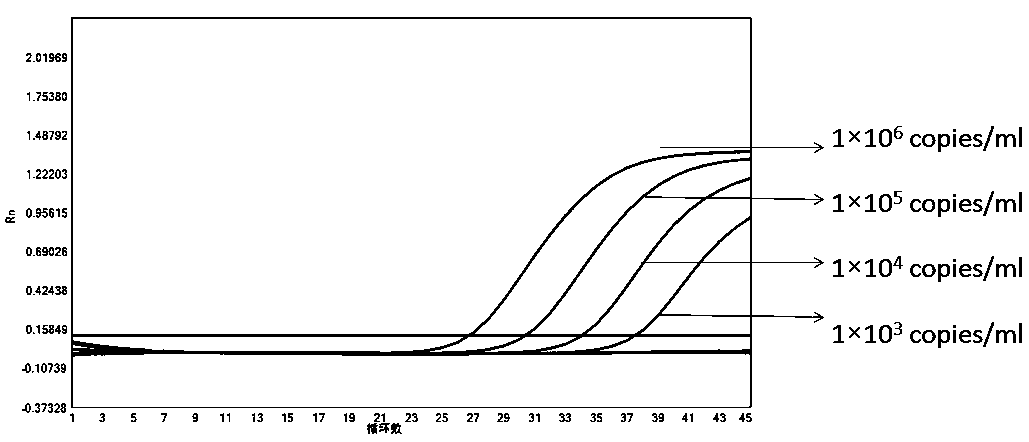
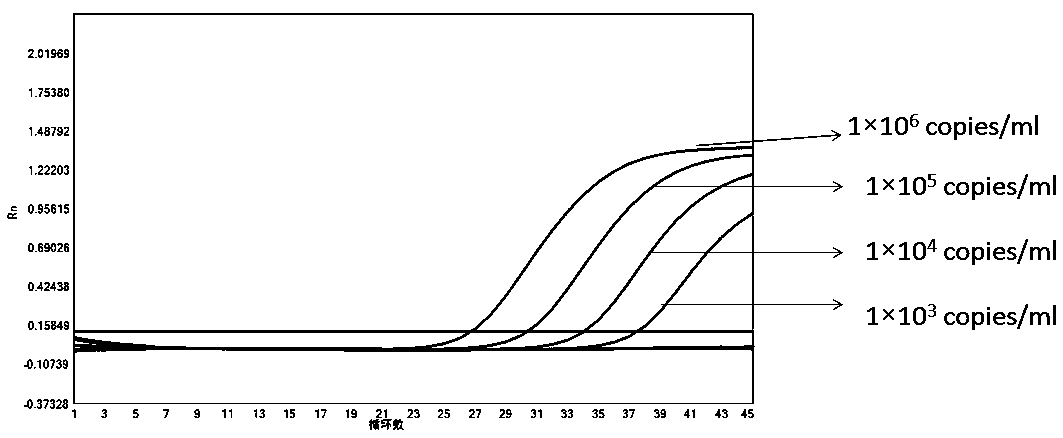
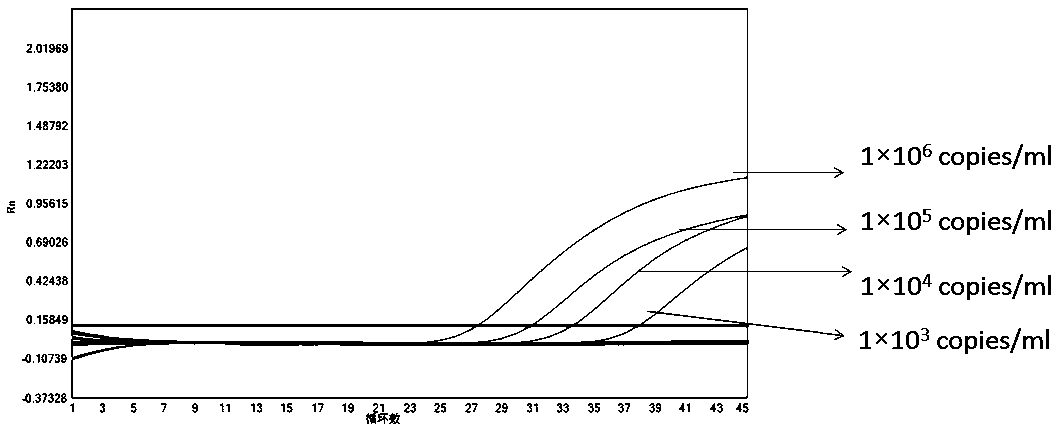
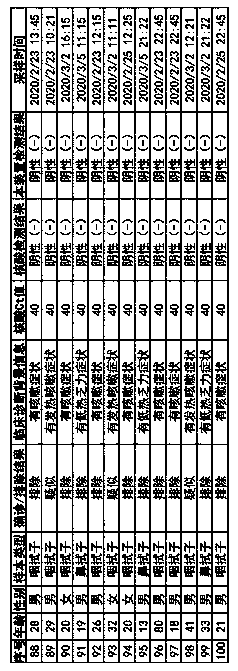
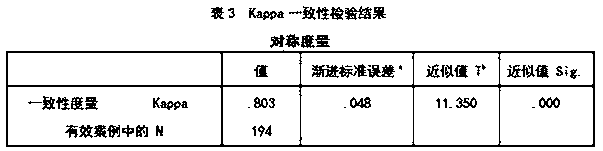
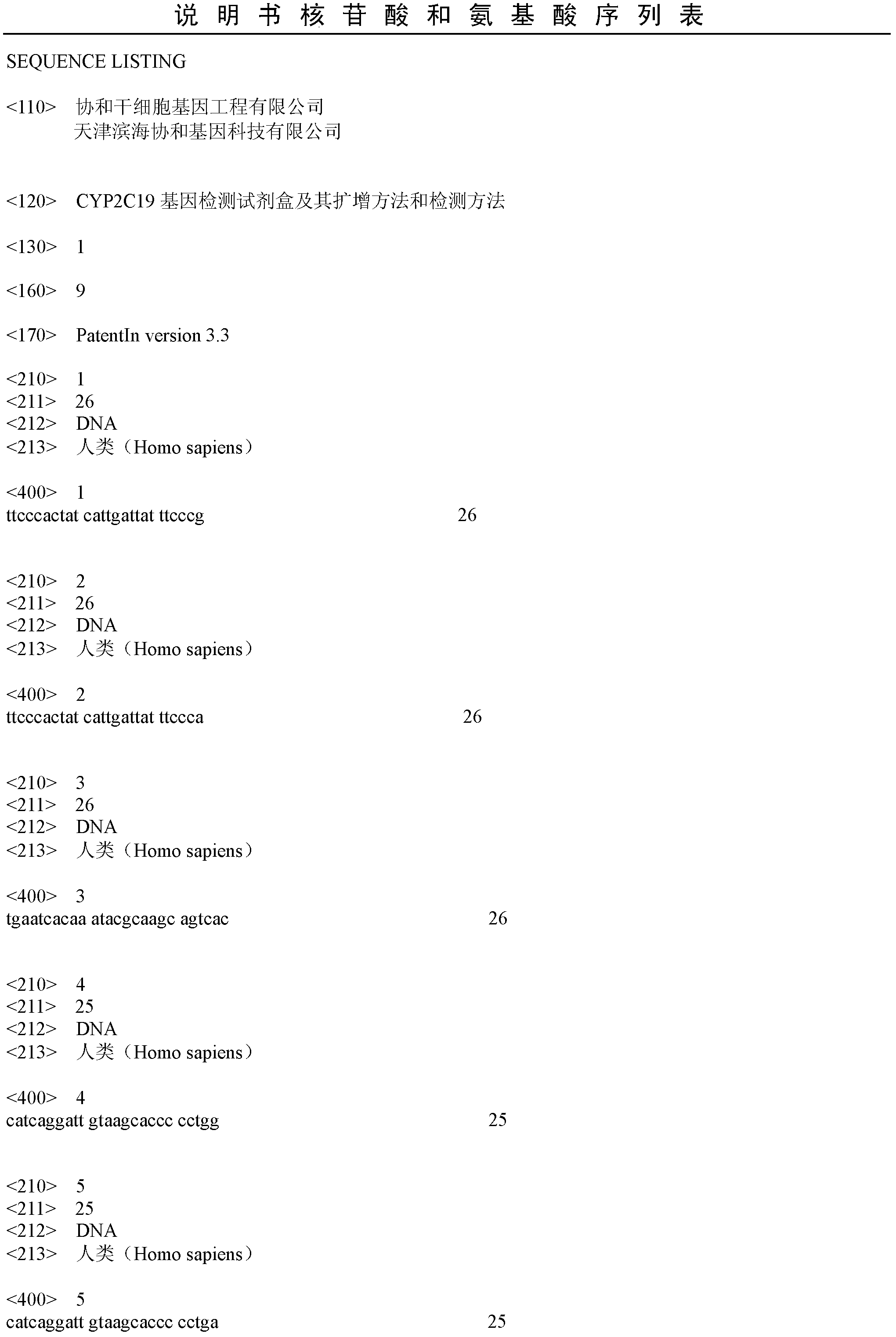Patents
Literature
204results about How to "Avoid false negative results" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
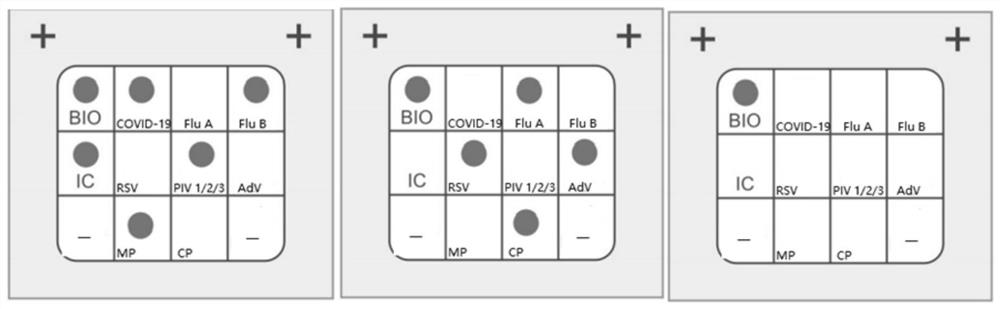
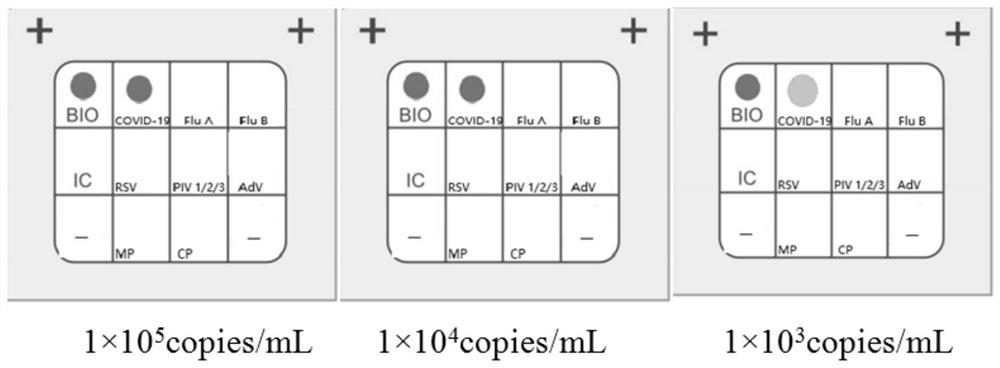
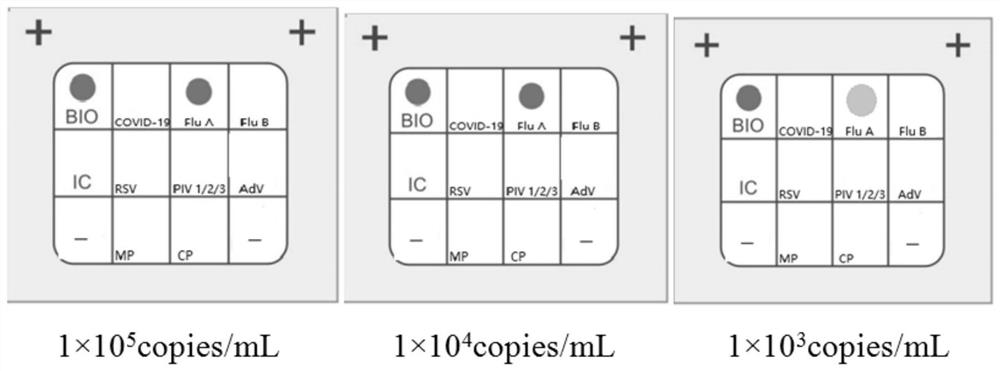
Detection kit for novel coronavirus, influenza A and B and respiratory syncytial virus
InactiveCN111088408AHigh detection sensitivityGood repeatabilityMicrobiological testing/measurementAgainst vector-borne diseasesRespiratory tract infectionsMolecular biology
The invention discloses a detection kit for a novel coronavirus, influenzas A and B and a respiratory syncytial virus. According to the detection kit, through combining primer probe design and adaptive detection system optimizing research, a group of nucleic acid combined detection primer and probe combinations and nucleic acid combined detection kits for the novel coronavirus (2019-nCoV / SARS-CoV2), an influenza A virus (FluA), an influenza B virus (FluB) and the respiratory syncytial virus (RSV) are developed, and sequences of primers and probes are shown in SEQ ID NO: 1-18. The kit has theadvantages of high detection sensitivity, good repeatability and low false negative and false positive and can effectively distinguish novel coronavirus infection from ordinary viral influenza and respiratory syncytial virus induced respiratory tract infection, so that precise diagnosis and subsequent precise treatment on a sufferer are achieved.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
Fluorescence immunochromatography device for detecting COVID-19 and using method thereof
InactiveCN111060691AImprove binding efficiencyHigh sensitivityBiological testingImmunoassaysVirologyDisease control
The invention discloses a fluorescence immunochromatography device for detecting a novel coronavirus COVID-19 and a using method of the fluorescence immunochromatography device. The fluorescence immunochromatography device disclosed by the invention is high in sensitivity and strong in specificity, the accuracy is high, the detection sensitivity can reach 10pg / ml, the detection speed is high, theoperation is simple, the device is portable, the requirement for personnel is low, the operation of professionals is not needed, the detection cost is low, the device can be applied to preliminary screening of various places such as hospitals, airports, customs and disease control centers, the detection time (10-15 min) is short, a simpler, more convenient and faster field detection means is provided for suspected patient investigation and asymptomatic infected person screening, and therefore epidemic spreading is prevented as soon as possible.
