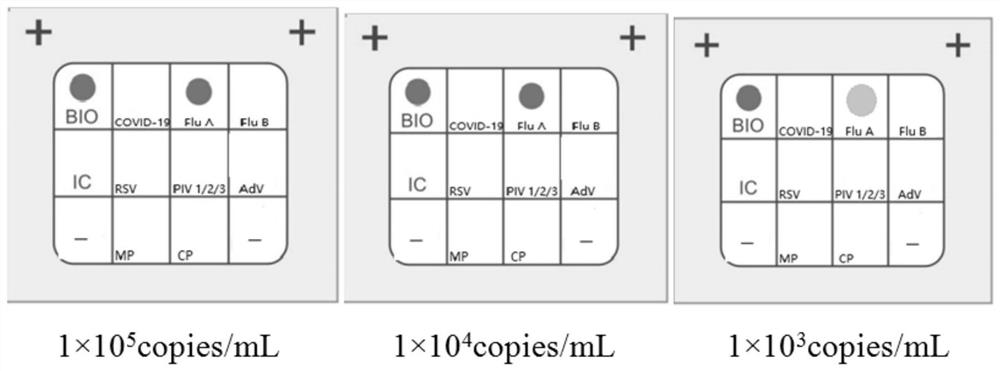
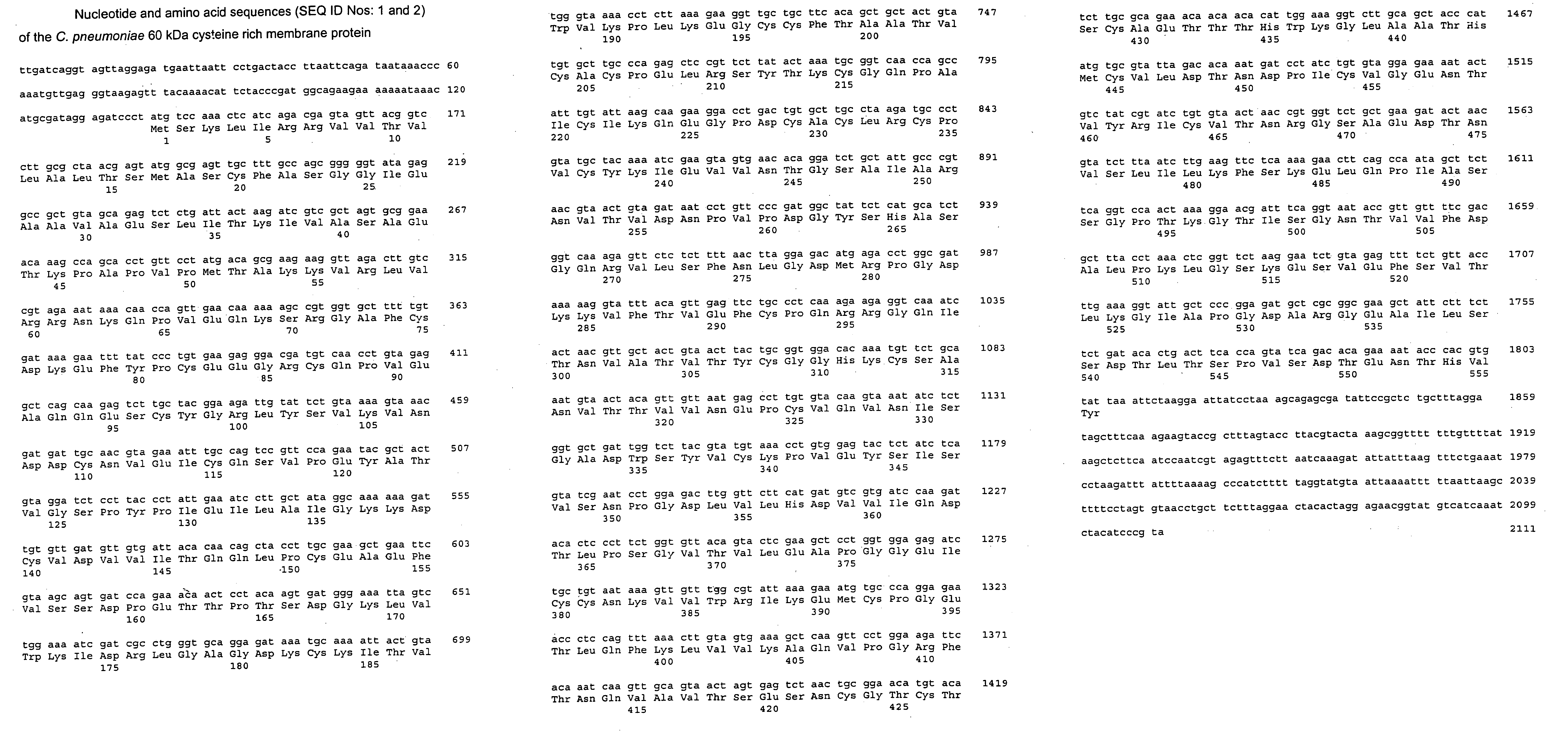
Patents
Literature
42 results about "Chlamydophila Pneumonia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
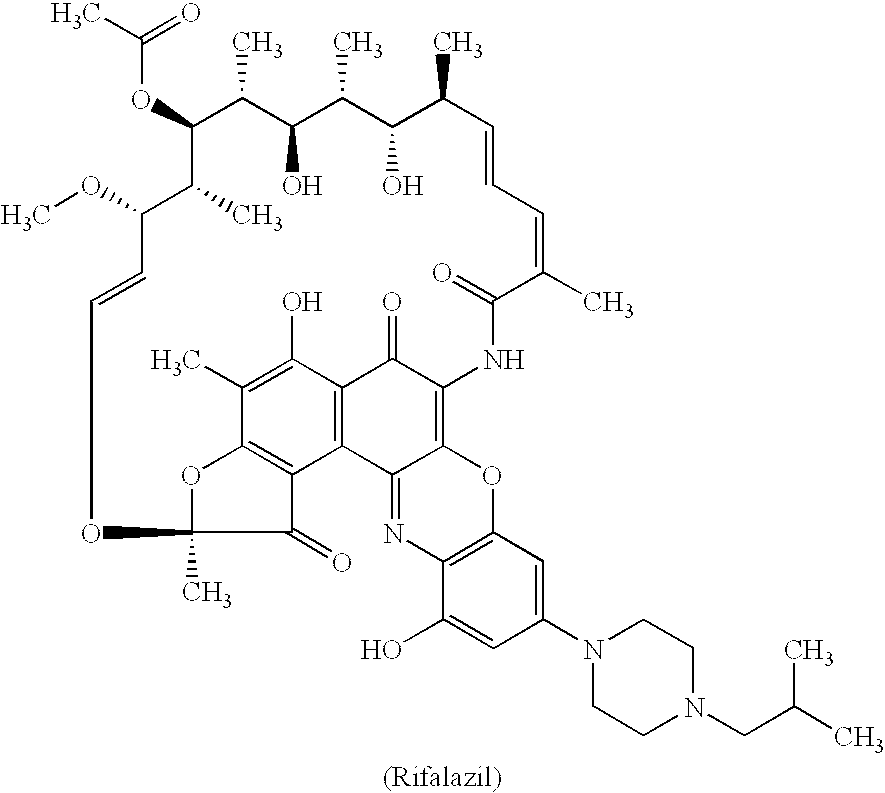
Method for treatment of bacterial infections with once or twice-weekly administered rifalazil
A method for treatment of bacterial infections with rifalazil administered once-weekly or twice-weekly. A method for treatment of tuberculosis caused by Mycobacterium tuberculosis, infections caused by Mycobacterium avium complex, infections caused by Chlamydia pneumoniae and infections caused by Helicobacter pylori by administering to a patient suffering from the bacterial infection 1-100 mg of rifalazil once or twice a week. In this dose regimen, the treatment is fast, efficacious and eliminates undesirable secondary symptoms observed with daily doses of 1-50 mg of rifalazil.
