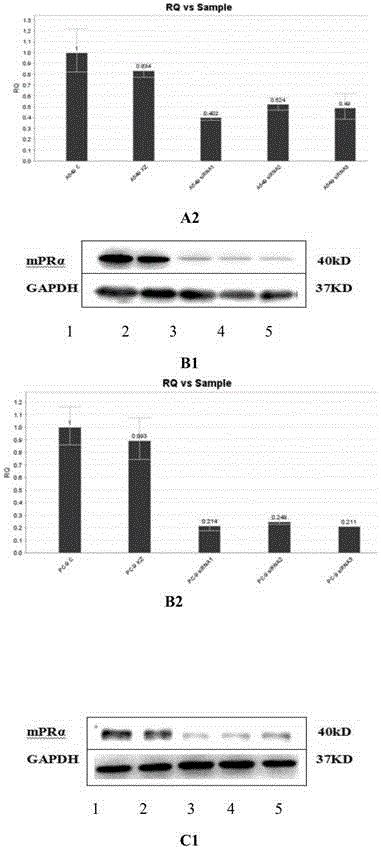
Method for adjusting sensibility of lung adenocarcinoma cells to EGFR-TKIs by mediating progesterone by mPR alpha
ActiveCN105420194AIncreased sensitivityMicrobiological testing/measurementBiological testingFemale sex hormonesProgesterones
The invention discloses a method for adjusting the sensibility of lung adenocarcinoma cells to EGFR-TKIs by mediating progesterone by mPR alpha, according to the method disclosed by the invention, the effects on the sensibility of lung adenocarcinoma cells of different EGFR mutation states to the EGFR-TKIs caused by the mPR alpha are compared, by inducing lung adenocarcinoma sensitive strain PC-9 cells into drug-resistant strain PC-9GR cells, and the relationship of a drug-resistant process and mPR alpha expression is dynamically observed. According to he method for adjusting the sensibility of lung adenocarcinoma cells to the EGFR-TKIs by mediating the progesterone by the mPR alpha, whether the sensibility of lung adenocarcinoma cells of different EGFR mutation states to the EGFR-TKIs can be improved or not after the progesterone is mediated by a membrane receptor is discussed from the point of view of female sex hormone, and a theoretical basis is provided for future research of a lung adenocarcinoma target drug sensitizer.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
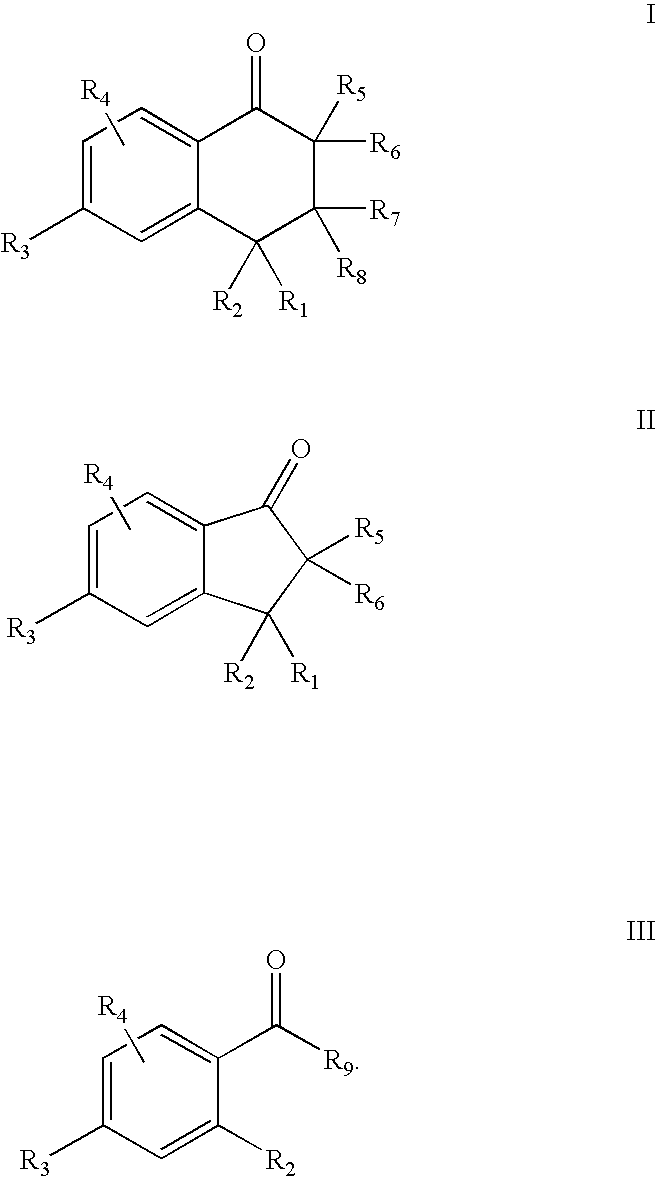
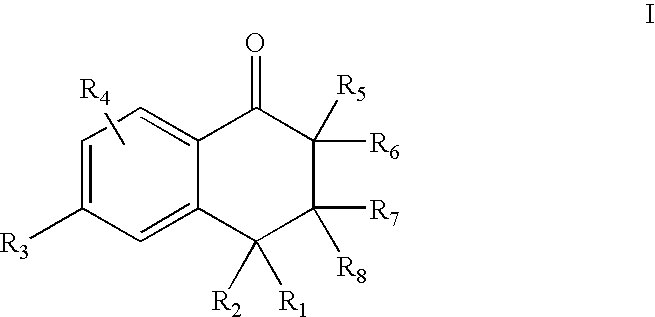
5-Aryl-indan-1-one and analogs useful as progesterone receptor modulators