Owner:SHENZHEN BIOEASY BIOTECHNOLOGY CO LTD
System and method for diagnosing diseases
ActiveUS20090208923A1Avoid false negative resultsMicrobiological testing/measurementBiological material analysisProtein targetHalf-life
One aspect of the invention provides a method of diagnosing a disease condition, comprising measuring presence or amount of a targeted protein or a degradation product of said protein in a collected biological sample as a marker for the disease condition. The targeted protein or degradation product is selected for measurement based on a prior identification of a measurable half-life at a predetermined time period, including the time at which said method is conducted, and correlating said measuring with the presence or absence of the disease condition. The targeted protein or degradation product may be identified by selecting a protein known or suspected to be a diagnostic marker for the disease condition, analyzing degradation of the protein in the collected biological sample, and selecting a protein or degradation product that exhibits a measurable half-life at a predetermined period of time. The analyzing may include identifying degradation product(s) of the protein as a function of time, and half-life of the protein and the degradation product(s).
Owner:BECTON DICKINSON & CO
Nucleic acid combined testing kit of respiratory tract infection pathogens
InactiveCN111378789AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention discloses a nucleic acid combined testing kit of respiratory tract infection pathogens. The invention develops a set of primer-probe combinations which can detect multiple types of respiratory tract infection pathogens such as novel coronavirus, influenza virus a, influenza virus b, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumonia and chlamydiapneumonia through combination of a multiple fluorescence quantitative PCR technology and a flow-through hybridization and gene chip technology, wherein nucleotide sequences thereof are shown by SEQ ID NO:1-36 respectively. The nucleic acid combined testing kit of the respiratory tract infection pathogens is established. The kid can realize synchronous combined testing of the 8 respiratory tract infection pathogens, is high in detection accuracy, specificity and sensitivity, good in repeatability, low in false negativity and false positivity, short in detection time and low in cost, can realize comprehensive detection of a patient, can locate a disease source accurately, can realize treatment in time or make corresponding quarantine measures and is of important significance to effective control of respiratory tract infection and subsequent prevention of outbreak of relevant contagion and infection.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
Kit for detecting aldehyde dehydrogenase 2 gene polymorphism and amplification method and detection method thereof
InactiveCN103184268AImprove detection efficiencyReduce testing costsMicrobiological testing/measurementGlycerolPolymerase L
The present invention discloses a kit for detecting aldehyde dehydrogenase 2(ALDH2) gene polymorphism by using the single-tube bidirectional allele-specific amplification technology combined with the SNP sensitivity molecular switch technology, and an amplification method and a detection method thereof. The kit genotypes the Glu487Lys(rs671) site on the aldehyde dehydrogenase 2. The kit includes amplification buffer, polymerase, cell lysis buffer and glycerol, and can complete the genotyping for the SNP site in one PCR reaction so as to understand the genotype of the aldehyde dehydrogenase 2 subject and the product activity thereof.
Owner:UNION STEMCELL & GENE ENG +1
Primers and probes for detecting mutation of cancer gene BRAFV600E
InactiveCN102242207AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationCancer genesForward primer
The invention relates to the field of biotechnology and aims to provide multiple primers and probes for detecting mutation of a cancer gene BRAFV600E. The primers and probes provided by the invention respectively comprise a mutation specific forward primer, a mutation non-specific forward primer, universal reverse primers and universal probes shown in SEQ NO.1-11. The detection sensitivity of themutation specific primer and probe provided by the invention can reach 500 copies / ml, thus the sensitivity is good. If a sample does not contain the cancer gene BRAFV600E, the ct value of the amplification curve of the sample is greater than or equal to 36, and the specificity is strong. Because the mutation non-specific primer is simultaneously designed in the invention to be used for detecting the total template quantity of samples, the false negative result is avoided and the quality control is convenient.
Owner:ZHEJIANG UNIV
Test for Microbial Blood Infections
InactiveUS20170349937A1Rapid and accurate informationEasy to useMicrobiological testing/measurementMicroorganismMicrobiome
The invention relates to methods for detecting microbial blood infections including sepsis in a subject, preferably a neonate or a non-neonate such as an adult, the method comprising real-time PCR reactions for detection of specific microorganisms. Said methods preferably include treatment of a subject following detection of a specific microorganism or group of microorganisms. The invention further relates to a kit of parts adapted for performing a method of the invention, a set of at least one forward or re verse primer or probe and a method for monitoring sepsis or for determining the efficacy of an anti-sepsis treatment.
Owner:MICROBIOME
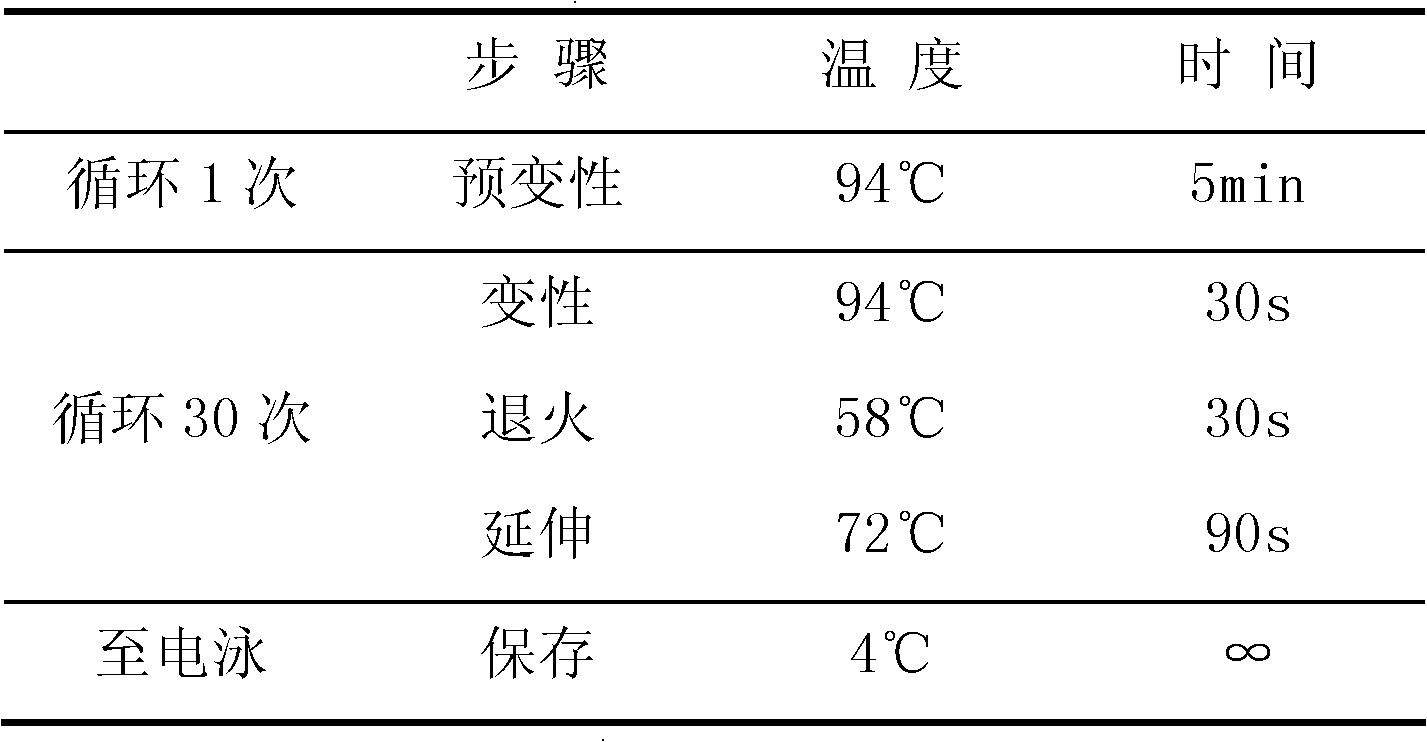
CYP2C19 gene detection kit, amplification method and detection method
InactiveCN103184265AImprove detection efficiencyReduce testing costsMicrobiological testing/measurementWild typePolymerase L
The present invention discloses a gene detection kit using the multiplex PCR technology combined with the SNP sensitive molecular switch technology for genotyping of cytochrome 4502C19 gene, an amplification method and a detection method. Three common polymorphic sites on the CYP2C19 gene significantly changing the product activity are genotyped by the kit, wherein the three common polymorphic sites include: rs4244285SNP site, rs4986893 site and rs12248560 site. The kit includes a wild-type 2*PCR buffer, a 2*mutant amplification buffer, and a polymerase. The two buffers respectively includes a sequence-specific primer and an internal reference primer corresponding to SNP wild and mutant phenotype, and can complete genotyping for the three SNP sites in two multiplex PCR reactions, and thus the CYP2C19 gene is genotyped, and molecular biological evidence is provided for the gene-expressed enzyme activity prediction.
Owner:UNION STEMCELL & GENE ENG +1
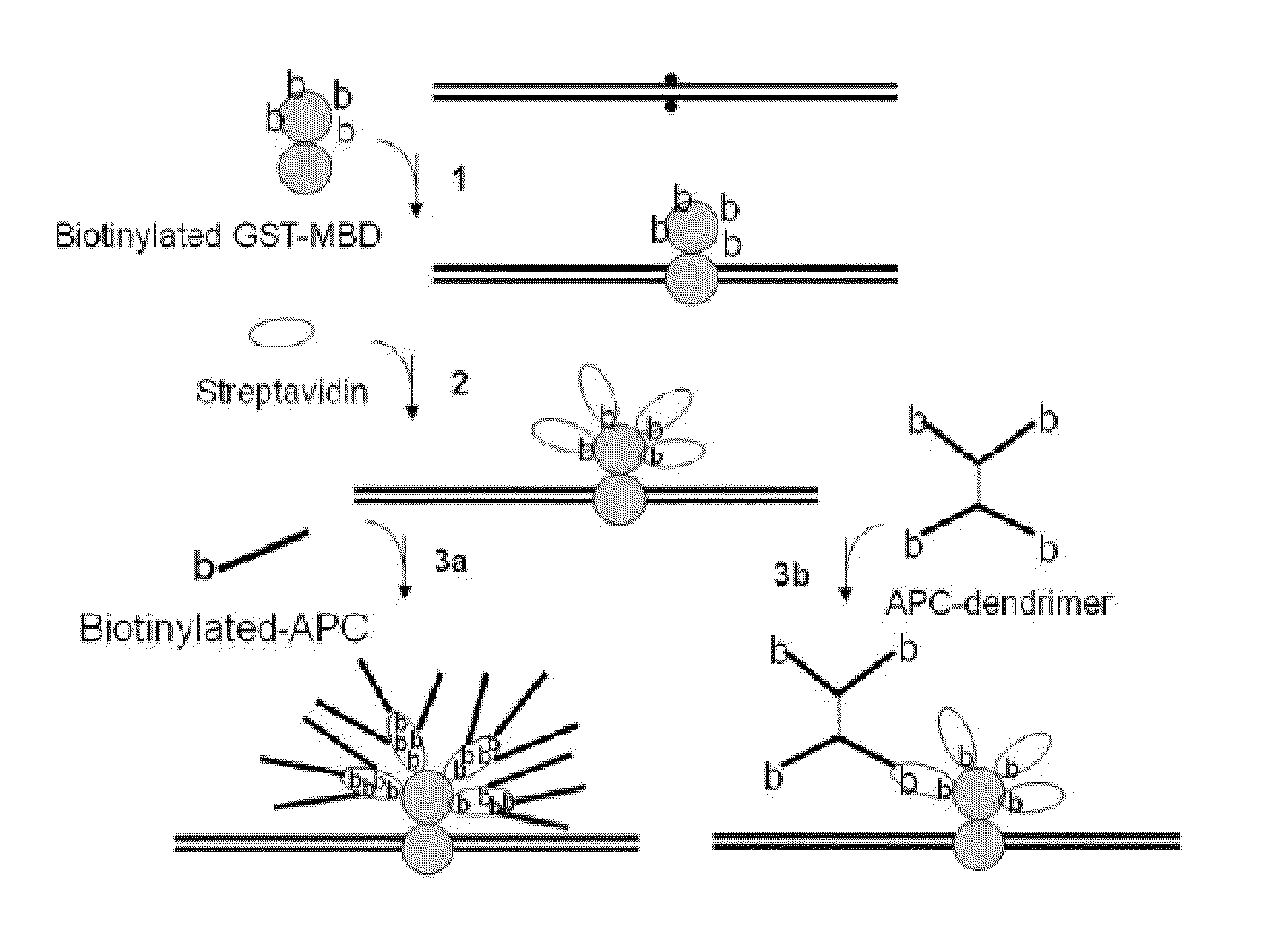
METHODS AND REAGENTS FOR DETECTING CpG METHYLATION WITH A METHYL CpG BINDING PROTEIN (MBP)
InactiveUS20090298080A1Simplify the development processSimplified suppressionMicrobiological testing/measurementTransferasesBinding domainSignal generator
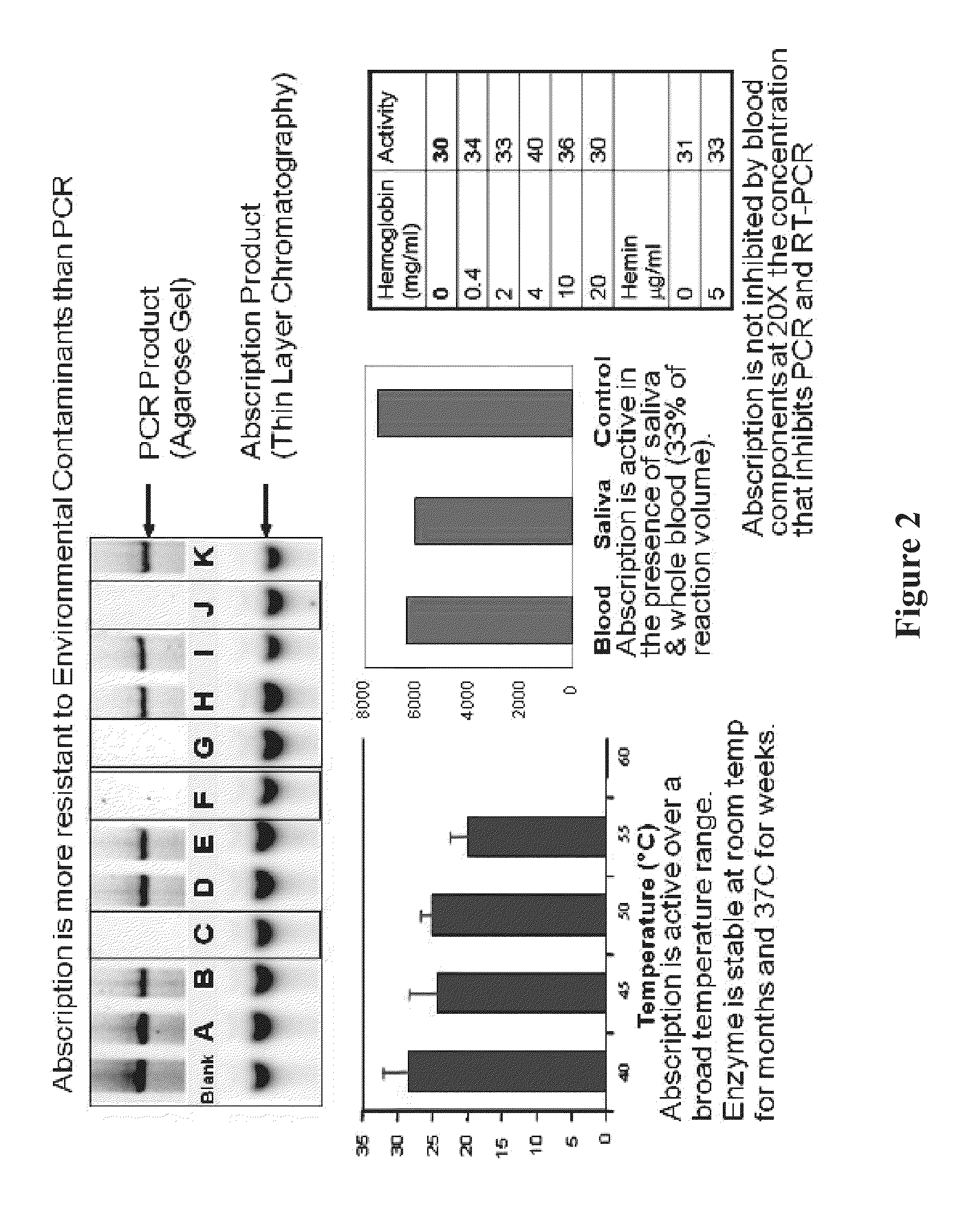
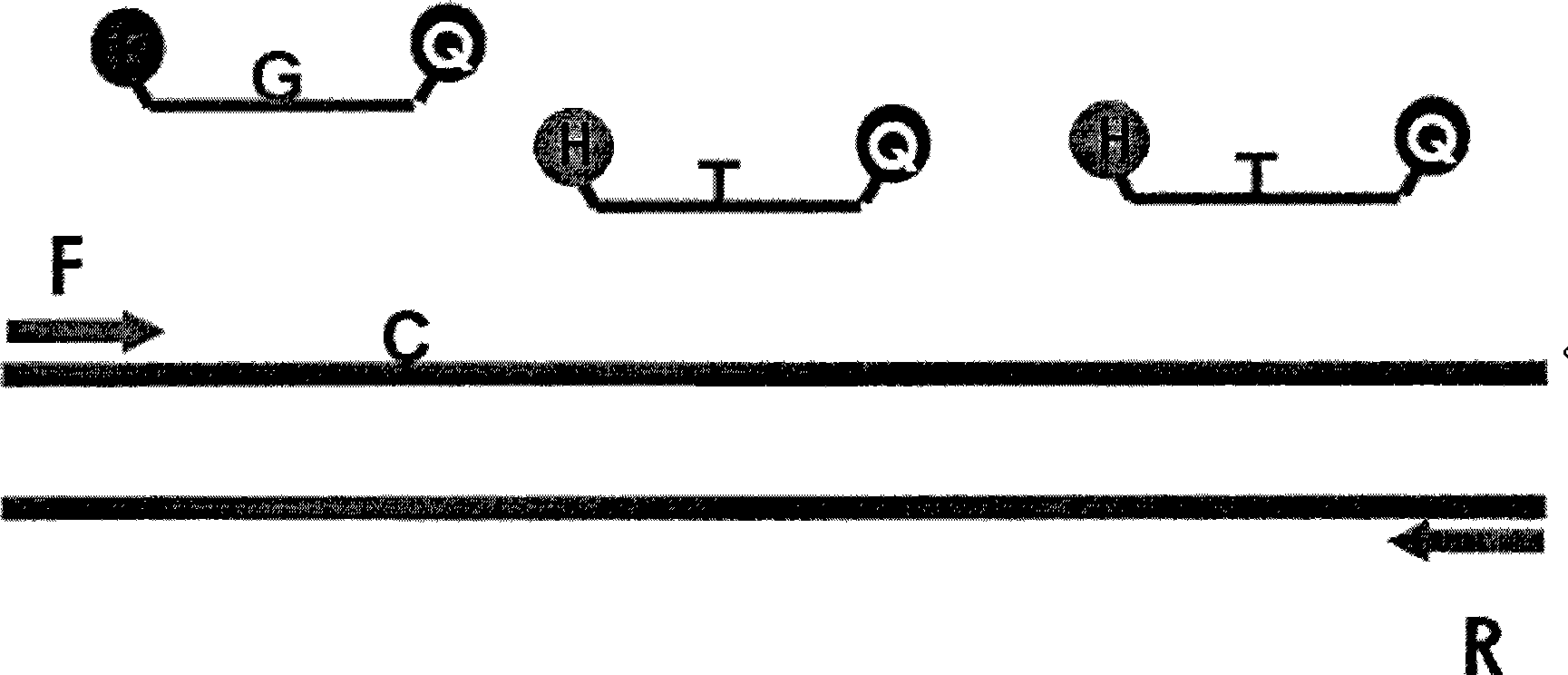