Owner:KANEKA CORP
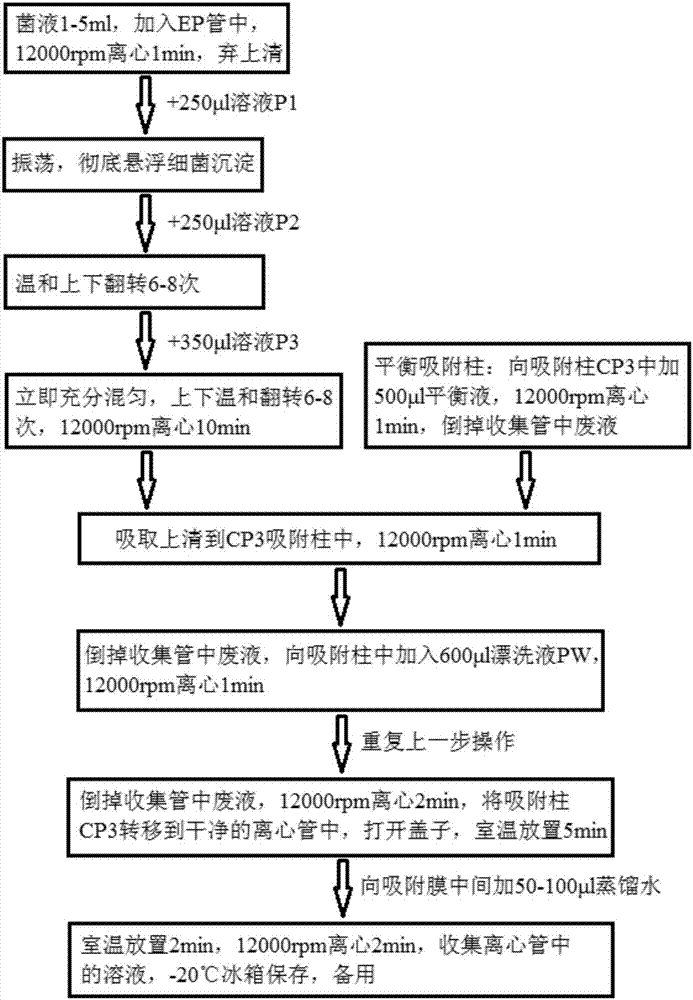
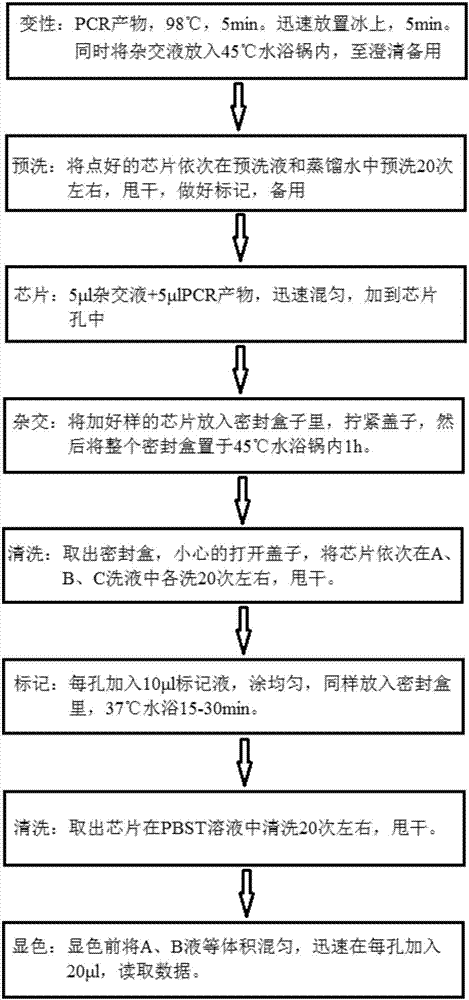
Kit for quickly detecting 15 pneumonia pathogenic bacteria
ActiveCN107338315AMicrobiological testing/measurementMicroorganism based processesBacteroidesStaphylococcus aureus
The invention discloses a kit for quickly detecting 15 pneumonia pathogenic bacteria. The kit can detect streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, baumanii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae which cover clinically common pneumonia pathogenic bacteria difficult to culture. 16S rDNA and specific gene sequences corresponding to the pneumonia pathogenic bacteria are detected by combining gene chips with multiple asymmetric PCR reactions, and the categories of the bacteria in a to-be-detected sample are identified in genus and species. The kit makes up for the defect that current clinical detection of pneumonia pathogenic bacteria is not in time or comprehensive and a novel detection means for early diagnosis and early treatment of patients suffering from pneumonia is provided.