Compounds of formula I or II are provided, wherein R1-R8 are defined herein, and pharmaceutical compositions and kits containing these compounds. Also provided are methods of inducing contraception, providing hormone replacement therapy, treating cycle-related symptoms, or treating or preventing benign or malignant neoplastic disease using the compounds of formula I, formula II, or formula III, wherein R1-R9 are defined herein:
Owner:WYETH LLC
11-alpha hydroxyprogesterone monoclonal antibody hybridoma cell strain and application thereof
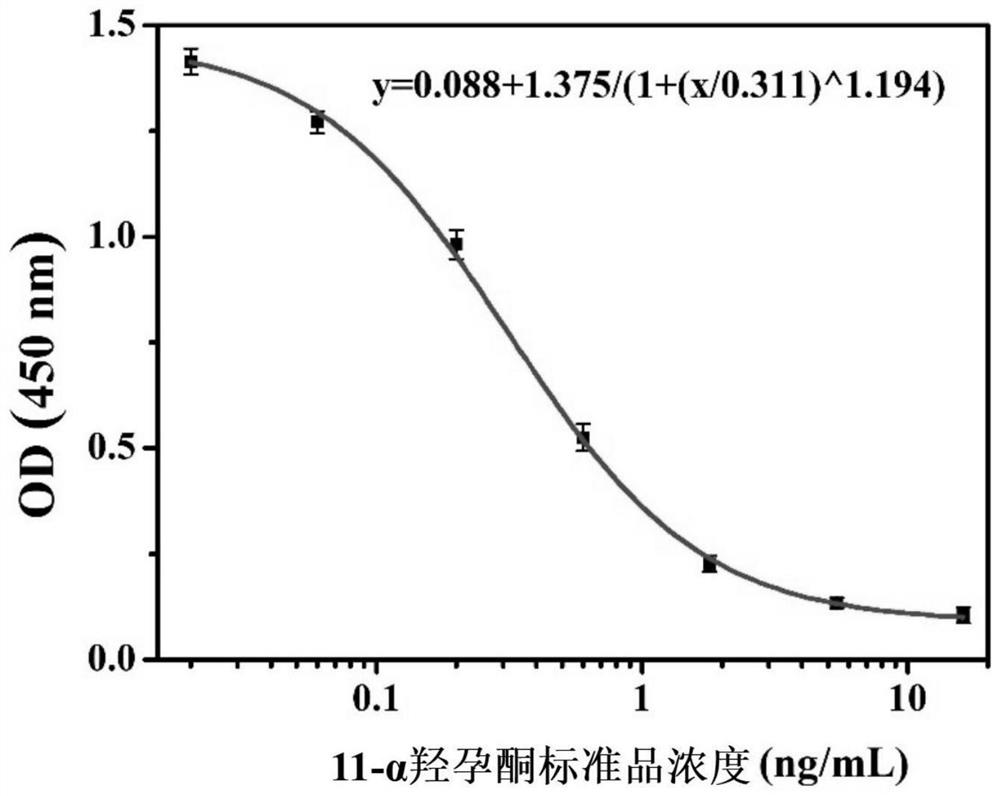
ActiveCN114836387AGood cross rateLow cross rateSerum albuminPeptide preparation methodsImmuno detectionHydroxyprogesterones
The invention relates to a 11-alpha hydroxyprogesterone monoclonal antibody hybridoma cell strain and application thereof, and relates to the technical field of immunodetection. The hybridoma cell strain is preserved in China General Microbiological Culture Collection Center (CGMCC) on March 3, 2022, the preservation address is No.3, No.1 yard, Beichen West Road, Chaoyang District, Beijing, and the preservation number is CGMCC No.45112. The hybridoma cell strain obtained by the invention belongs to a monoclonal cell strain. The 11-alpha hydroxyprogesterone monoclonal antibody disclosed by the invention has better specificity to 11-alpha hydroxyprogesterone (the cross-over rate to sex hormones such as progesterone, 17-alpha hydroxyprogesterone, medroxyprogesterone, estradiol and the like is less than 0.02%) and detection sensitivity (the IC50 value is 0.311 ng / mL) to the 11-alpha hydroxyprogesterone. And an immunological method is provided for detecting the content of 11-alpha hydroxyprogesterone in a clinical blood sample.