The present invention provides a simple and sensitive technology for the detection of CpG methylation in DNA without chemical modification of sample DNA by bisulfite treatment or PCR amplification. Signal generation is based on an Abscription (Abortive Transcription) technology in which DNA signal generators called Abortive Promoter Cassettes (APCs) are bound to target mCpG sites via mCpG target specific probes based on methyl binding polypeptides or methyl binding domains thereof. RNA polymerase produces uniform, short RNA molecules from synthetic promoters in APCs as signals of the presence of methylated CpGs. Detection of CpG methylation and hypermethylation of DNA targets such as CpG islands provides a convenient means for detecting and monitoring cancer in a subject.
Owner:RIBOMED BIOTECHNOLOGIES INC
Design method for realtime fluorescent quantitative PCR experiment interior label
InactiveCN101475988AEffective monitoring errorAvoid false negative resultsMicrobiological testing/measurementInternal standardFluorescence
A design method of real-time fluorescence quantitative PCR internal standard includes the following steps: (1) designing a primer sequence according to the activating genes to be tested, select the corresponding internal standard genes, whose primer sequence is same to that of the activating genes; (2) designing the probe of the activating genes, determining the concentration, and labeling a fluorescent reporting group at the 5' end of the probe; (3) designing two probes of the internal standard genes, one probe corresponding to the internal standard genes, the other probe corresponding to another sequence of the activating genes, and respectively labeling a same fluorescent reporting group at the 5' end of the two probes; (4) extracting and purifying the activating nucleic acid, and performing real-time fluorescence quantitative PCR amplification. The present invention can effectively monitor errors occurring in the nucleic acid extraction, amplification and product analysis processes, thus avoiding false-negative results.
Owner:戴立忠
Method for assaying cedinafei and derivative thereof
ActiveCN101021479ANo interference detectedStrong specificityMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationCITRATE ESTERTest sample
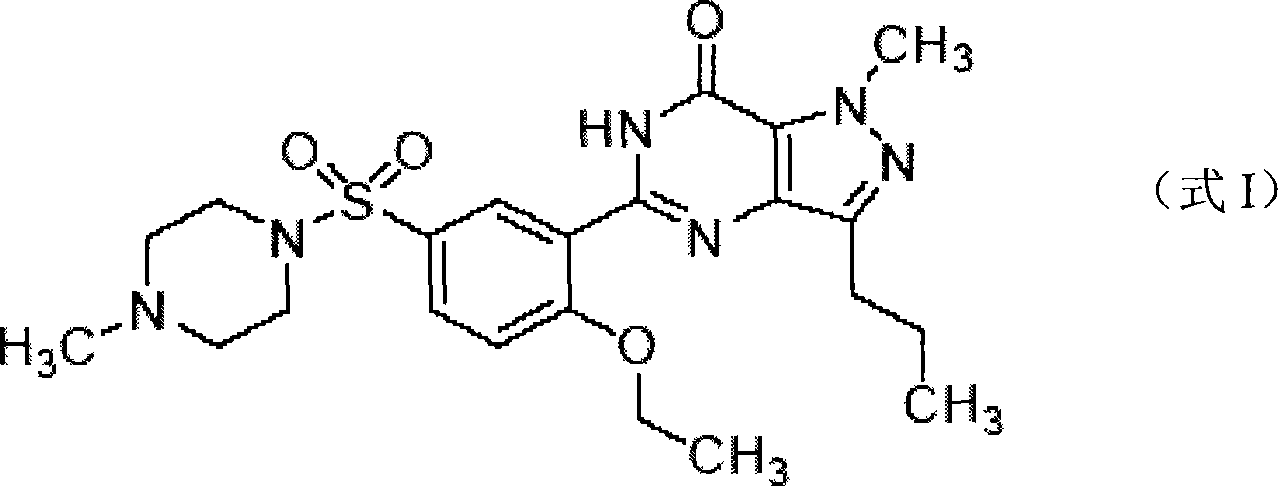
The invention discloses a method to test Sildenafil citrate and its ramifications. It contains steps: (1) Color reaction. Make color reaction of test sample with bismuth potassium iodide, silico-tungstic acid and Hager's reagent to observe result. (2) Result estimation. If above three color reactions appear deposit, there are Sildenafil citrate and its ramifications in test samples. If just one, two or none color reaction appear deposit, there is no Sildenafil citrate and its ramifications in test samples. The method has merits of quick, simple, strong property, high accuracy, extensive application and testing range.
Owner:BEIJING INST FOR DRUG CONTROL
KIR and ligand genetic typing experimental method
InactiveCN108624665ASmall sample sizePromote amplificationMicrobiological testing/measurementDiseaseNatural Killer Cell Inhibitory Receptors
The invention discloses a KIR and ligand genetic typing experimental method. By the technology, the time and labor are saved, and meanwhile, DNA sample capacity is further saved. A multi-PCR technology is adopted, meanwhile, 36 pairs of primers are further combined according to the sizes of PCR products, and finally, amplified reaction is finished in 12 reaction holes. The KIR and ligand genetic typing experimental method is conveniently applied to amplification of 96 pore plates, conventional Taq enzyme is used, typing of all KIR genes and ligands thereof can be finished at a time under the same reaction conditions, and the practicality of the KIR and ligand genetic typing experimental method is greatly improved. Two pairs of primers are used for a KIR gene, the accuracy is improved, anda false negative result is avoided. The KIR gene can be combined to MHC-I type ligand molecules on the surfaces of targeting cells, inhibiting or activating signals are transmitted to regulate the activity of NK cells and T cells, and the KIR and ligand genetic typing experimental method plays an important regulation role in hematopoietic stem cell transplantation, feto-matemal tolerance, anti-infectious immunity, tumor immunity and autoimmune diseases. Therefore, KIR genetic typing facilitates understanding of influences of KIR to tumor immunity, hematopoietic stem cell transplantation and autoimmune diseases.
Owner:韩瑜
Respiratory tract infection pathogen nucleic acid combined detection kit
PendingCN112280897AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementAgainst vector-borne diseasesNucleic acidChlamydophila Pneumonia
The invention discloses a respiratory tract infection pathogen nucleic acid combined detection kit. The invention develops a primer and probe combination for detecting various respiratory tract infection pathogens such as novel corona virus, influenza A virus, influenza B virus, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumoniae and chlamydia pneumoniae by combining a multiple fluorescent quantitative PCR technology and a diversion hybridization gene chip technology. The nucleotide sequences of the primer and probe combination are shown in SEQ ID NO: 1-36in sequence. The respiratory tract infection pathogen nucleic acid combined detection kit is constructed. Synchronous joint detection of eight respiratory tract infection pathogens can be realized, the detection accuracy is good, the specificity is strong, the sensitivity is high, the repeatability is good, false negative and false positive are low, the detection time is short, the cost is low, apatient can be comprehensively detected, the pathogens can be accurately positioned, timely treatment is carried out or corresponding isolation measures are carried out, and the kit has important significance for effectively controlling respiratory tract infection to prevent related infectious infection outbreak.
Owner:SHANGHAI CITY PUDONG NEW DISTRICT ZHOUPU HOSPITAL +2
Primer probe set, kit and detection method for detecting SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus) and MERS-CoV (Middle East Respiratory Syndrome Coronavirus)
InactiveCN108060266AQuick judgmentShort timeMicrobiological testing/measurementAgainst vector-borne diseasesMiddle East respiratory syndrome coronavirusRecombinase Polymerase Amplification
The invention discloses a primer probe set, kit and detection method for detecting SARS-CoV and MERS-CoV based on Recombinase Polymerase Amplification (RPA) detection. The primer probe set for detecting SARS-CoV and MERS-CoV based on the RPA detection comprises primers for nucleotide sequences as shown in SEQ ID NO.1-4 and probes for nucleotide sequences as shown in SEQ ID NO. 5-6. The invention further provides the kit for detecting SARS-CoV and MERS-CoV based on the RPA detection, wherein the kit comprises the primer probe set. By adopting the technical scheme, the sensitivity, specificity and simplicity for detecting SARS-CoV and MERS-CoV are obviously improved.
