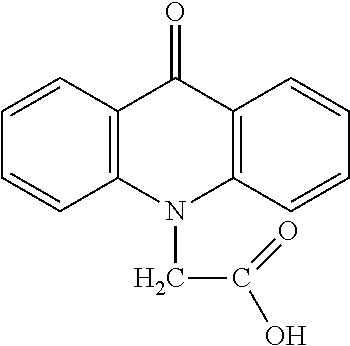
Method for Treatment of Primary Hormone Resistant Endometrial and Breast Cancers
a breast cancer and hormone-resistant technology, applied in the field of primary hormone-resistant endometrial and breast cancer treatment, can solve the problems of no treatment option for patients with recurrent or metastatic endometrial carcinoma, and the depletion of prs in the target tissue of progestin treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
tion of PR-Agonist and Sodium Cridanimod has Limited Efficacy in Secondary PR-Negative Endometrial Cancer with Acquired Resistance to PR-Agonist but is Highly Effective in Primary PR-Negative Endometrial Cancer
[0095]Eight patients with advanced and metastatic endometrial cancer (FIGO stage III-IV; Mutch. Gynecol Oncol. 2009, 115:325-328) were included to the study. All patients were not amenable for treatment for surgery, chemo- or radiotherapy. Tumor tissue samples from the patients were assessed for PR level. Primary progesterone receptor-negative (PR−) cancer was registered in 3 patients; secondary progesterone receptor-negative (PR−) cancer was registered in 5 patients. All patients were treated with oral PR agonist medroxyprogesterone acetate (MPA) at dose of 500 mg once a day and sodium cridanimod (SC) intramuscular injections at dose of 500 mg twice a week. The responses (complete response, partial response or stable disease) according to RECIST 1.0 (www.recistcom; Therasse e...
example 2
tion of PR-Agonist and Sodium Cridanimod has Significant Antitumor Effect in an Animal Model of Human Primary PR-Negative Endometrial Cancer and Limited Efficacy in an Animal Model of Human Secondary PR-Negative Endometrial Cancer
[0097]In order to confirm the above clinical findings, cridanimod / PR-agonist therapy was tested in animal models of human primary and secondary PR-negative cancers:
[0098]Mouse Model of Primary PR-Negative Endometrial Cancer:
[0099]40 female 8-week-old immunodeficient BNX nu / nu mice (Harlan Laboratories) were bilaterally s.c. injected with 5×106 of human primary PR-negative endometrial cancer cells HEC-1B in 0.1 ml of Matrigel forming two tumors per mouse. Treatment was started on the next day after the injection and discontinued after 5 weeks. Cohorts (10 mice / group) received either:
1) Vehicle control group: vehicle only, 0.9% sodium chloride for injection i.m each alternate day and i.p. for 5 days / week.
2) MA only control group: megestrol acetate (MA) (10 mg...
example 3
tion of Tamoxifen and Sodium Cridanimod is Highly Effective for Treatment of Primary ER-Negative Breast Cancer
[0107]Nine women (48-69 years old) with primary PR / ER-negative (diagnosed using LBA and ICH methods) and HER2 negative (as determined with IHC Dako DakoCytomation's HercepTest), i.e., triple negative breast cancer (TNBC) of stage III-IV not amenable for surgery, chemo- or radiotherapy were treated with tamoxifen (40 mg / day orally) in combination with sodium cridanimod solution injected intramuscularly (i.m.) twice a week at a dose of 500 mg during the first four weeks. After discontinuation of sodium cridanimod, patients continued to take tamoxifen only. The response was assessed each 8 weeks according to RECIST 1.0 criteria (www.recistcom; Therasse et al., J. Natl. Cancer Inst., 2000, 92(3):205-216). The interim results of the trial are shown in Table 4, below.
TABLE 4Patient PR / ER Tamoxifen dailyClinical Progression-free#statusdose, mgeffectperiod, months1Negative20PR82Nega...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com