Owner:JIANGNAN UNIV
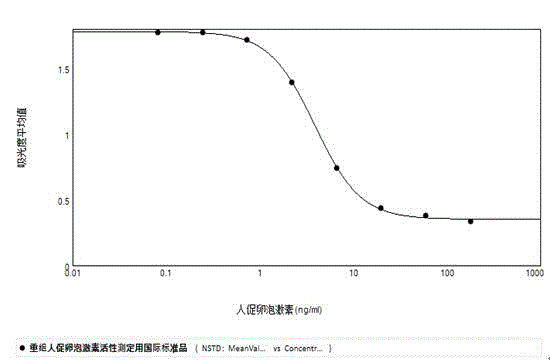
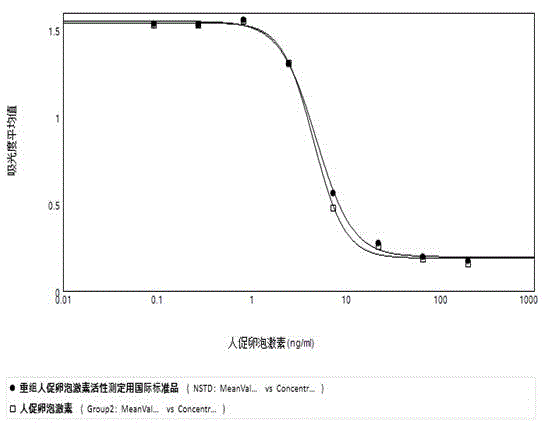
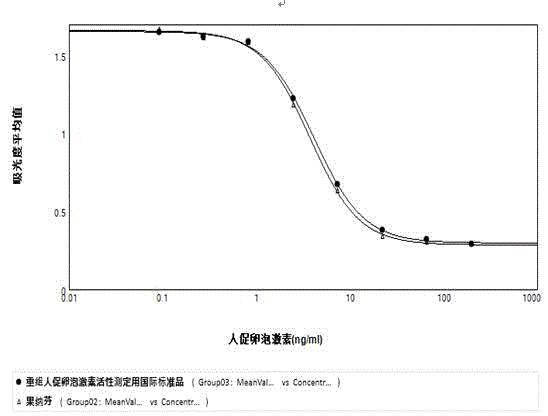
Analytical method for evaluating in-vitro biological activity of human follicle stimulating hormone
InactiveCN104569440AMeet ethical requirementsAvoid harmBiological testingGranular leucocyteOvarian Granulosa Cell
The invention discloses an analytical method of utilizing adherent cell-human ovarian granular cell tumor cell strain (KGN) to evaluate in-vitro biological activity of human follicle stimulating hormone. The method comprises the following steps: using the human follicle stimulating hormone to stimulate the human ovarian granular cell tumor cell, measuring the content of generated progesterone, so as to reflect the activity of the human follicle stimulating hormone. The analytical method of using the human ovarian granular cell tumor adherent cell to evaluate the in-vitro biological activity of the human follicle stimulating hormone, disclosed by the invention, is effective, stable and quick, and avoids the limitations of long time consumption, complicated operation and poor result repeatability in the animal in-vivo ovarian weight increment method; meanwhile, the progesterone content is determined by a commercial enzyme linked immunosorbent assay (ELISA) kit, so that the biological activity of the human follicle stimulating hormone can be reflected truly and more accurately.