Owner:北京卓诚惠生生物科技股份有限公司
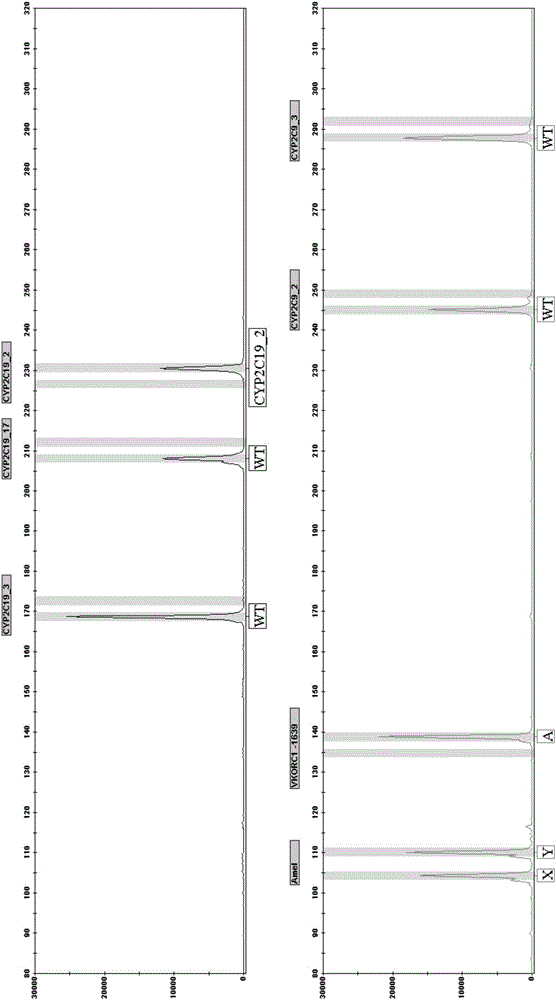
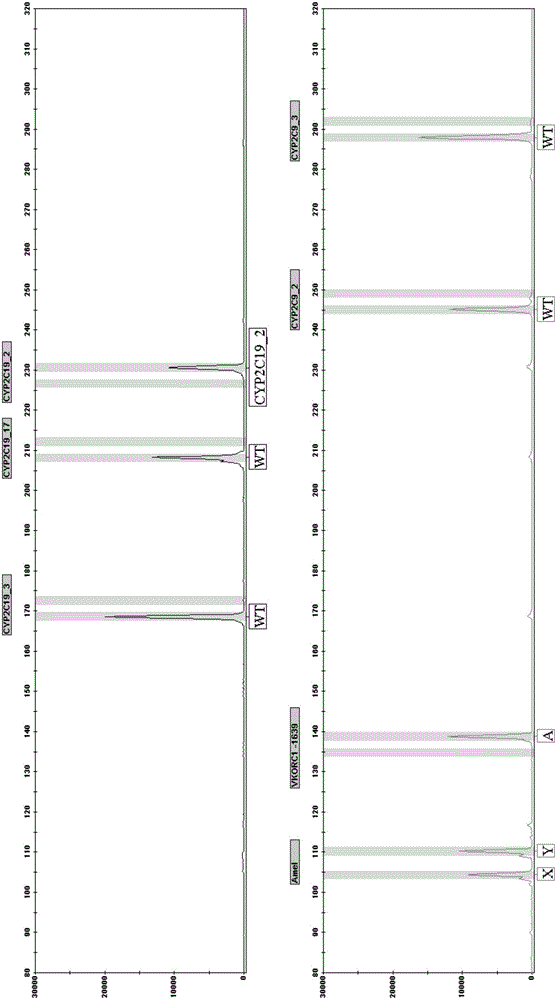
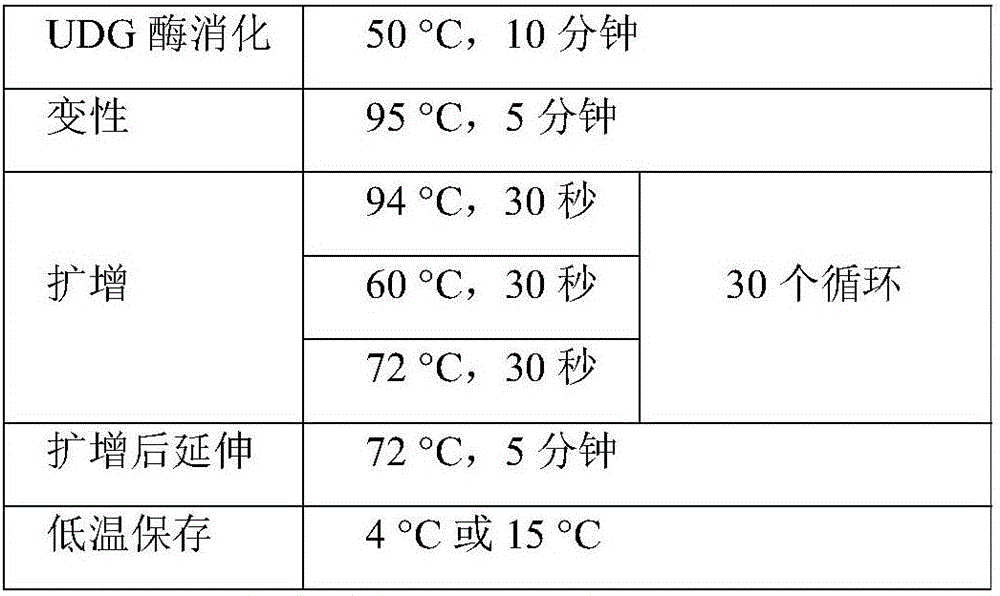
CYP2C19, CYP2C9 and VKORC1 genotyping multiplex amplification system and detection kit
InactiveCN105861739AHigh detection sensitivityImprove detection resolutionMicrobiological testing/measurementForward primerNucleotide
The invention discloses a CYP2C19, CYP2C9 and VKORC1 genotyping multiplex amplification system and detection kit. The amplification system comprises two forward primers and a reverse fluorescent primer of six SNPs (single nucleotide polymorphisms) respectively and can simultaneously amplify the six SNPs. The system is characterized by achieving one-tube amplification of three SNPs on the gene CYP2C19, two SNPs on the gene CYP2C9 and one SNP on the gene VKORC1 through multiple PCR (polymerase chain reaction). In particular, the amplification system can achieve direct amplification of blood and blood spot samples and dispenses with the step of extracting DNA (deoxyribonucleic acid). The amplification system can integrate the UDG-dUTP antipollution measure and can effectively prevent product pollution. The detection system is comprehensive in site detection, is simple and convenient to operate, has high specificity, high sensitivity and strong reliability, is low in cost and has the capacity of mass detection.
Owner:BEIJING MICROREAD GENE TECH
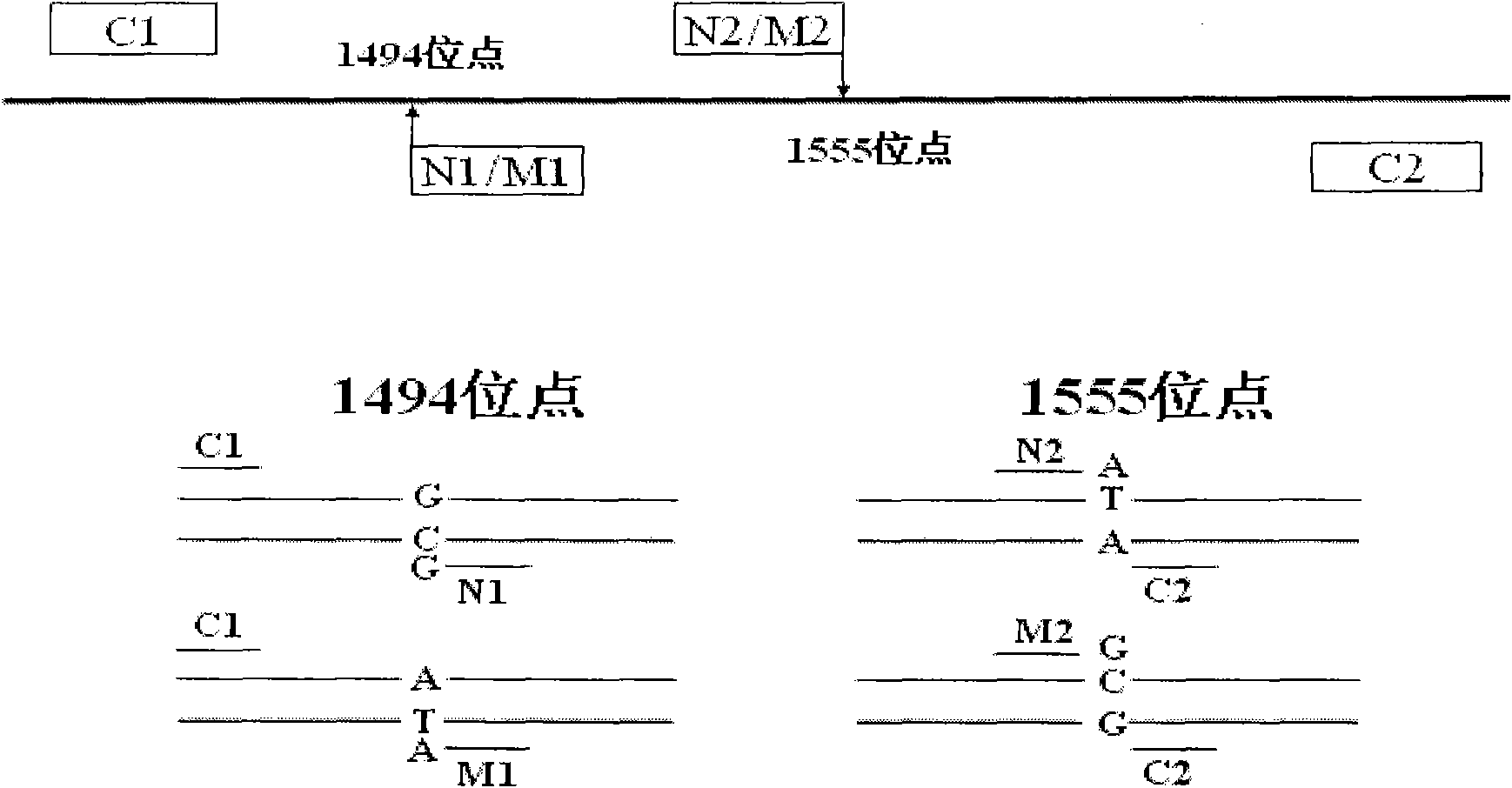
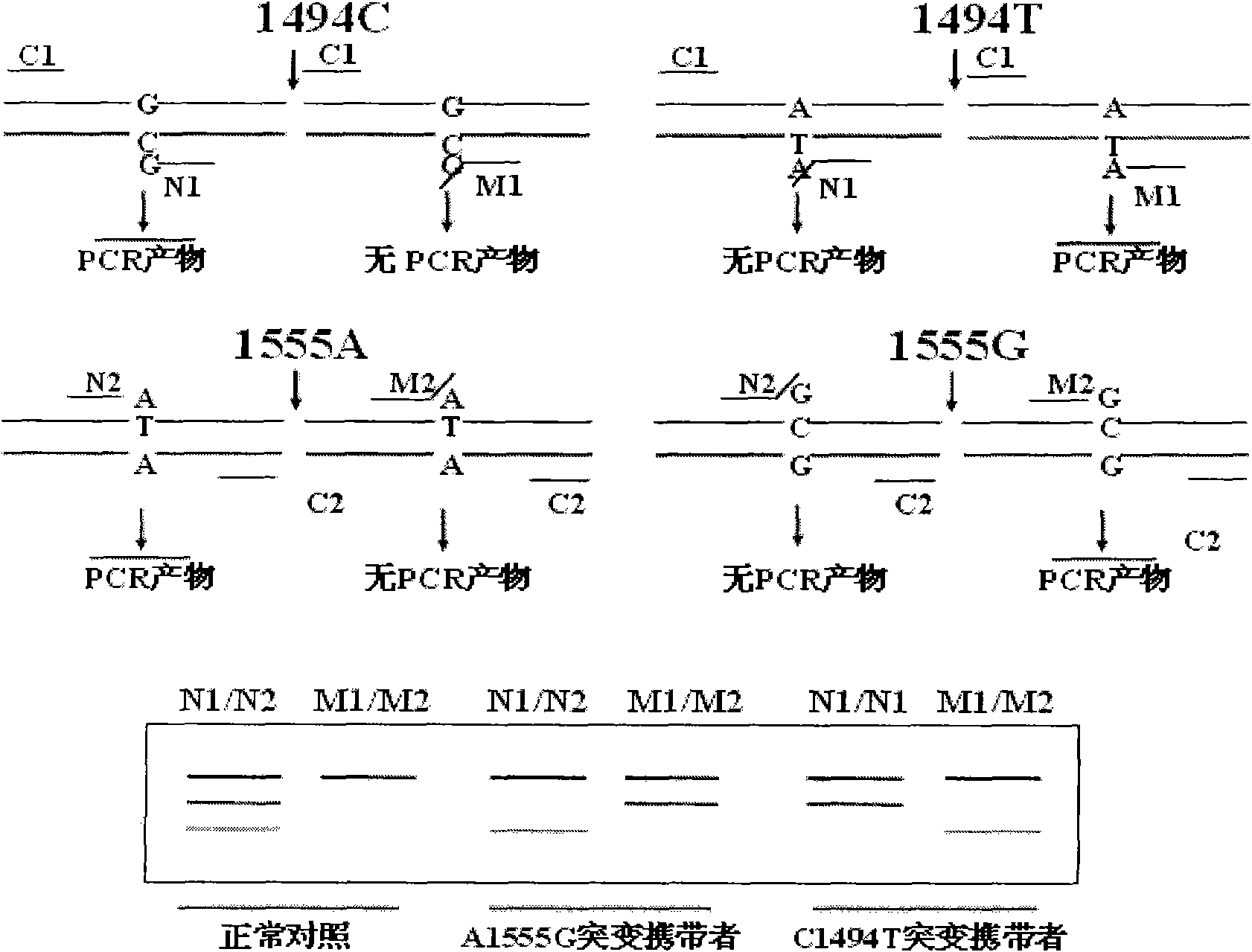
Kit for simultaneously detecting mutations in mitochondria DNA A1555G and C1494T and using method thereof
InactiveCN101768637ARelieve painSolve the problem of extracting DNAMicrobiological testing/measurementPositive controlGenomic DNA
The invention provides a kit for simultaneously detecting mutations in mitochondria DNA A1555G and C1494T related to maternally inherited drug-induced deafness and a using method thereof. The kit comprises a reagent for extracting sample genomic DNA, a PCR amplification reactive reagent, a primer mixed liquor, a positive control template and a negative control template. The using method of the kit mainly comprises the following steps: using blood, hair with follicle, oral mucosa doctor blade, saliva, and the like, as a sample; adopting a proteinase K digestion pyrolysis method to extract genomic DNA; and then simultaneously detecting mutations in A1555G and C1494T by multiple allele specific PCR. The kit is used for detecting the mutations in mitochondria DNA A1555G and C1494T related to maternally inherited drug-induced deafness and is more rapid, economical and simpler than the single detection for mutation in A1555G or C1494T, and the kit has low requirements for equipment and environment and is conducive to promotion and application.