Owner:GENERAL HOSPITAL OF PLA +1
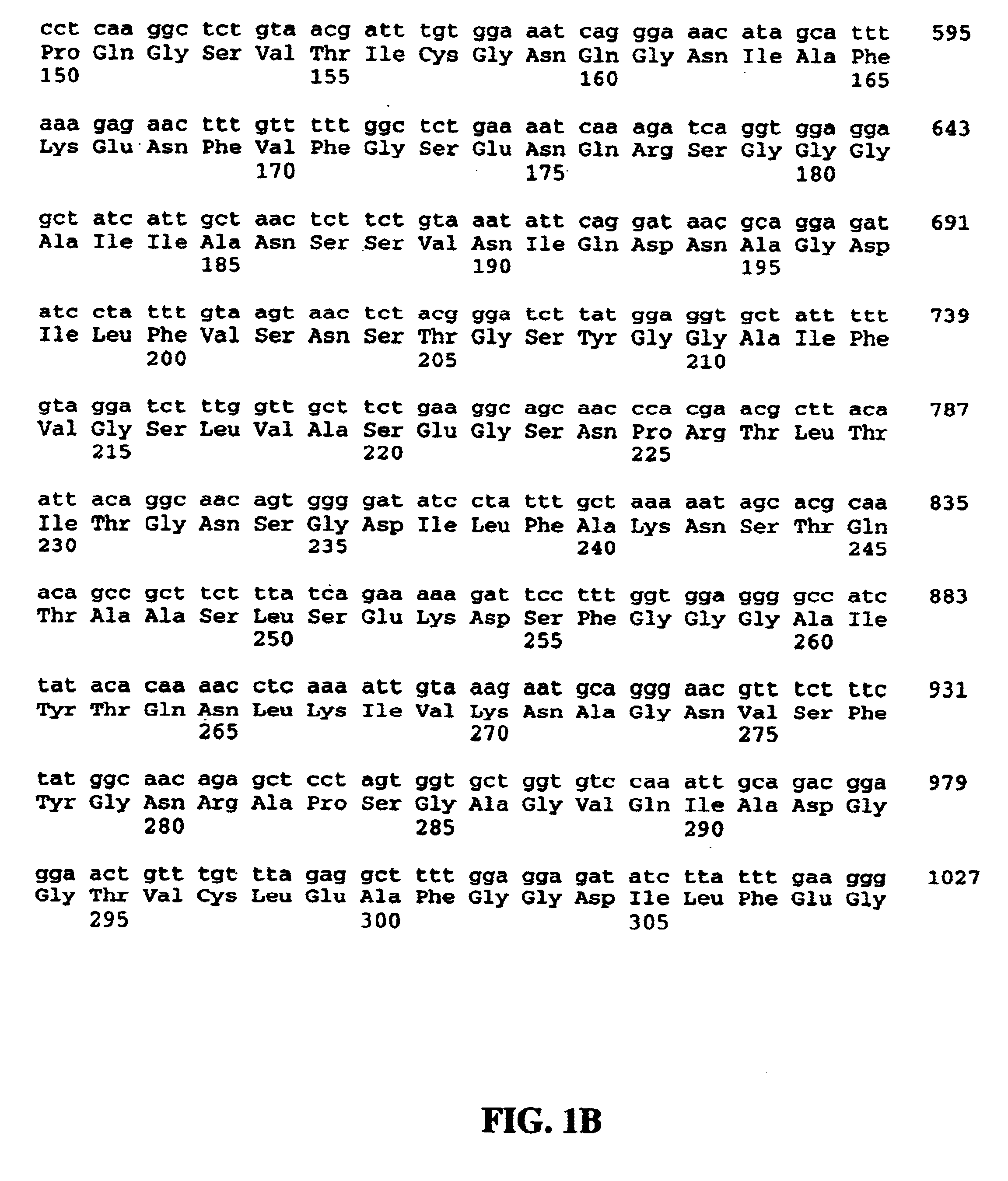
Chlamydia antigens and corresponding DNA fragments and uses thereof
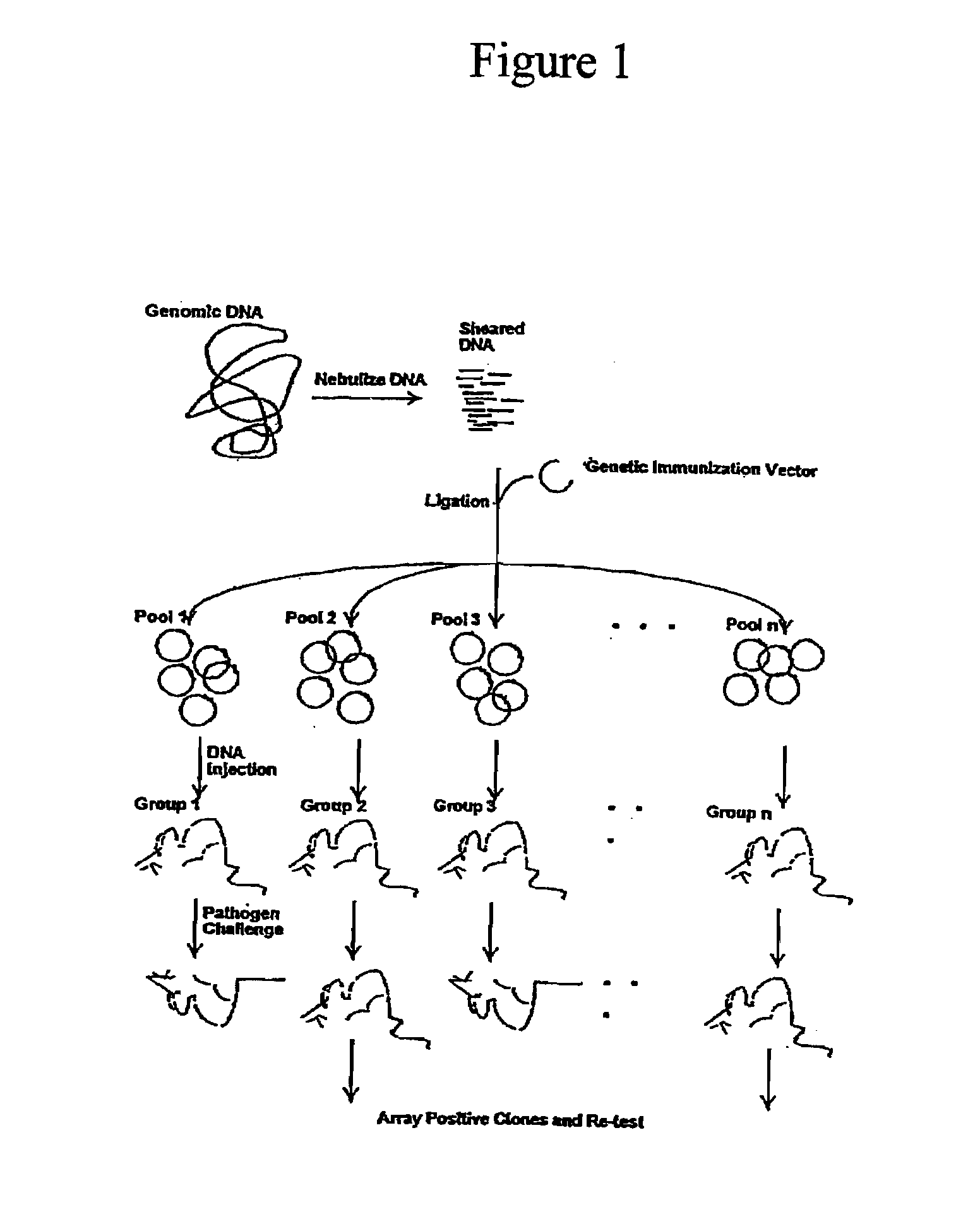
The present invention provides a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. pneumoniae, employing a vector containing a nucleotide sequence encoding full-length, 5′-truncated or 3′-truncated 76 kDa protein of a strain of Chlamydia pneumoniae and a promoter to effect expression of the 76 kDa protein gene in the host. Modifications are possible within the scope of this invention.