Owner:BEIJING TIDE PHARMA
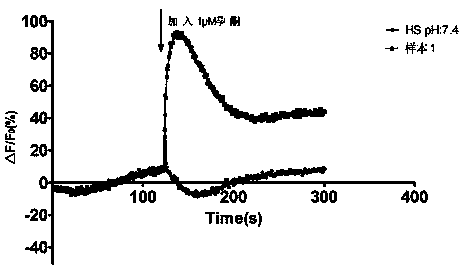
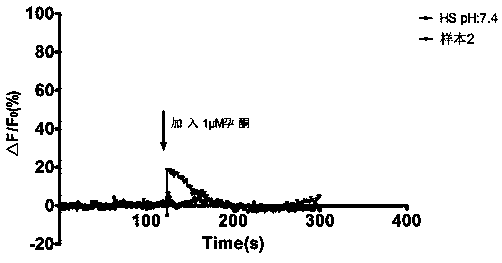
High-throughput screening method for progesterone response defect infertility human sperm samples
InactiveCN110873706AImprove stabilityAvoid time differenceBiological testingFluorescence/phosphorescenceObstetricsHigh-Throughput Screening Methods
The invention discloses a high-throughput screening method for progesterone response defect infertility human sperm samples. According to the invention, healthy reproductive men are used as normal control, a male patient with infertility caused by unknown reasons is used as a research object, the effect of progesterone among different human sperm samples on the concentration ([Ca<2+>]i) of free Ca<2+> in head cells is observed and screened at high throughput, human sperm infertility samples with progesterone response defects are efficiently and rapidly screened while the dosing time differenceis avoided, the influence of progesterone on the human sperm function and the molecular mechanism of progesterone are further explored, and meanwhile, the clinical significance of sperm membrane progesterone receptor function defects in evaluation of male infertility causes is expected to be illuminated.
Owner:NANTONG UNIVERSITY
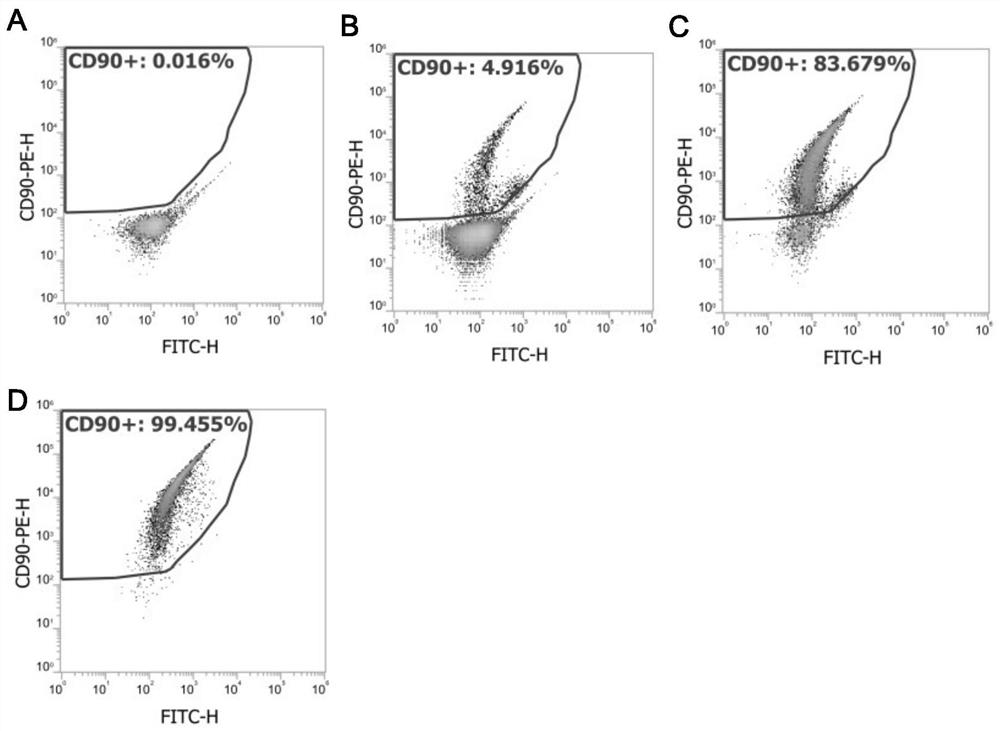
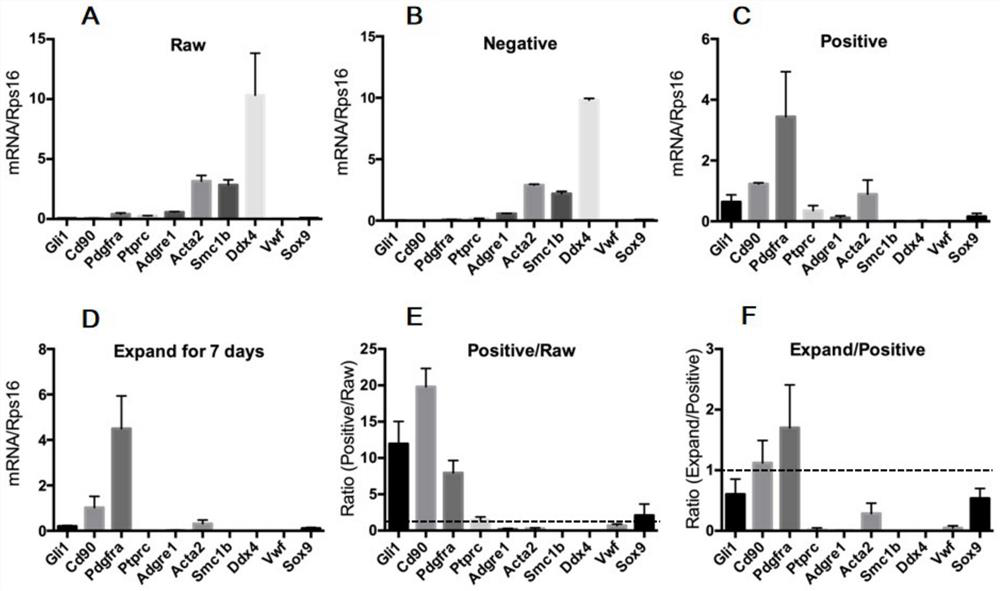
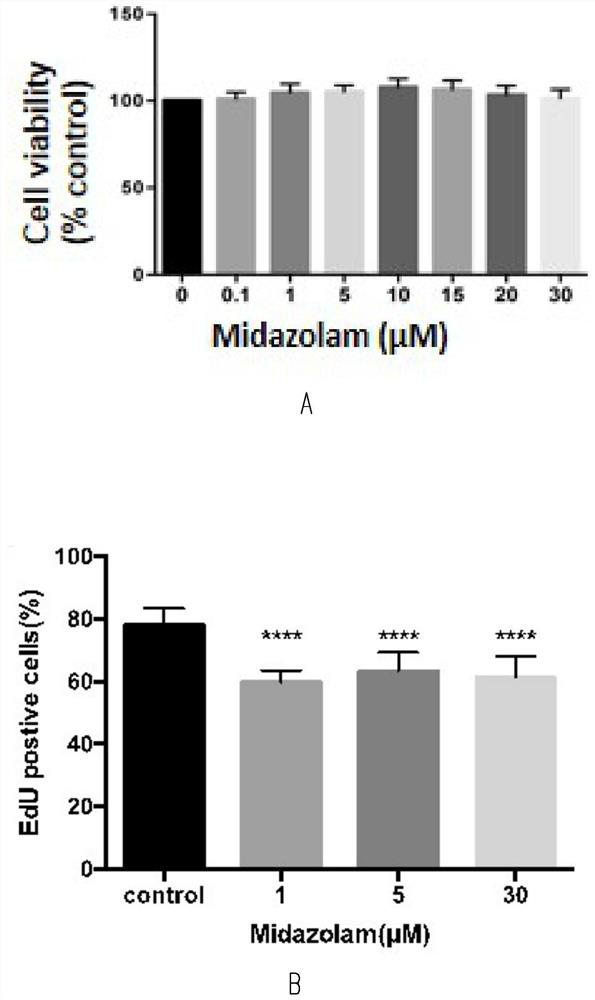