Owner:WENZHOU MEDICAL UNIV
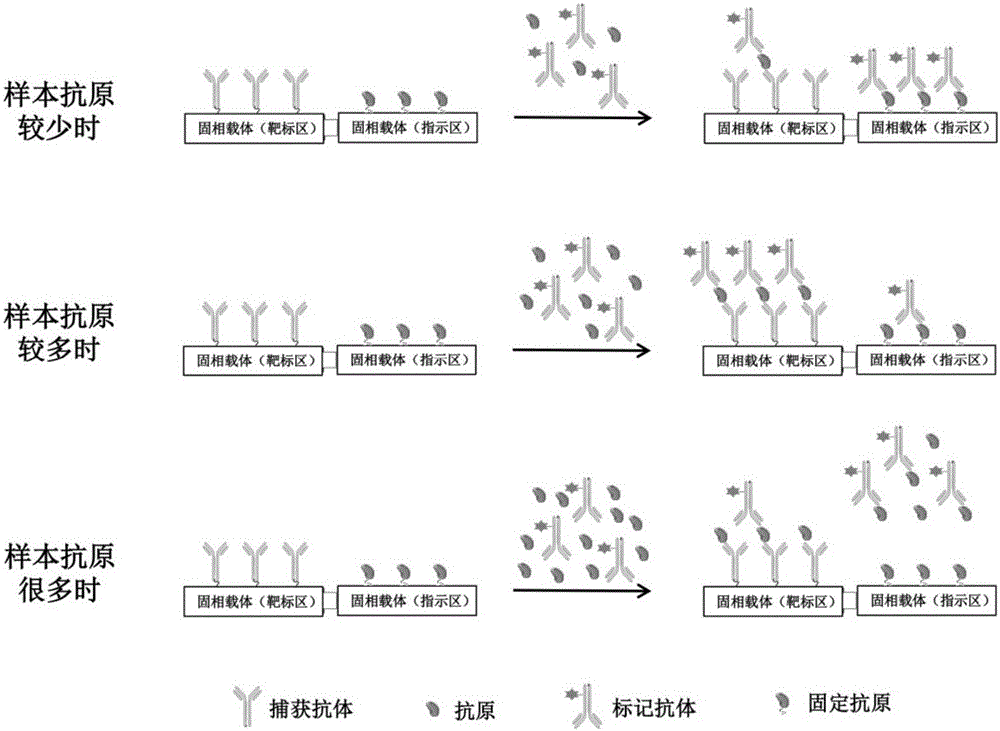
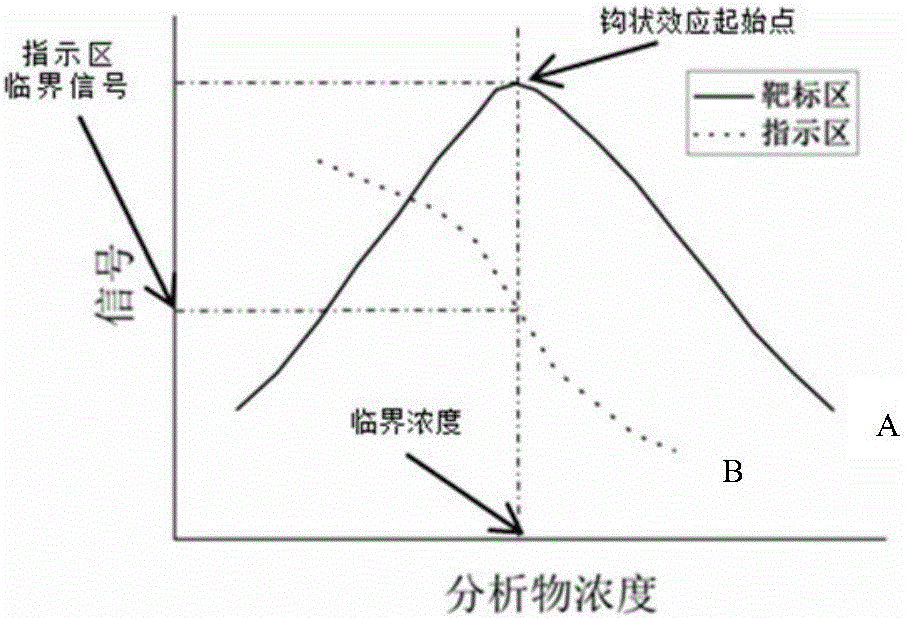
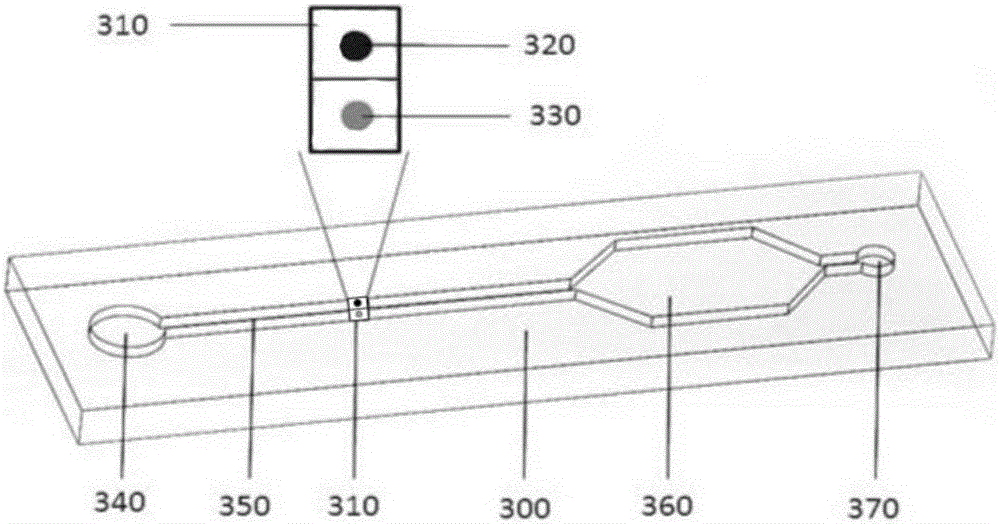
Biochip utilizing hook effect to enlarge detection range and detection method thereof
ActiveCN105823880AWide detection rangeHigh detection sensitivityMaterial analysisTime efficientAnalyte
The invention discloses a biochip utilizing the hook effect to enlarge the detection range and a detection method thereof. The biochip comprises at least one target area, a capturing antibody is fixed in the target area, the capturing antibody is combined with an analyte, through the labeling antibody combined with the analyte, detectable signals can be generated and can be used to quantitatively analyze the analyte in a sample; the biochip also comprises at least one indicating area, an antigen is fixed in the indicating area; and the fixed antigen is not combined with the analyte and is combined with labeling antibody that is not combined with the analyte to generate a detectable signal, which can be used to judge whether a hook effect is generated or not. The biochip has the advantages of flexible and convenient operation, time saving, and high sensitivity, largely enlarges the detection range of biological molecules, and avoids the false negative results.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI +1
Nucleic acid reagent, kit, system and method for detecting invasive fungi
InactiveCN110551840AQuick checkAccurate detectionMicrobiological testing/measurementMicroorganism based processesCandida tropicalisCandida famata
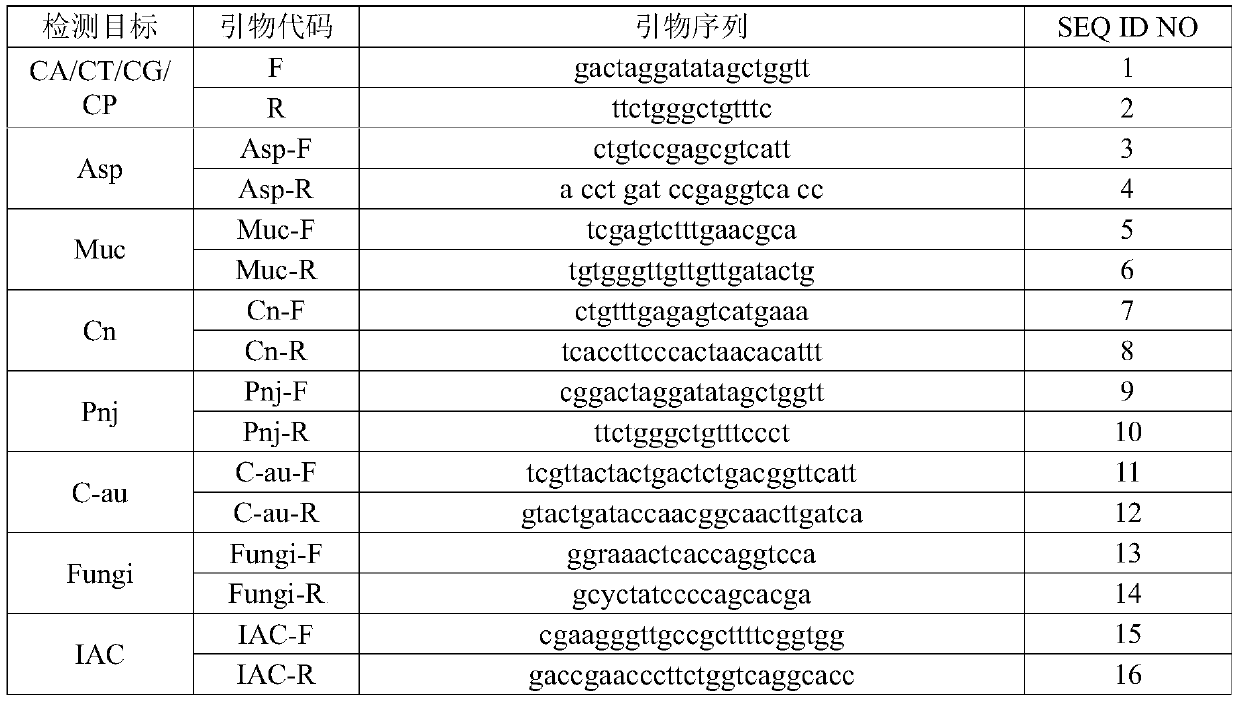
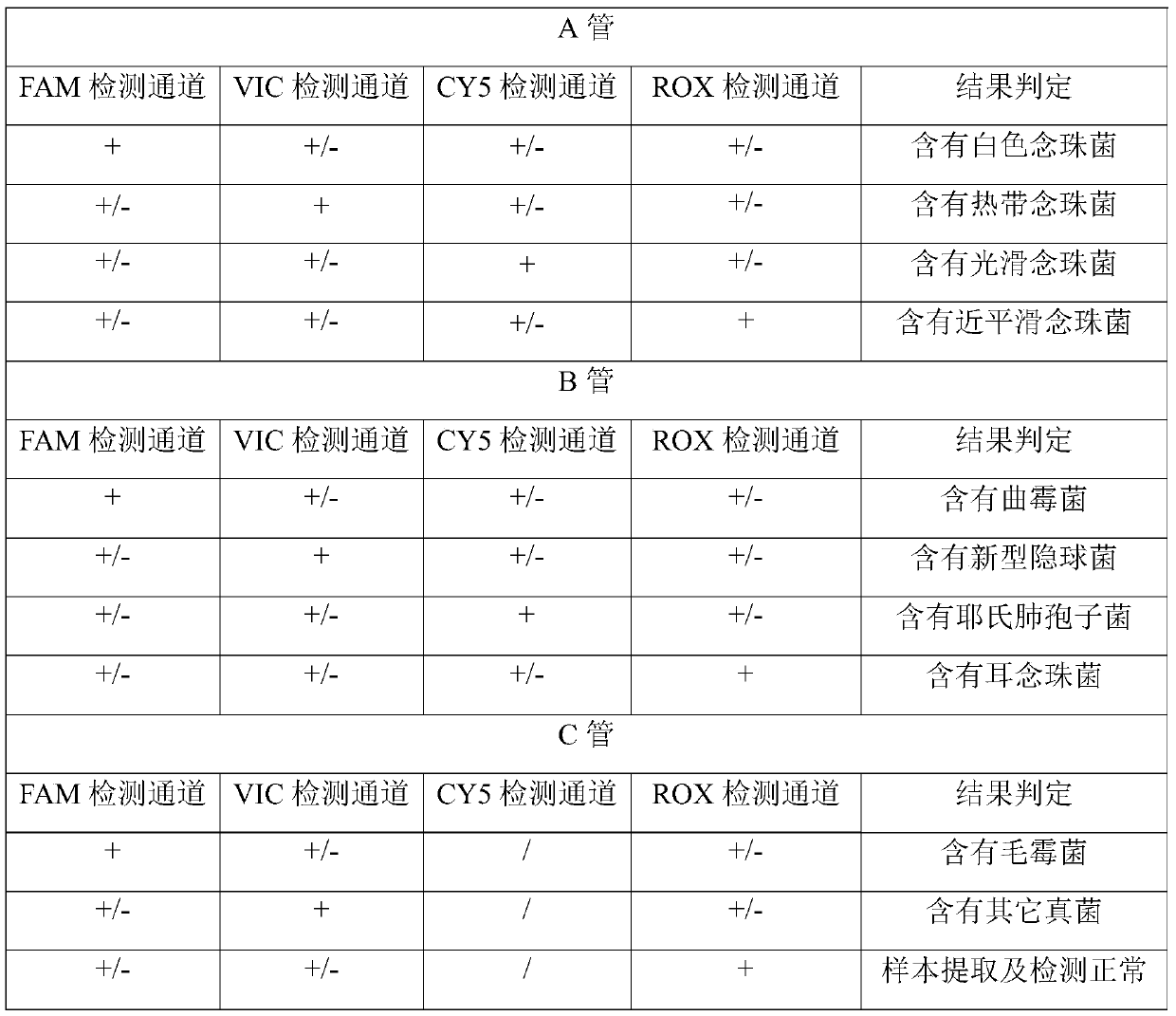
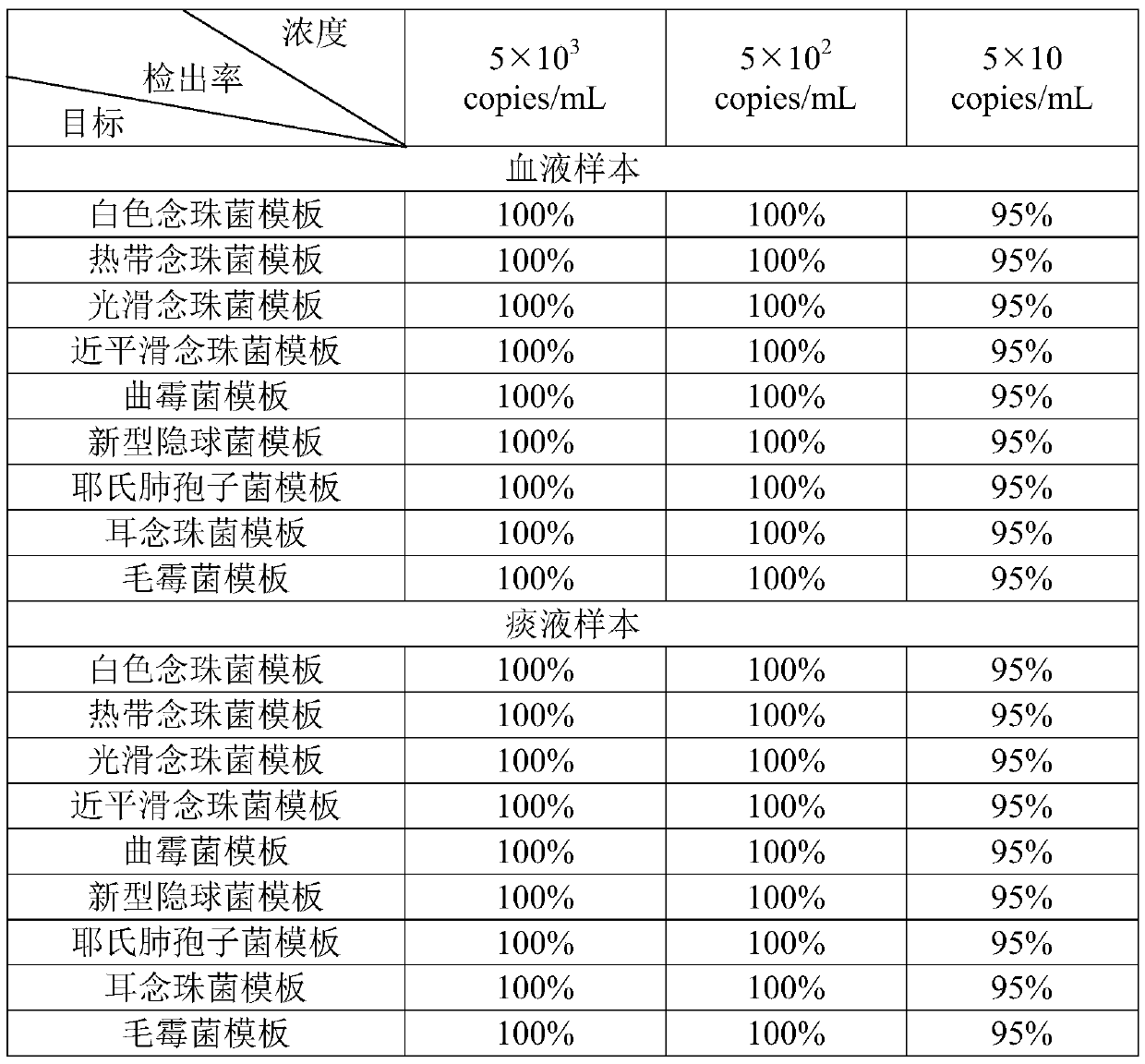
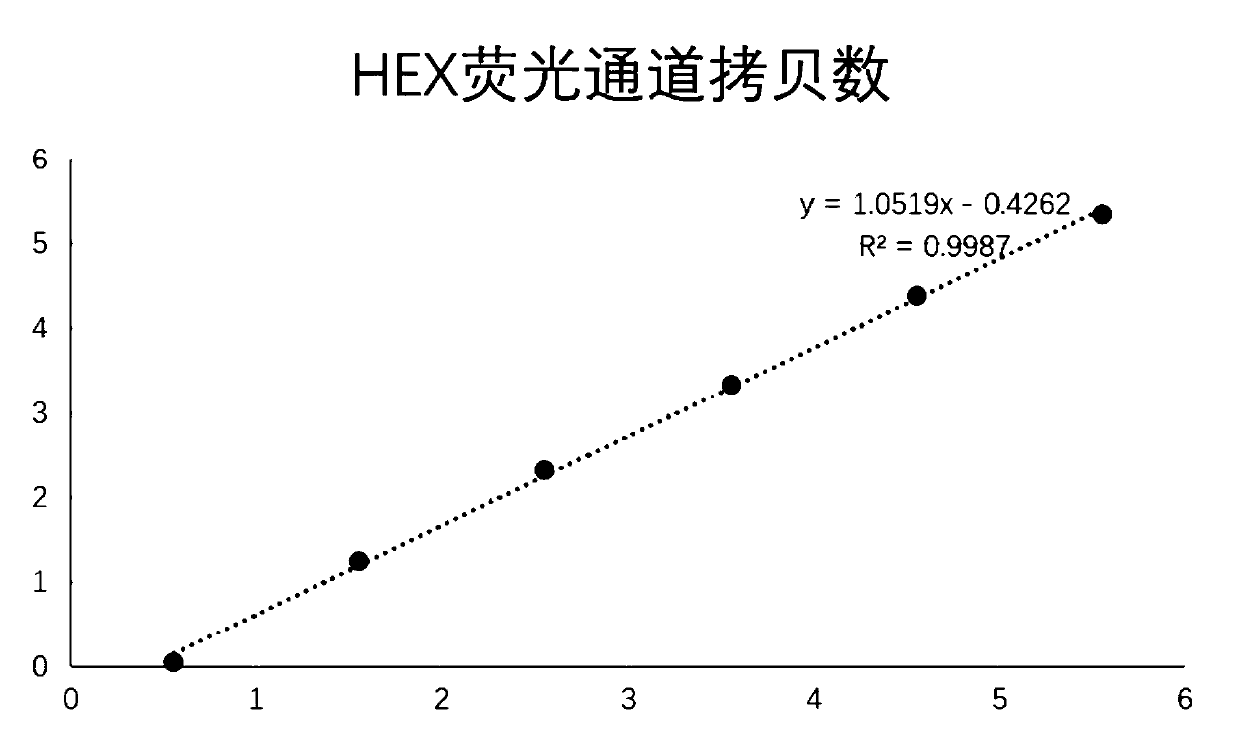
The invention relates to a nucleic acid reagent, kit, system and method for detecting invasive fungi. The nucleic acid reagent comprises a primer shown in SEQ ID NO.1-14 and a probe shown in SEQ ID NO.17-26, and the primer and the probe are stored independently with each other correspondingly or mixed with each other at will. Through the primer and the probe, the nucleic acid reagent, the kit, thesystem and the method of at least 9 invasive fungi of candida albicans, candida tropicalis, candida glabrata, candida parapsilosis, mucor, aspergillus, cryptococcus neoformans, pneumocystis jeroveciand candida auris, fast, comprehensive, sensitive, specific and automatic detection result determination can be achieved, and the sensitivity, specificity, simplicity and convenience of simultaneous detection of the detection target genome are significantly improved.
Owner:北京卓诚惠生生物科技股份有限公司
Novel coronavirus 2019-nCoV nucleic acid kit and virus nucleic acid acquisition method