Owner:SANOFI PASTEUR LTD
Chlamydia antigens and corresponding DNA fragments and uses thereof
The present invention provides nucleic acids, proteins and vectors for a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. pneumoniae. The method employs a vector containing a nucleotide sequence encoding a transmembrane protein of a strain of Chlamydia pneumoniae and a promoter to effect expression of the transmembrane protein gene product in the host. Modifications are possible within the scope of this invention.
Owner:AVENTIS PASTEUR LTD
Nucleic acid combined testing kit of respiratory tract infection pathogens
InactiveCN111378789AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention discloses a nucleic acid combined testing kit of respiratory tract infection pathogens. The invention develops a set of primer-probe combinations which can detect multiple types of respiratory tract infection pathogens such as novel coronavirus, influenza virus a, influenza virus b, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumonia and chlamydiapneumonia through combination of a multiple fluorescence quantitative PCR technology and a flow-through hybridization and gene chip technology, wherein nucleotide sequences thereof are shown by SEQ ID NO:1-36 respectively. The nucleic acid combined testing kit of the respiratory tract infection pathogens is established. The kid can realize synchronous combined testing of the 8 respiratory tract infection pathogens, is high in detection accuracy, specificity and sensitivity, good in repeatability, low in false negativity and false positivity, short in detection time and low in cost, can realize comprehensive detection of a patient, can locate a disease source accurately, can realize treatment in time or make corresponding quarantine measures and is of important significance to effective control of respiratory tract infection and subsequent prevention of outbreak of relevant contagion and infection.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
Immunogenic compositions for protection against Chlamydial infection
InactiveUS20050065106A1Improve efficacySugar derivativesChlamydiaceae ingredientsNucleotideProtection sex
Owner:MURDIN ANDREW +1
Respiratory tract infection pathogen nucleic acid combined detection kit
PendingCN112280897AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementAgainst vector-borne diseasesNucleic acidChlamydophila Pneumonia
The invention discloses a respiratory tract infection pathogen nucleic acid combined detection kit. The invention develops a primer and probe combination for detecting various respiratory tract infection pathogens such as novel corona virus, influenza A virus, influenza B virus, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumoniae and chlamydia pneumoniae by combining a multiple fluorescent quantitative PCR technology and a diversion hybridization gene chip technology. The nucleotide sequences of the primer and probe combination are shown in SEQ ID NO: 1-36in sequence. The respiratory tract infection pathogen nucleic acid combined detection kit is constructed. Synchronous joint detection of eight respiratory tract infection pathogens can be realized, the detection accuracy is good, the specificity is strong, the sensitivity is high, the repeatability is good, false negative and false positive are low, the detection time is short, the cost is low, apatient can be comprehensively detected, the pathogens can be accurately positioned, timely treatment is carried out or corresponding isolation measures are carried out, and the kit has important significance for effectively controlling respiratory tract infection to prevent related infectious infection outbreak.
Owner:SHANGHAI CITY PUDONG NEW DISTRICT ZHOUPU HOSPITAL +2
Primer probe combination and detection kit for detecting mycoplasma pneumoniae, chlamydia pneumoniae and adenovirus
PendingCN112410472ASolve efficiency problemsSolve the characteristicsMicrobiological testing/measurementMicroorganism based processesMicrobiologyVirology
The invention relates to the technical field of biology, in particular to a primer probe combination and a detection kit for detecting mycoplasma pneumoniae, chlamydia pneumoniae and adenovirus. The primer probe combination comprises a specific primer pair of mycoplasma pneumoniae shown in SEQ ID NO: 1-2, a specific probe of mycoplasma pneumoniae shown in SEQ ID NO:3, a specific primer pair of chlamydia pneumoniae shown in SEQ ID NO: 4-5, a specific probe of chlamydia pneumoniae shown in SEQ ID NO:6, a specific primer pair of adenovirus shown in SEQ ID NO: 7-8 and a specific probe of adenovirus shown in SEQ ID NO: 9. The primers and the probes have good specificity, and rapid, accurate and sensitive identification of MP, CP and ADV can be achieved in combination with a real time PCR detection method.
Owner:AUTOBIO DIAGNOSTICS CO LTD
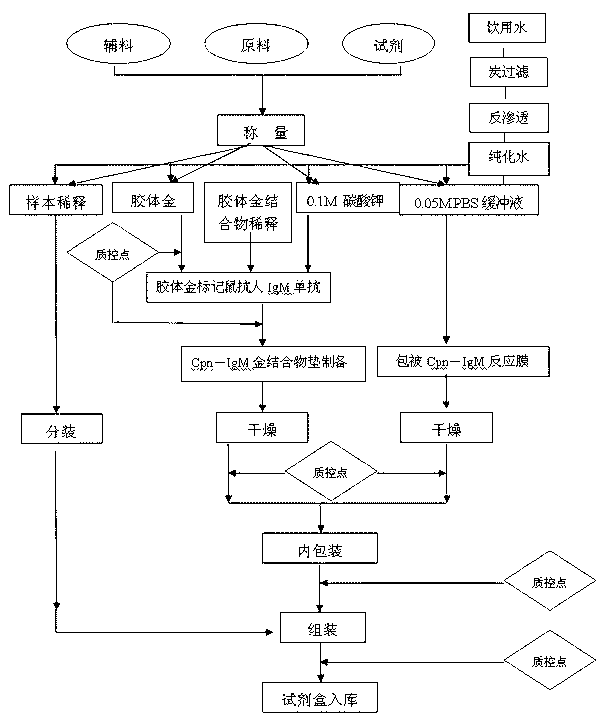
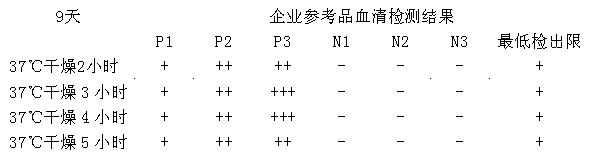
Chlamydia pneumoniae IgM (immunoglobulin M) colloidal golden method kit and preparation method thereof
InactiveCN102749446AObvious detectabilityReduce the effectMaterial analysisMurine antibodyChlamydiae
The invention discloses a chlamydia pneumoniae IgM (immunoglobulin M) colloidal golden method kit which comprises recombinant chlamydia pneumoniae antigens enveloped on a nitrocellulose membrane detection line, goat anti-rat IgG (immunoglobulin G) antibodies enveloped on a quality control line and rat anti-human IgM monoclonal antibodies which have colloidal gold labels and are enveloped on a gold label pad, the concentration of the chlamydia pneumoniae antigens ranges from 1mg / ml to 2mg / ml and is measured by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), the concentration of the goat anti-rat IgG (immunoglobulin G) antibodies ranges from 1mg / ml to 3mg / ml, and the concentration of the rat anti-human IgM monoclonal antibodies ranges from 5g / mL to 30g / mL and is measured by the SDS-PAGE. The chlamydia pneumoniae IgM colloidal golden method kit has the advantages that the kid is speedy, simple, convenient and accurate and is high in sensitivity, and a judgment result can be read after integral operation time of dozens of minutes; and a colloidal gold quick detecting paper strip is made of the multi-epitope recombinant antigens and is simple and convenient to operate, low in cost, good in specificity and high in sensitivity, can be used for single-component detection and is popularized easily, and detection and control effects for chlamydia pneumoniae IgM are obvious.
