Nucleic acid combined testing kit of respiratory tract infection pathogens
A combined detection and respiratory technology, applied in the fields of biomedicine and clinical diagnosis, can solve the problems of high detection cost, easy missed diagnosis of early disease diagnosis, low sensitivity and specificity of immunological methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Combined Detection of Respiratory Tract Infection Pathogen Nucleic Acid
[0064] 1. Experimental method
[0065] (1) Design and screening of primers and probes
[0066] Multiplex PCR experiments are not simply mixing multiple pairs of specific primers into one reaction system. The reason why multiplex PCR is difficult is that the amplification conditions between multiple targets are incompatible, and each target needs to be matched with other primers next to it. The multiple pairs of primers that are required to be added in multiplex PCR experiments can neither combine with each other, nor can they combine with regions other than the target fragment on the template DNA. There should be no greater complementarity between each primer and other amplified fragments, and no greater homology between the amplified fragments; the length of each primer is generally 18 to 24 bases, and the primers cannot be complementary, especially avoid 3' Complementary ends. Long...
Embodiment 2
[0109] Example 2 Construction of Respiratory Tract Infection Pathogen Nucleic Acid Combined Detection Kit
[0110] 1. Composition of nucleic acid joint detection kit for pathogens of respiratory tract infection
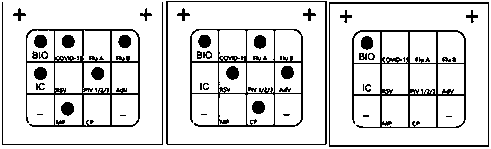
[0111] The present invention constructs a novel coronavirus (2019-nCoV), influenza A virus (FluA), influenza B virus (FluB), respiratory syncytial virus ( RSV), human parainfluenza virus (PIV1 / 2 / 3), adenovirus (AdV), Mycoplasma pneumoniae (MP) and Chlamydia pneumoniae (CP) nucleic acid combined detection kit, including primer and probe combinations, PCR buffer, enzyme Mixed solution, blank control and positive control, the specific components are as follows:
[0112] (1) Primer and probe combination
[0113] By analyzing clinical sample data and gene sequence query analysis, the inventor obtained SEQ ID NO: 1, which has a nucleotide sequence complementary only to the 2019-nCoV ORF1ab target gene, as shown in Tables 7-8. The primer of ~2 and the probe of SEQ ID NO: ...
Embodiment 3
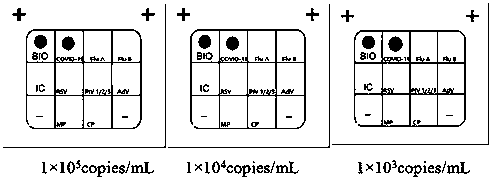
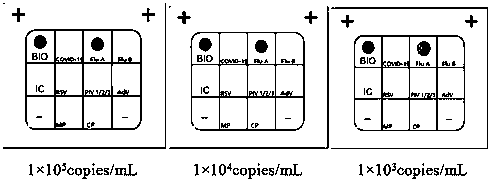
[0134] Example 3 Detection of actual samples by the nucleic acid joint detection kit for respiratory tract infection pathogens
[0135] 1. Experimental method
[0136] In order to verify that the respiratory infection pathogen nucleic acid joint detection kit constructed in Example 2 of the present invention is aimed at novel coronavirus (2019-nCoV), influenza A virus (FluA), influenza B virus (FluB), respiratory syncytial virus (RSV ), human parainfluenza virus (PIV1 / 2 / 3), adenovirus (AdV), Mycoplasma pneumoniae (MP) and Chlamydia pneumoniae (CP) detection accuracy, specificity and sensitivity, with influenza A virus (FluA) Quality Control, Influenza B Virus (FluB) Quality Control, Respiratory Syncytial Virus (RSV) Quality Control, Human Parainfluenza Virus (PIV1 / 2 / 3) Quality Control, Adenovirus (AdV) Quality Control, Mycoplasma pneumoniae (MP) quality control, Chlamydia pneumoniae (CP) quality control (dilute different concentrations: 1×10 5 copies / mL, 1×10 4 copies / mL, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com