Method for testing influence of midazolam on development of testicular interstitial cells cultured in vitro
ActiveCN114181885APromote differentiationPromote productionCell dissociation methodsMicrobiological testing/measurementTesticular Interstitial CellsMidazolam
The invention discloses a method for testing influence of midazolam on development of testicular interstitial cells cultured in vitro. Comprising the following steps: culturing rats, separating CD90 positive cells, culturing testicular mesenchymal stem cells (SLC), detecting the influence of midazolam on proliferation and differentiation, amplifying the SLCs and detecting the cell activity, carrying out DAPI dyeing and counting, analyzing cells by a flow cytometer, analyzing testosterone (T) and progesterone (P4), carrying out mRNA and protein expression detection through QPCR (Quantitative Polymerase Chain Reaction) and WB (White Brown) experiments, and determining significant differences and drawing a chart through SNK (Sodium Nitrogen Kinase) detection. The steps are simple, and the influence of midazolam medicine on proliferation and differentiation of the testicular mesenchymal stem cells can be effectively verified.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Transdermal Hormone Delivery System: Compositions And Methods
A transdermal hormone delivery system (THDS) is disclosed. The THDS is useful for control of fertility and as therapy for a variety of diseases and conditions treatable by robust delivery of progestin and estrogen hormones, particularly the progestin, levonorgestrel. The THDS comprises a backing layer, an adjoining adhesive polymer matrix comprising an effective amount of at least a progestin hormone, delivery of which is enhanced by one or more skin permeation enhancing agents present in pre-determined amounts. The THDS is capable of providing effective daily doses of progestin and estrogen hormones from a small surface area in contact with the skin, e.g., less than 20 square centimeters. Methods of fertility control and various types of hormone replacement therapy utilizing the THDS are also disclosed.
Owner:AGILE THERAPEUTICS
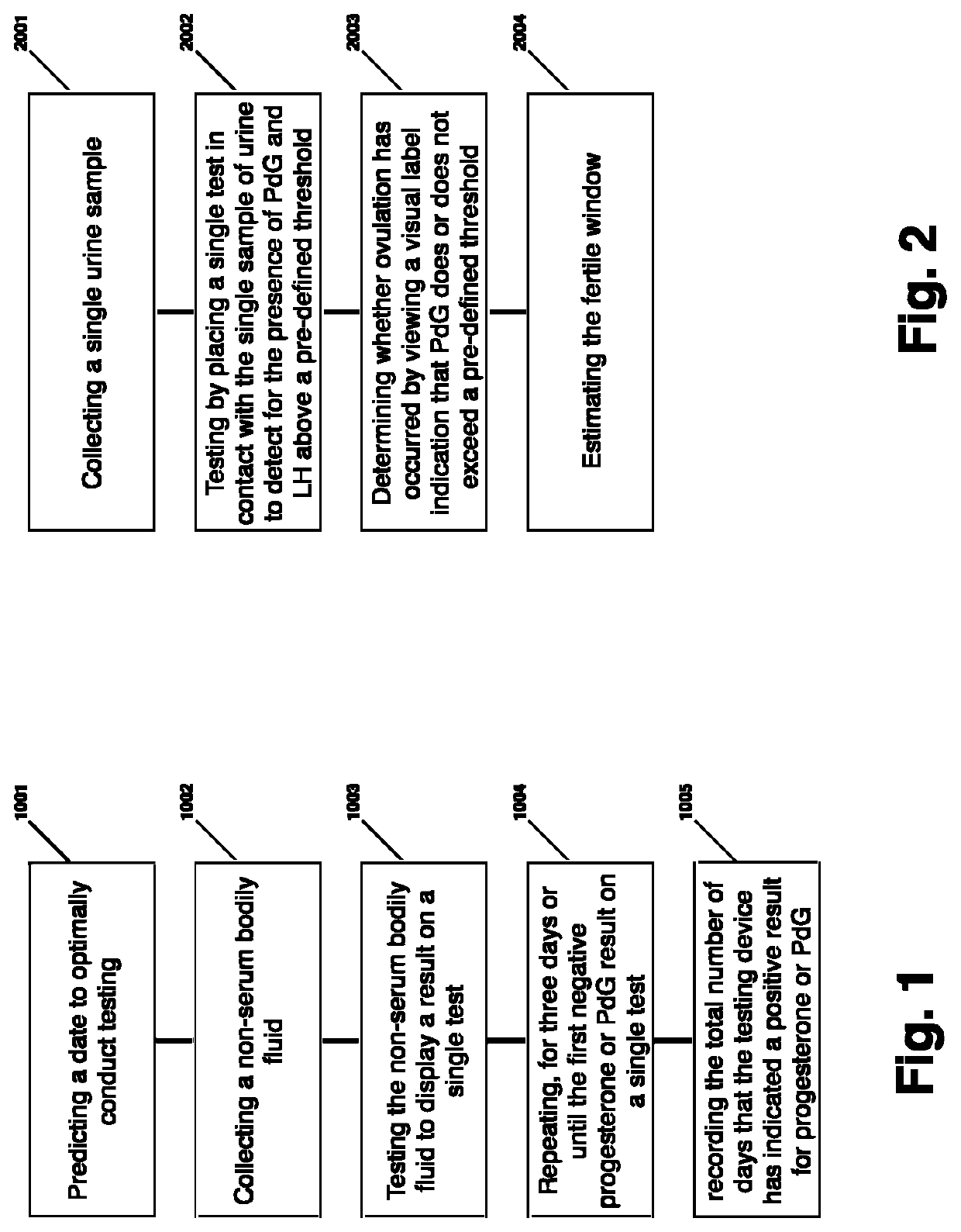
Method for evaluating urine of a subject to estimate the fertile window by evaluating for the presence of analytes of estrogen and progesterone
Disclosed herein are devices, systems, methods and kits for performing immunoassay tests to detect for at least progesterone or analytes of progesterone on a sample in association with diagnosing problems and issues associated with corpus luteum functionality. The immunoassay devices and methods may be used in conjunction with diagnostic reader systems and / or a base unit for obtaining a sensitive readout of the immunoassay results. The methods disclosed herein may also incorporate steps associated with evaluating the urine of a sample for the presence of an estrogen metabolite and / or luteinizing hormone.
Owner:MFB FERTILITY INC
Fluorescent quantitative detection kit for progesterone, estradiol and beta-human chorionic gonadotropin
The invention provides a fluorescent quantitative detection kit for progesterone, estradiol and beta-human chorionic gonadotropin, and relates to the technical field of in vitro diagnosis. The kit comprises a test strip, wherein the test strip comprises a substrate, and a sample pad, a CP pad, an NC membrane and an absorption pad which are sequentially adhered to the substrate; the CP pad is coated with a mouse anti-progesterone monoclonal antibody, a mouse anti-estradiol monoclonal antibody, a mouse anti-beta human chorionic gonadotropin monoclonal antibody and a fluorescein labeled conjugateof chicken IgY; and a detection line coated with a progesterone antigen, an estradiol antigen and a beta-HCG monoclonal antibody and a quality control line coated with a rabbit anti-chicken IgY monoclonal antibody are respectively arranged on the NC membrane. According to the kit, three early pregnancy indexes can be rapidly, accurately and quantitatively detected at the same time through one-time sampling, and a more accurate reference basis is provided for dynamically detecting early pregnancy hormone level changes and predicting the pregnancy outcome of early threatened abortion patients.
Owner:RELIA BIOTECH JIANGSU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com