Owner:北京中检安泰诊断科技有限公司
Compositions, methods and kits for determining the presence of chlamydophila pneumoniae in a test sample
The present invention relates to oligonucleotides useful for determining the presence of Chlamydophila pneumoniae in a test sample. The oligonucleotides of the present invention may be incorporated into detection probes, capture probes and amplification oligonucleotides, and used in various combinations thereof.
Owner:GEN PROBE INC
Chlamydia antigens and corresponding DNA fragments and uses thereof
In summary of this disclosure, the present invention provides a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. pneumoniae, employing a vector, containing a nucleotide sequence encoding an 98 kDa outer membrane protein of a strain of Chlamydia pneumoniae and a promoter to effect expression of the 98 kDa outer membrane protein gene in the host. Modifications are possible within the scope of this invention.
Owner:AVENTIS PASTUER LTD
Application of heterocyclic compound in preparation of medicine for treating pneumonia
ActiveCN111358787AExcellent anti-pneumonia pharmacological activityAntibacterial agentsAntimycoticsMycoplasma pneumoniaBiomedicine
The invention discloses an application of heterocyclic compounds in preparation of medicines for treating pneumonia, and relates to the technical field of biomedicine. According to the application ofthe heterocyclic compounds in the preparation of the medicines for treating the pneumonia provided by the invention, the heterocyclic compounds have anti-pneumonia activity, wherein the pneumonia includes but is not limited to viral pneumonia, bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia. The anti-pneumonia pharmacological activity of the structurally modified and transformedheterocyclic compounds is tested, the pharmacological activity of the compounds is tested in multiple pneumonia models, and data of anti-pneumonia activity are provided to confirm that the heterocyclic compounds all have excellent anti-pneumonia activity, that is, the heterocyclic compounds with antipneumonia activity are screened out, so that a novel skeleton can be provided for screening a novel compound for treating the pneumonia, and a theoretical foundation is laid for development of a novel lead compounds.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Compositions and methods for determining the presence of Chlamydophila pneumoniae in a test sample