PendingCN111378783AGuaranteed reliabilityIncrease the positive detection rate of nucleic acidMicrobiological testing/measurementAgainst vector-borne diseasesFluoProbesViral nucleic acid
The invention discloses a novel coronavirus 2019-nCoV nucleic acid kit and a virus nucleic acid acquisition method. The method comprises the following steps: according to novel coronavirus 2019-nCoV ORF1ab and an N gene as amplification target areas, designing specific primers and fluorescent probes for detecting novel coronavirus 2019-nCoV nucleic acid in samples; and performing Digital PCR (polymerase chain reaction) absolute quantification on 2019-nCoV nucleic acid RNA (ribonucleic acid) by using a one-step method by using a prepared Super premix reaction liquid A together with Super premixreaction liquids B and C, and performing Digital PCR detection on RNA of a diluted positive virus sample. The kit and the method have the beneficial effects that by using the novel virus nucleic acidacquisition method, and together with a Digital PCR probe method, the nucleic acid positive detection rate of infection of the novel coronavirus 2019-nCoV can be increased, the process is ensured tobe specified and standardized, the influence of artificial non-standard operation upon detection results can be reduced, result reliability can be ensured, and meanwhile, false negative results causedby detection of sputum and throat swab sampling together with real-time fluorescent PCR methods can be avoided.
Owner:杭州美中疾病基因研究院有限公司
BVDV (bovine viral diarrhea virus) internal control typing fluorescent PCR (polymerase chain reaction) detection kit and preparation thereof
InactiveCN103725796AShort detection timeShorten detection timeMicrobiological testing/measurementFluorescence/phosphorescenceBovine Viral Diarrhea VirusesComplementary deoxyribonucleic acid
The invention discloses a BVDV (bovine viral diarrhea virus) internal control typing fluorescent PCR (polymerase chain reaction) detection kit and a preparation thereof. By means of the primer design, a BVDV I, a BVDV II and a PCR template for monitoring internal control are obtained through single PCR amplification, a BVDV internal control typing fluorescent PCR detection system is established through the primer design, PCR amplification and optimization of reaction conditions, the BVDV I and the BVDV II can be independently detected or synchronously detected, and quality monitoring can be performed on the detection result. The prepared kit comprises two parts of a cDNA (complementary deoxyribonucleic acid) synthesis system and an SYBR fluorescent PCR amplification system. The minimum detection limit of the kit to three types of genes is 102 copy, the kit has better specificity and repeatability, the detection quality is ensured, and the kit has a better application value.