The present invention relates to oligonucleotides useful for determining the presence of Chlamydophila pneumoniae in a test sample. The oligonucleotides of the present invention may be incorporated into detection probes, capture probes and amplification oligonucleotides, and used in various combinations thereof.
Owner:GEN PROBE INC
Chlamydia pneumoniae vaccine and methods for administering such a vaccine
The present application relates to antigens and nucleic acids encoding such antigens obtainable by screening the Chlamydia pneumoniae genome. In more specific aspects, the present application relates to methods of isolating such antigens and nucleic acids and the methods of using such isolated antigens for producing immune responses. The ability of an antigen to produce an immune response may be employed by vaccination or antibody preparation techniques.
Owner:AUBURN UNIV
Preparation method and application method of chlamydia pneumoniae recombinant antigen
InactiveCN111087453AStrong specificityImprove stabilityBiological material analysisDepsipeptidesProkaryotic expressionImmunogenicity
The invention discloses a preparation method and an application method of a chlamydia pneumoniae (CP) recombinant antigen, wherein the preparation method of the CP recombinant antigen comprises the following specific steps: selection of CP recombinant antigen sequences; prokaryotic expression of the CP recombinant antigen; purification and renaturation of CP recombinant antigen; and verification of CP recombinant antigen. A serological detection method is developed on the premise of preparing an effective CP antigen, a preparation process of a CP natural antigen is complicated and high in cost, and there is a risk of infection for preparation personnel. Using the recombinant antigen with strong immunogenicity and good specificity as a substitute of the natural antigen not only reduces production cost, but also lays a foundation for developing a detection kit for detecting CP antibodies, IGG and IgM. The method is simple in operation and low in cost, the CP antigen is strong in specificity and good in stability, capable of achieving mass production and suitable for popularization and application.
Owner:南京拂晓生物科技有限公司
Chlamydia antigens and corresponding DNA fragments and uses thereof
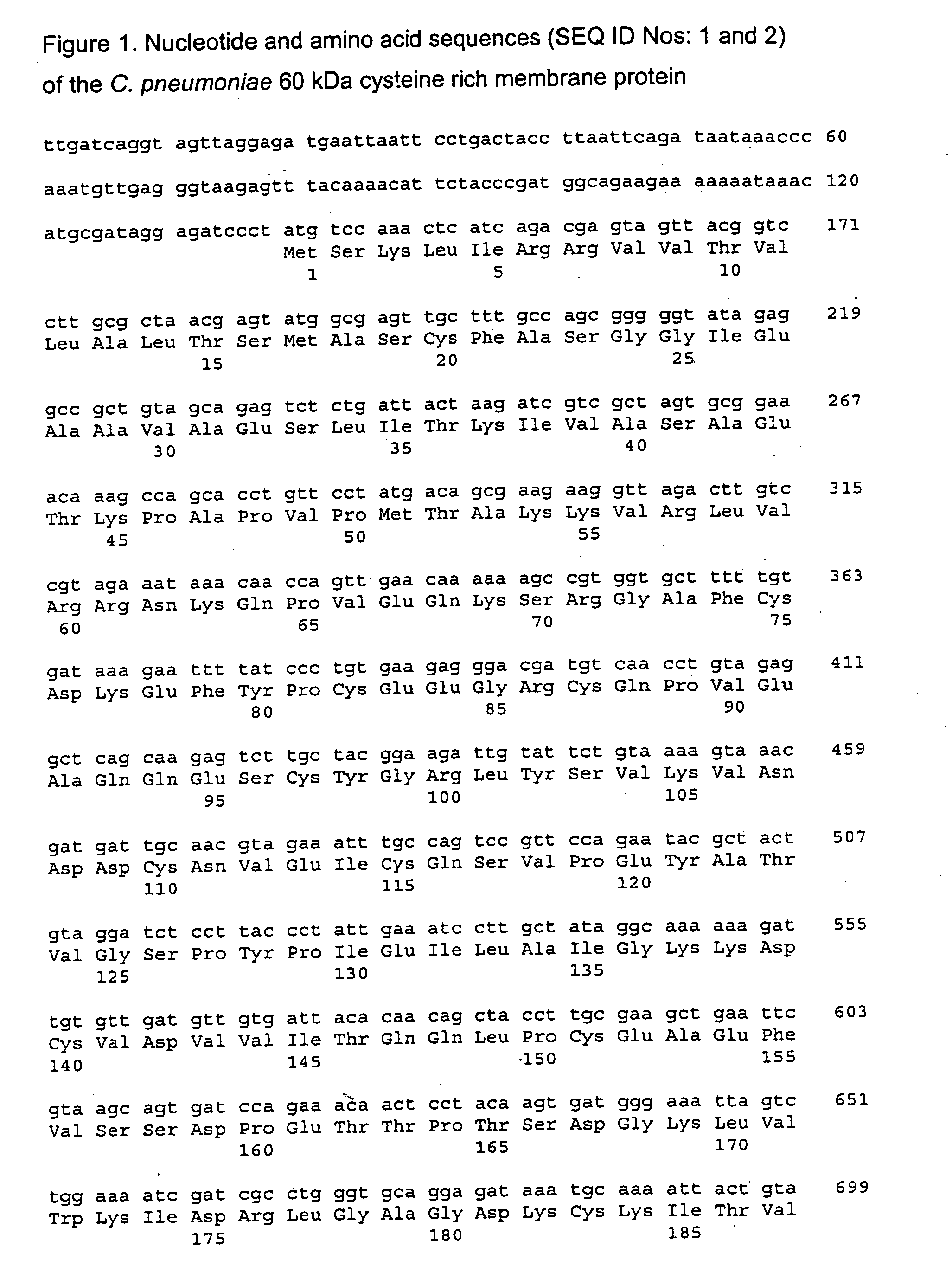
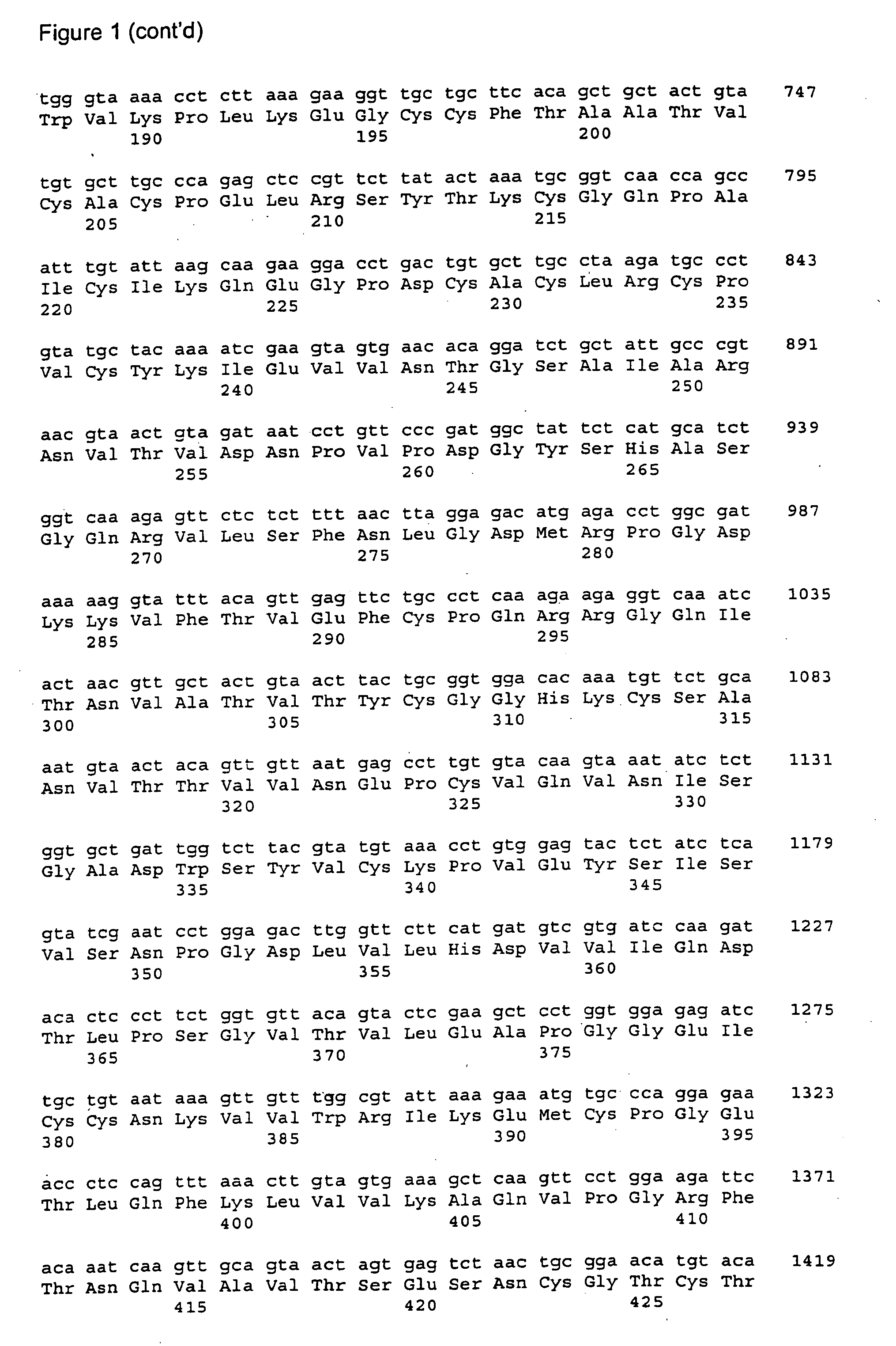
The present invention provides vaccines and methods for immunizing a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. pneumoniae. The vaccine and method employ a 60 kDa cysteine-rich membrane protein of a strain of Chlamydia pneumoniae. Modifications are possible within the scope of this invention.
Owner:SANOFI PASTEUR LTD
Mycoplasma pneumoniae and chlamydia pneumoniae nucleic acid combined detection kit and application thereof
ActiveCN110904194AEasy to degradeLower requirementMicrobiological testing/measurementAgainst vector-borne diseasesRNA extractionNucleic acid detection
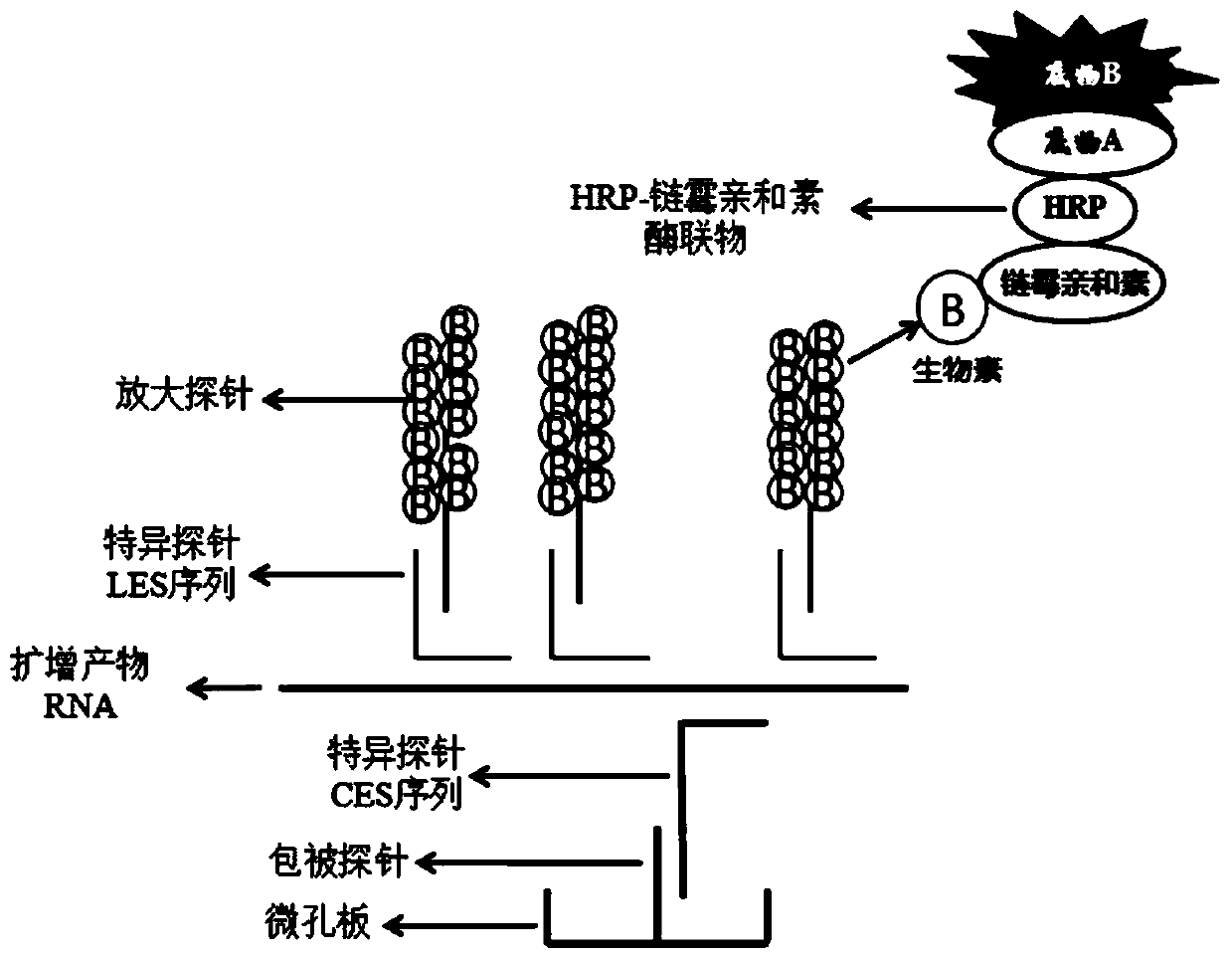
The invention discloses a mycoplasma pneumoniae and chlamydia pneumoniae nucleic acid combined detection kit and an application thereof. A collected sample is split by a cell lysis solution to releasepathogen nucleic acid, and amplification of pathogen nucleic acid fragments is realized through reverse transcription and transcription processes. The amplified RNA product is added into a microporecoated with a coating probe, and a specific probe and an amplification probe are added at the same time, wherein the coating probe can be combined with one end of the specific probe CES to fix the amplified product RNA. One end of the specific probe LES is combined with the RNA product, and the other end of the specific probe LES is combined with the amplification probe to realize signal amplification. The amplification probe marked with multiple biotins is then combined with a streptavidin-HRP enzyme conjugate. Finally, an HRP enzyme chemiluminiscence substrate is added, and detection is carried out on a chemiluminiscence instrument. According to the invention, RNA extraction is not needed, and pollution is not likely to happen in the detection process; and the kit has the advantages of high sensitivity and high specificity, and can be widely applied to mycoplasma pneumoniae and chlamydia pneumoniae nucleic acid combined detection.
Owner:武汉中帜生物科技股份有限公司
Coating liquid for improving stability of chlamydia pneumoniae antigen/mycoplasma antigen in immunochromatographic reagent and preparation method thereof
The invention relates to the field of biological reagents, in particular to coating liquid for improving the stability of chlamydia pneumoniae antigen / mycoplasma antigen in an immunochromatographic reagent and a preparation method thereof. The coating solution comprises a buffer solution, wherein the buffer solution comprises a first component for immobilizing a chlamydia antigen or a mycoplasma antigen on a nitrocellulose membrane, a stabilizer, an antioxidant and a preservative. The first component is beneficial to immobilization of the antigen on the nitrocellulose membrane. The stabilizercan improve the stability of the mycoplasma antigen and the chlamydia antigen. The antioxidant can prevent the mycoplasma antigen and the chlamydia antigen from being oxidized. The preservative can play a role in preventing mycoplasma antigens and chlamydia antigens from being corroded. All the components in the coating liquid cooperate with one another to protect the stability of the antigen in the immunochromatographic reagent.
Owner:ZHUHAI LIVZON DIAGNOSTICS
Chlamydia antigens and corresponding DNA fragments and uses thereof
The present invention provides a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. pneumoniae, employing a vector containing a nucleotide sequence encoding full-length, 5'-truncated or 3'-truncated 76 kDa protein of a strain of Chlamydia pneumoniae and a promoter to effect expression of the 76 kDa protein gene in the host. Modifications are possible within the scope of this invention.