Owner:XINJIANG AGRI UNIV +1
Kit for identifying new coronavirus by applying mass spectrum system and using method thereof
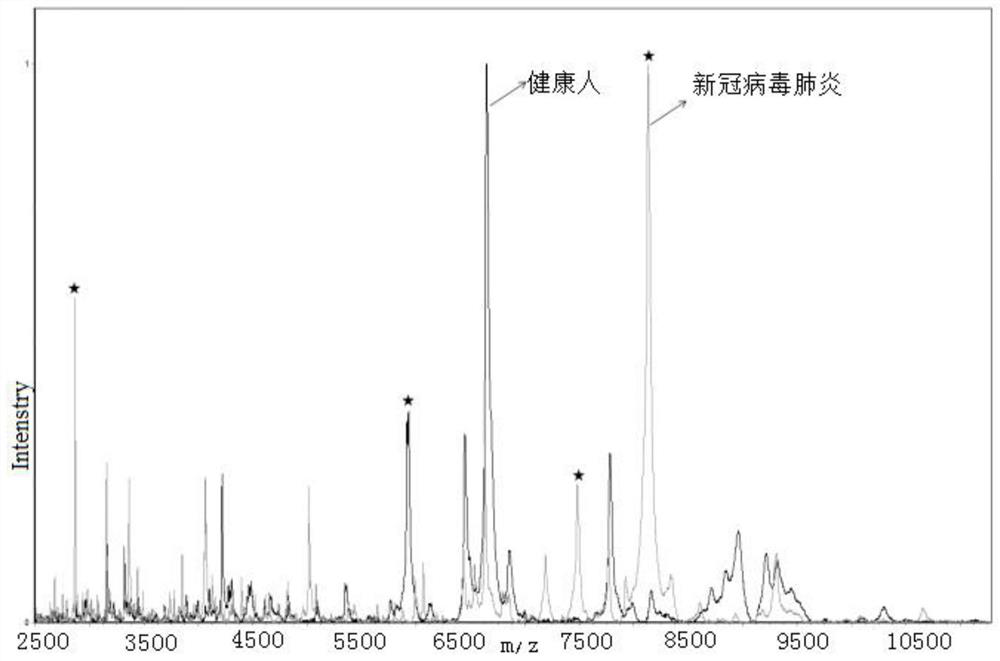
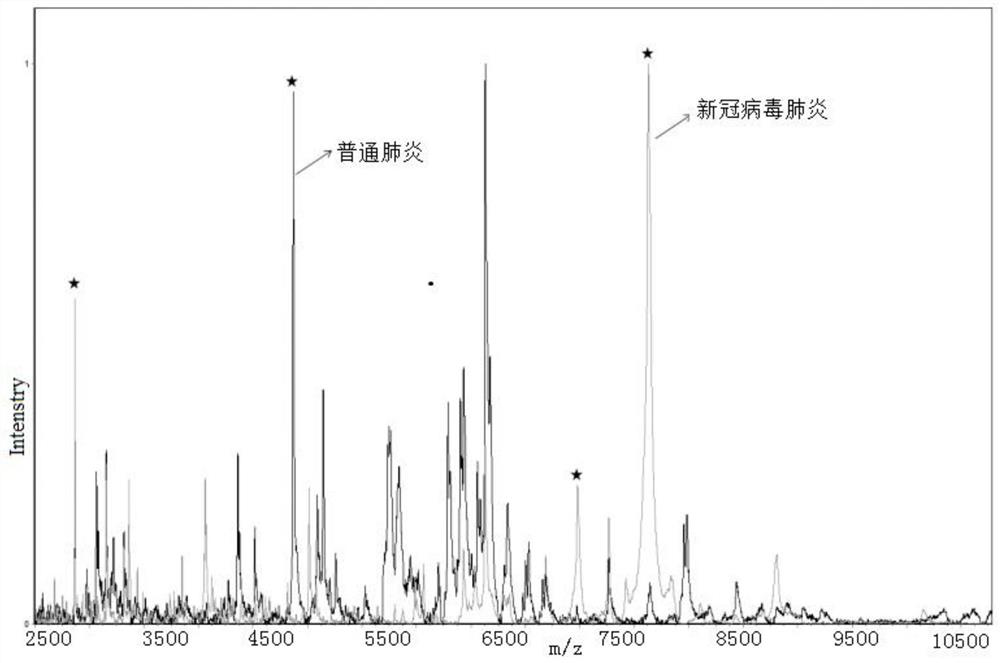
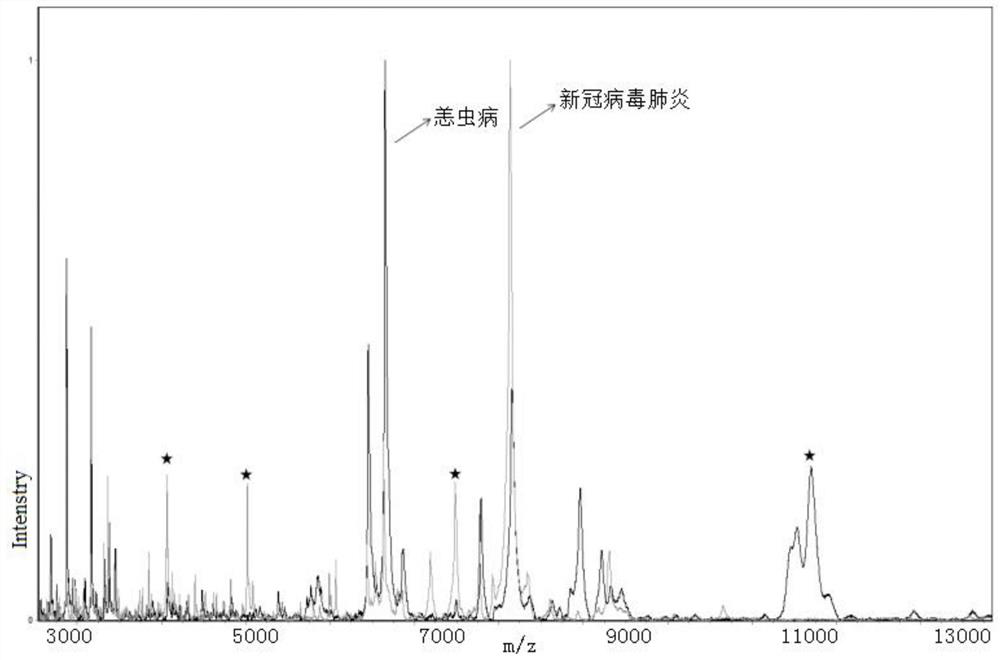
ActiveCN111830120AFast and effective comprehensive detectionAccurate detectionPreparing sample for investigationMaterial analysis by electric/magnetic meansSodium acetateMetalloprotein
The invention discloses a kit for identifying a new coronavirus by applying a mass spectrum system and a use method of the kit. The kit comprises the following components: a U9 buffer solution, a U1 buffer solution and a sodium acetate buffer solution. The using method of the kit comprises the following steps: S1, activating a metalloprotein chip target plate; S2, pretreating a serum sample; S3, extracting protein in the serum sample by using the metalloprotein chip target plate; and S4, acquiring and processing a spectrogram by using an MALDI-TOF mass spectrum system, and analyzing the read spectrogram by using software. Therefore, the kit is high in sensitivity, good in accuracy, simple and convenient to operate, short in consumed time and very suitable for clinical diagnosis.
Owner:北京东西分析仪器有限公司
Novel coronavirus (COVID-19) antigen detection kit and detection method thereof
PendingCN112129937AStrong specificityThe detection process is fastBiological testingImmunoassaysProtein.monoclonalEpidemic spread
The invention relates to the technical field of novel coronavirus detection, and discloses a novel coronavirus (COVID-19) antigen detection kit. The novel coronavirus (COVID-19) antigen detection kitcomprises a cuboid paper box package, wherein a detection card, a sterile swab, a sample extraction solution, a sample extraction tube, a suction head and a specification are arranged in the cuboid paper box package; the detection card comprises a card shell and a test strip; the test strip comprises a sample pad, a marking pad, an NC film, absorbent paper and a PVC bottom plate; and the marking pad is provided with a colloidal gold-marked murine N protein monoclonal antibody I. The detection kit disclosed by the invention is high in specificity, high in detection speed and simple and convenient to operate, does not need special equipment or professional operation, can be applied to preliminary screening of various places such as communities, primary hospitals, airports, customs and even families, can judge results within several minutes, and provides a simpler, more convenient and faster field detection means for suspected patient investigation and asymptomatic infected person screening, thereby preventing epidemic spread as soon as possible.
Owner:深圳容金科技有限公司
Kit for simultaneously detecting four pathogenic bacteria and non-diagnostic detection method thereof
ActiveCN105950759AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesTime efficientYersinia pestis
The invention discloses a kit for simultaneously detecting four pathogenic bacteria Yersinia pestis, Frencisella tularensis, Burkholderia pseudomallei and Brucella by using fluorescent quantitative PCR (polymerase chain reaction) and a non-diagnostic detection method. The kit comprises specific primers and probes corresponding to the four pathogenic bacteria. The kit disclosed by the invention is convenient to use, and has the advantages of low reagent consumption, low cost, high detection specificity and high sensitivity. The detection method can detect four pathogenic bacteria for one sample, thereby greatly simplifying the operational process, reducing the repetitive operation steps, saving the time, reducing the labor consumed by repetitive operation, effectively saving the cost and implementing quick screening.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
CRAS-PCR detection method of single base mutation of gene
InactiveCN104164478ASatisfy thermodynamic characteristicsGuaranteed accuracyMicrobiological testing/measurementWild typeSingle strand
The invention provides a CRAS-PCR detection method of single base mutation of a gene. The detection method uses single-brand or double-brand allele specific scorpion primers capable of specifically identifying the wild and mutant nucleic acid sequences of the single base mutation, and the common reference of the allele specific scorpion primers. The different genotypes of the single base mutation can be qualitatively and quantitatively detected in a single PCR reaction tube by analyzing the type and intensity of fluorescence signals generated by all competitive allele specific scorpion primers in a PCR amplification process, the detection results are accurate and reliable, have good linear range and sensitivity, and do not comprise false positive results caused by non-specific amplification and false negative results can be completely avoided.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Preparation method and application of single cell or tissue particle suspension
ActiveCN101957282ASimplify detection stepsEasy to storePreparing sample for investigationDead animal preservationPositive controlBiology
The invention relates to a single cell or tissue particle suspension, belonging to the technical field of biomedicine. The suspension is prepared by the following steps: 1) tissue sample is collected; 2) single cell or tissue particle buffer solution is prepared; 3) the single cell or tissue particle buffer solution is washed; 4) the single cell or tissue particle suspension is prepared. In the invention, multiple single cells or tissue particles which have positive control significance or single cell or tissue particle which has multiple positive control significances are combined, control can be formed while only a drop of the suspension is dropped, and no slicing is required, thus detection procedure is simplified; the suspension can be applied to in-situ hybridization, FISH technologyand dyeing of pathogens, thus having wide application range; the single cell or tissue particle suspension is convenient to store, is easy to be stored for a long time and can be repeatedly used; anddetection result is ensured to be accurate, thus being convenient for popularization and being helpful to comprehensively and easily solve the problem of quality control of in situ detection in immunohistochemistry.
Owner:浙江易柏生物技术有限公司
Multiple qPCR (quantitative Polymerase Chain Reaction) method for rapidly detecting five kinds of diarrheogenic Escherichia coli, kit and application thereof
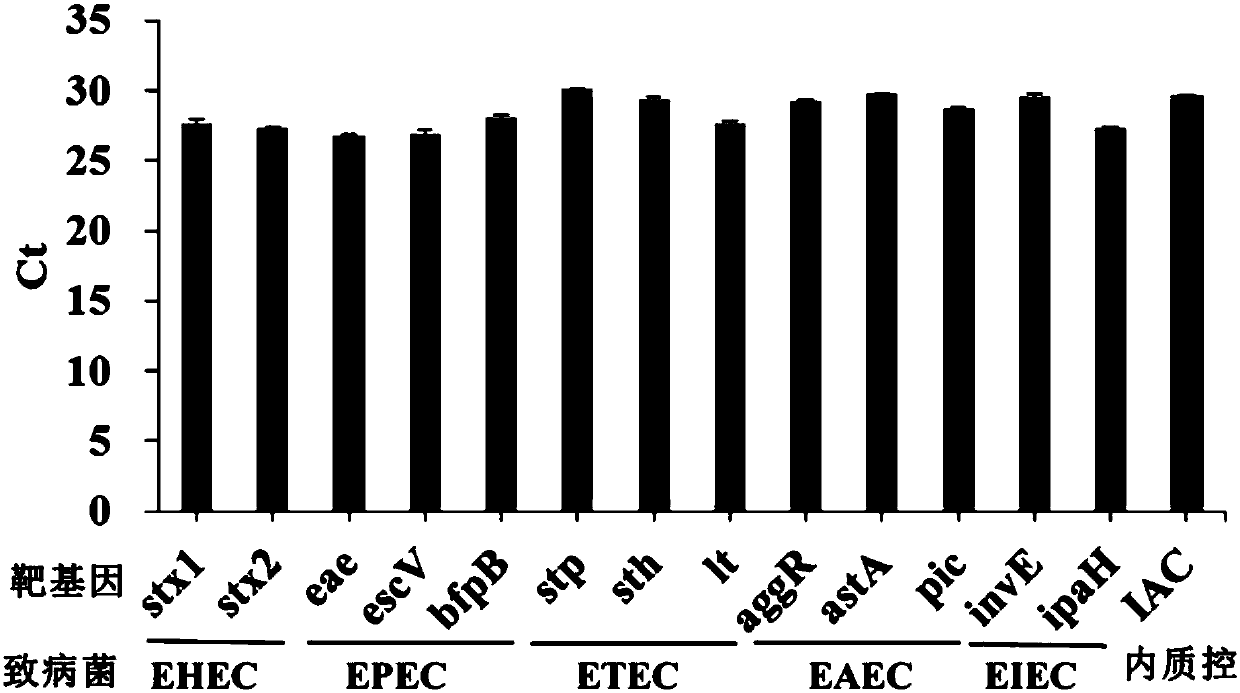
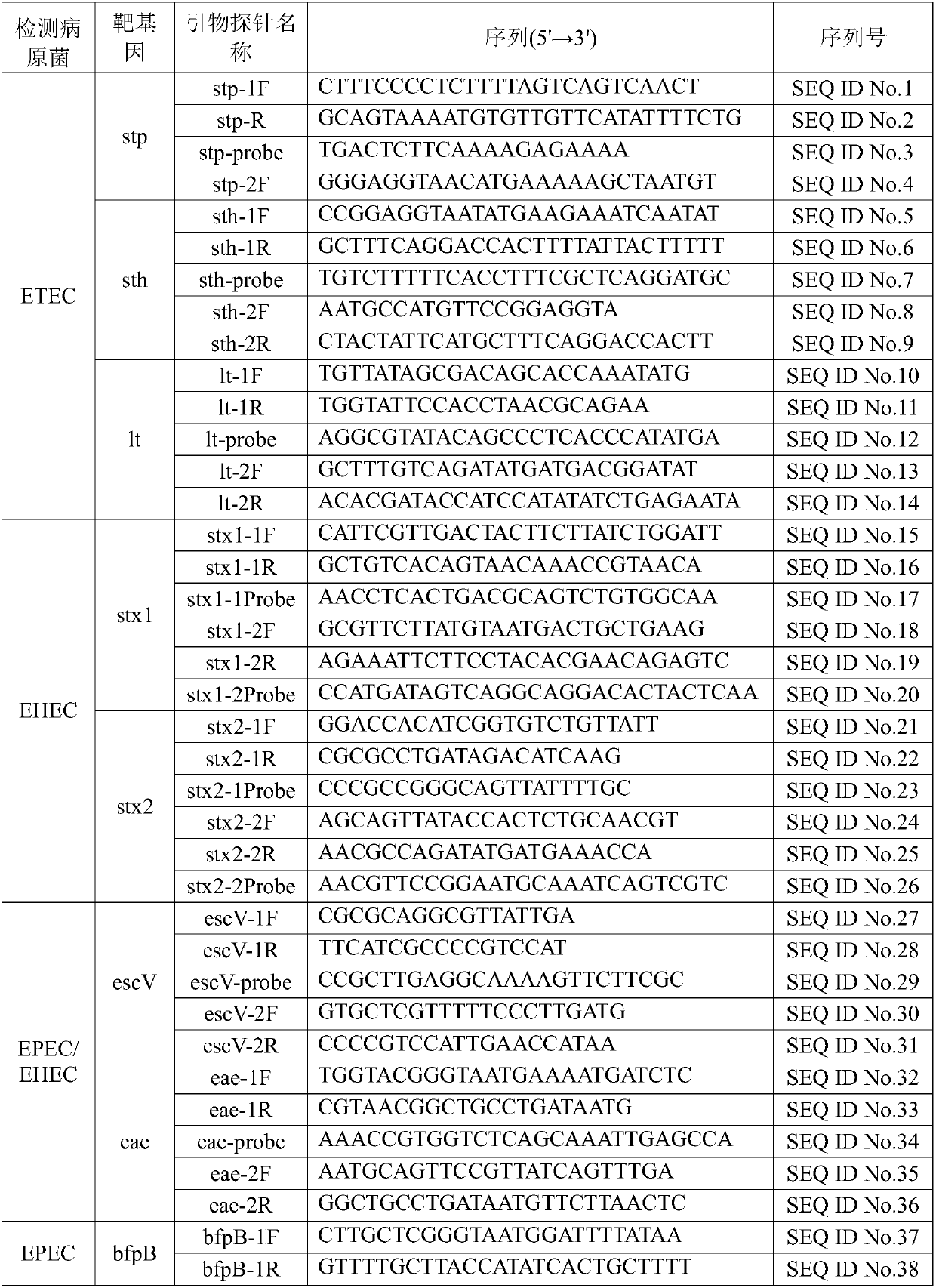
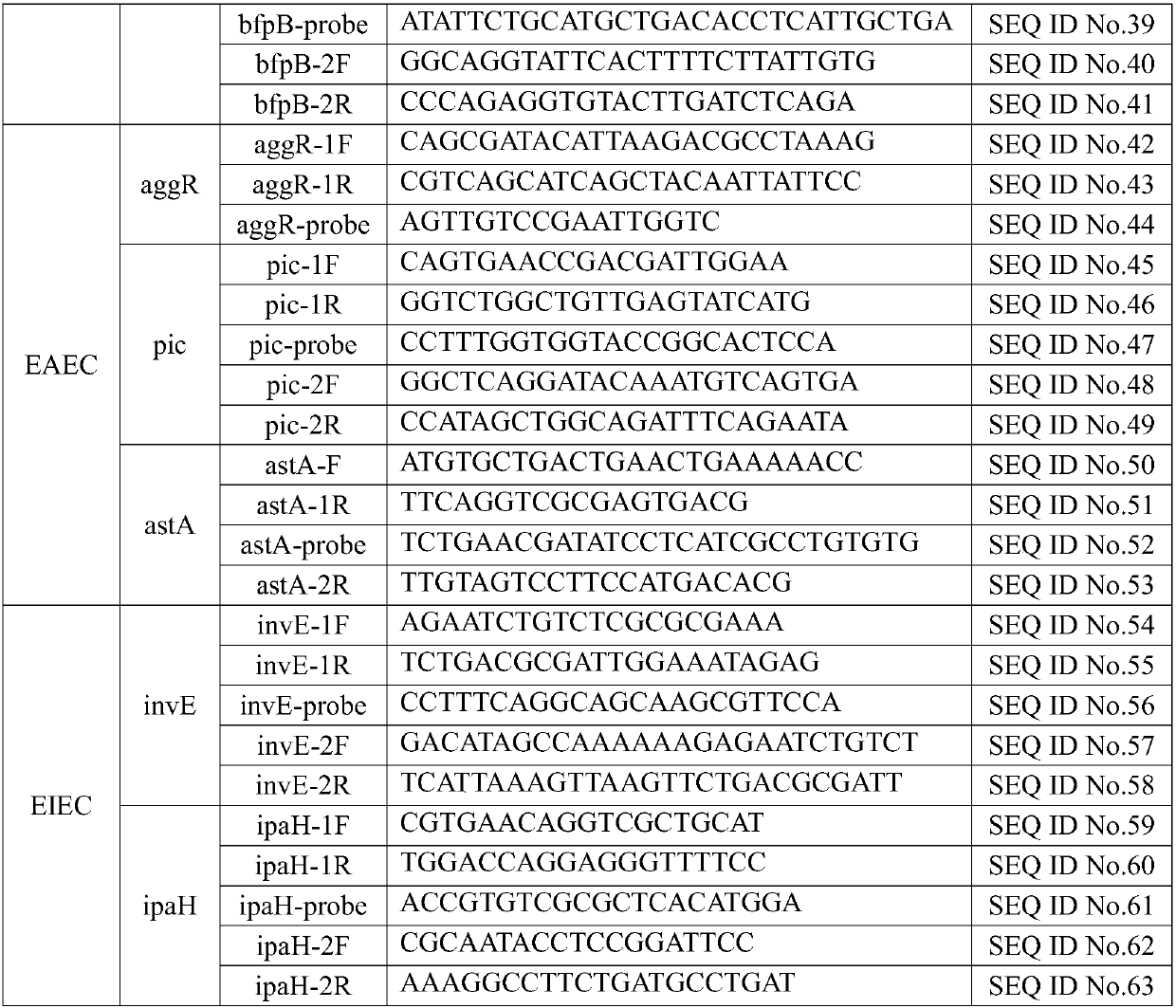
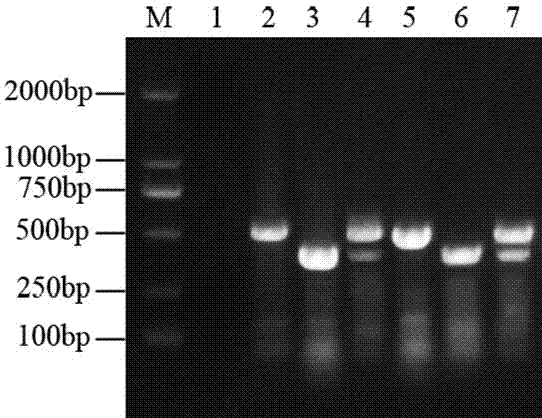
InactiveCN107760766AComprehensive detection coverageAvoid missing detectionMicrobiological testing/measurementDNA/RNA fragmentationEscherichia coliFluorescence
The invention discloses a kit for rapidly detecting five kinds of diarrheogenic Escherichia coli through multiple qPCR (quantitative Polymerase Chain Reaction). The kit comprises primer and probe groups capable of respectively performing specific amplification on eae genes of EPEC or EHEC, escV genes of EPEC or EHEC, bfpB genes of EPEC, stx1 or stx2 genes of EHEC lt, sth or stp genes of ETEC, aggR, astA or pic genes of EAEC and internal quality control IAC genes. Moreover, the invention further discloses a method and application for rapidly detecting the five kinds of diarrheogenic Escherichiacoli by using the kit. A method for simultaneously detecting the five kinds of diarrheogenic Escherichia coli in foods in the same reaction tube by virtue of fourteen real-time fluorescence quantitative PCR is realized, all characteristic genes in the national standard of the five DEC (diarrheogenic Escherichia coli) can be totally detected, and phenomena such as missing detection and false negative are avoided. The kit has the advantages of being short in detection time, simple and convenient to operate, easily decipherable in results, high in sensitivity, excellent in specificity and the like.
Owner:GWP BIOTECHNOLOGIES INC
RT-PCR primers and kit for detecting high-pathopoiesia porcine reproductive andrespiratory syndrome and classic porcine reproductive andrespiratory syndrome
InactiveCN103205507AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesHighly pathogenicNucleotide
The invention discloses a pair of RT-PCR ( reverse transcription-polymerase chain reaction) primers and a kit for detecting high-pathopoiesia pig blue ear disease and classic pig blue ear disease. The upstream primer nucleotide sequence of the primers is GGCGACAATGTCCCTAAC, the downstream primer nucleotide sequence of the primers is GATGGCTTGAGCTGAGTAT, and the kit contains the primers. The primers and the kit for detecting high-pathopoiesia porcine reproductive andrespiratory syndrome and classic porcine reproductive andrespiratory syndrome have the advantages of high sensibility, strong specificity, rapidness and convenience, low detecting cost and the like.
Owner:广西壮族自治区动物疫病预防控制中心
Multiple-PCR rapid detection method of salmonella and escherichia coli
ActiveCN104711365AReduce the proportionHigh densityMicrobiological testing/measurementMicroorganism based processesEscherichia coliNucleotide
The invention discloses a multiple-PCR rapid detection method of salmonella and escherichia coli. A primer for performing multiple-PCR rapid detection on salmonella and escherichia coli O78 comprises (1) a nucleotide sequence shown in SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:4 and SEQ ID NO:5; or (2) a nucleotide sequence having the basic sequence homology with the nucleotide sequence in (1) and having the function as same as that of the nucleotide sequence in (1). According to the detection method, specific genes of salmonella and escherichia coli O78 and a part of sequences of bacteria 16s rDNA are amplified once by using the multiple-PCR technology, and a PCR amplification product is detected by means of agarose gel electrophoresis. According to the method, the problems of long period (4-7 days) of a traditional pathogenic bacteria detection technology, tedious and complicated operations and relatively-low specificity and sensitivity are solved.
Owner:青岛智测检验检测有限公司
Pregnancy test device & method
ActiveUS20180088136A1Avoid false negative resultsImprove the level ofBiological material analysisBiological testingObstetricsPregnancy test
Disclosed is a test device to detect pregnancy In a human female subject, the test device comprising: an assay means to measure the absolute or relative amount of hCG m a sample from the subject; an assay means to measure the absolute or relative amount of FSH in a sample from the subject; and m assay means to measure the absolute or relative amount of one or mere progesterone metabolites I>> a sample from the subject.
Owner:SPD SWISS PRECISION DIAGNOSTICS
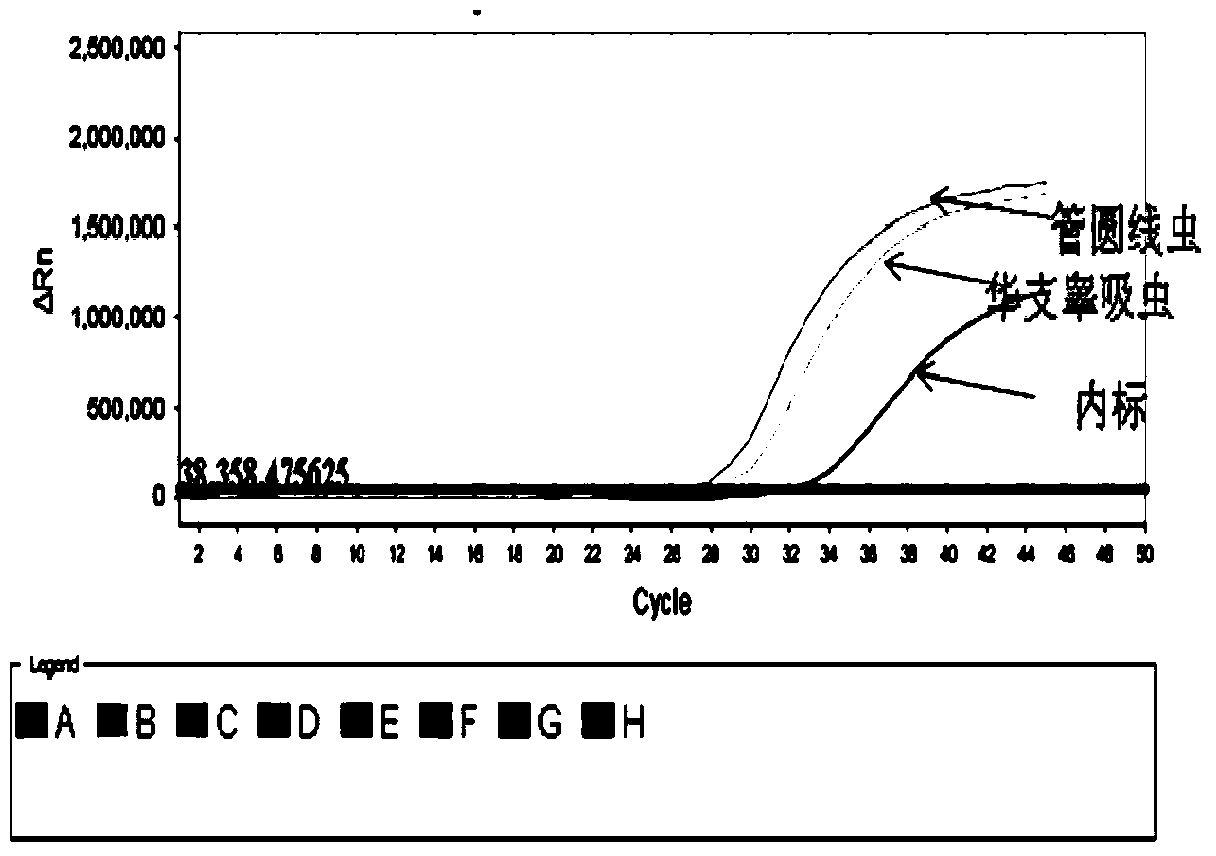
Clonorchiasis sinensis and angiostrongyliasis cantonensis real-time fluorescence PCR (Polymerase Chain Reaction) detection reagent, and kit and detection method thereof
ActiveCN103773861AImprove throughputQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceClonorchiasis
The invention discloses a clonorchiasis sinensis and angiostrongyliasis cantonensis real-time fluorescence PCR (Polymerase Chain Reaction) detection reagent, and a kit and a detection method thereof, wherein the detection reagent comprises two groups of compositions taking conserved segments of clonorchiasis sinensis ITS1 gene and angiostrongyliasis cantonensis ITS2 gene as targets, and the two groups of compositions respectively comprise a pair of specific primers, a specific probe and an internal standard probe. The kit comprises PCR mixed solution, Taq DNA polymerase, DEPC-H2O, a positive quality control product, a negative quality control product and pseudovirus internal standard solution; the PCR mixed solution contains the detection reagent. The detection method comprises the steps of total DNA extraction, reaction composition preparation, amplification and result judgment. The reagent and the kit are strong in specificity, high in sensitivity, pollution-free, high-throughput, and capable of accurately detecting parasite larvae, invisible to the human eyes, in food, and the detection method is fast, specific and sensitive and capable of simultaneously detecting a great amount of samples.
Owner:深圳澳东检验检测科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com