Owner:SANOFI PASTEUR LTD
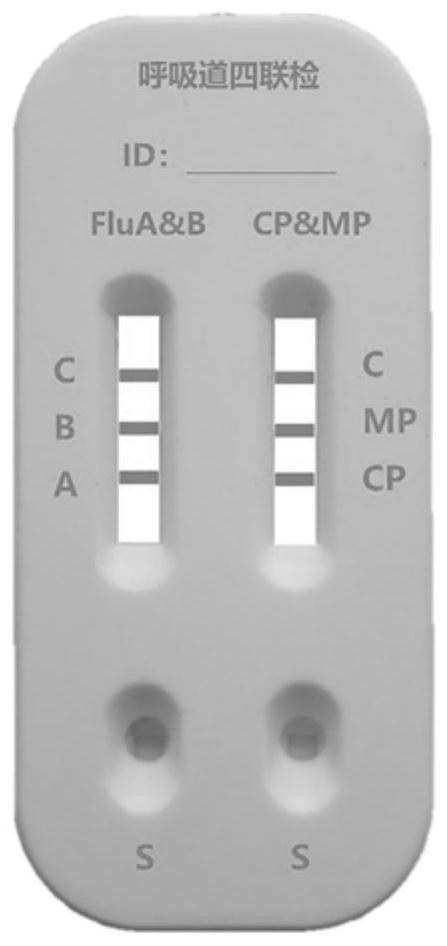
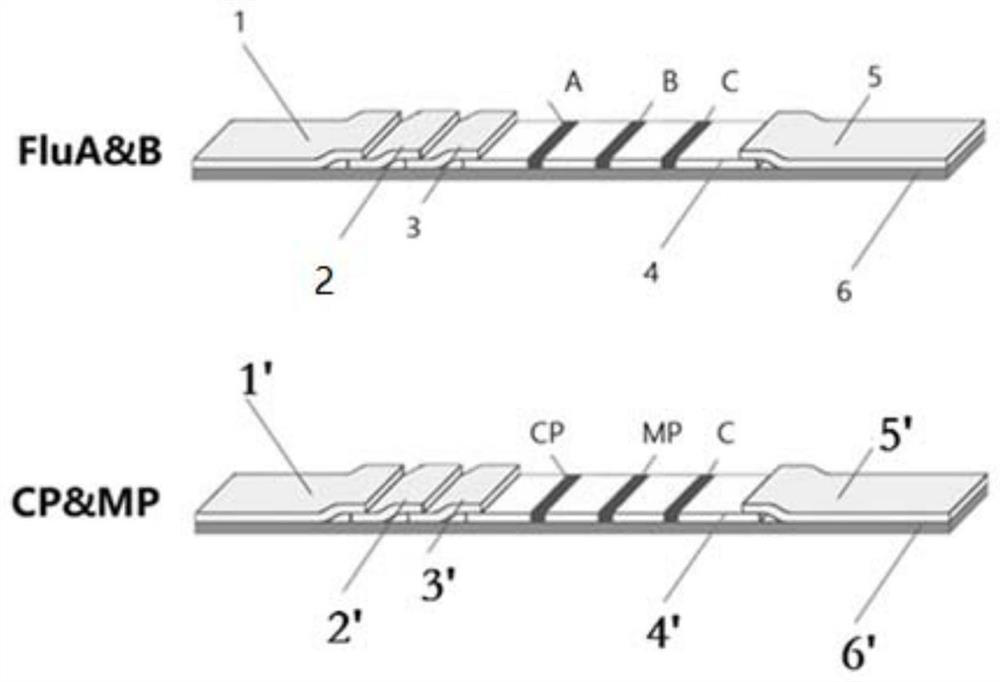
Combined device and detection method for synchronously detecting influenza A virus, influenza B virus, chlamydia pneumoniae IgM antibody and mycoplasma IgM antibody
PendingCN111610329ASynchronize independent test resultsIndependent Testing ProcessMaterial analysisReceptorIgm antibody
The invention belongs to the field of medical detection equipment, and provides a combined device for synchronously detecting IgM antibodies of influenza A and B viruses and chlamydia pneumoniae and mycoplasma pneumoniae. The device comprises a double-channel clamping shell, a test strip FluA & B and a test strip CP & MP which are arranged in the double-channel clamping shell in parallel; influenza virus A detection lines arranged on a nitrocellulose membrane of the test strip FluA & B at intervals are coated with high-specificity influenza virus A antigens, influenza virus B detection linesare coated with high-specificity influenza virus B antigens, and first quality control lines are coated with quality control line coating receptors; chlamydia pneumoniae detection lines (CP) arranged on a nitrocellulose membrane of the test strip CP & MP at intervals are coated with high-specificity chlamydia pneumoniae antigens, mycoplasma pneumoniae detection lines (MP) are coated with high-specificity mycoplasma pneumoniae antigens, and second quality control lines are coated with quality control line coating receptors.
Owner:北京柏兆嘉业科技有限公司
Fluorescent quantitative PCR method for detecting toxigenic chlamydia pneumoniae and corresponding kit
PendingCN111676300AHigh amplification efficiencyAchieving correct detectionMicrobiological testing/measurementMicroorganism based processesMicrobiologyPcr method
The invention discloses a fluorescent quantitative PCR method for detecting toxigenic chlamydia pneumoniae and a kit. The method ingeniously applies specific gene detection to distinguish chlamydia pneumoniae from other chlamydia and toxigenic and non-toxigenic chlamydia pneumoniae, and obtains accurate bacterial information through comprehensive judgment. Compared with the existing mainstream detection kit, the kit for detecting toxigenic chlamydia pneumoniae has the advantages of high sensitivity, quickness, convenience, good specificity, rigorous and accurate judgment and the like, and hasgood application prospect and market value.
Owner:广东美格基因科技有限公司
Compositions for treating infective arterial diseases and related conditions
PendingUS20210052557A1Enhance host defence mechanismAntibacterial agentsTetracycline active ingredientsDiseaseMycoplasma penetrans
In alternative embodiments, provided are pharmaceutical compositions and methods for treatment, amelioration and prevention of infection-associated blood vessel diseases, and also for the treatment, amelioration and prevention of non-vessel diseases affected by infective agents which can be treated by these compositions. In alternative embodiments, one common pathogen targeted by compositions and methods as provided herein is Chlamydia and Chlamydophila species, including pneumoniae, trachomatis and psittaci species which infect humans, including Chlamydophila penumoniae which also infects humans. In alternative embodiments, pathogens targeted and infections (diseases) treated ameliorated, or prevented by compositions and methods as provided herein include Mycoplasma, Listeria, Leptospirosis, Q fever or Coxiella burnetii infection, Lyme disease or Lyme borreliosis or any Borrelia infection, and Bartonella or of the family Bartonellaceae, including cat scratch disease.
Owner:CENT FOR DIGESTIVE DISEASES PTY LTD
Method for detecting respiratory tract pathogens in to-be-detected sample and kit and application thereof
PendingCN113355454AMicrobiological testing/measurementMicroorganism based processesInfluenza Viruses Type APneumonitis
The invention discloses a method for detecting respiratory pathogens in a to-be-detected sample and a kit and application thereof, and relates to the technical field of biology. The method comprises the step of detecting a to-be-detected sample with a first reagent for detecting influenza A virus, a second reagent for detecting influenza B virus, a third reagent for detecting mycoplasma pneumoniae and / or a fourth reagent for detecting chlamydia pneumoniae, and a new way is provided for effectively detecting respiratory pathogens or simultaneously detecting multiple respiratory pathogens.
Owner:GUANGDONG FAPON BIOTECH CO LTD
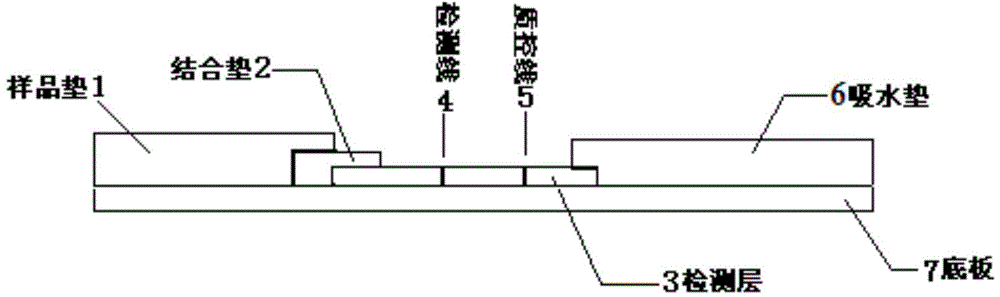
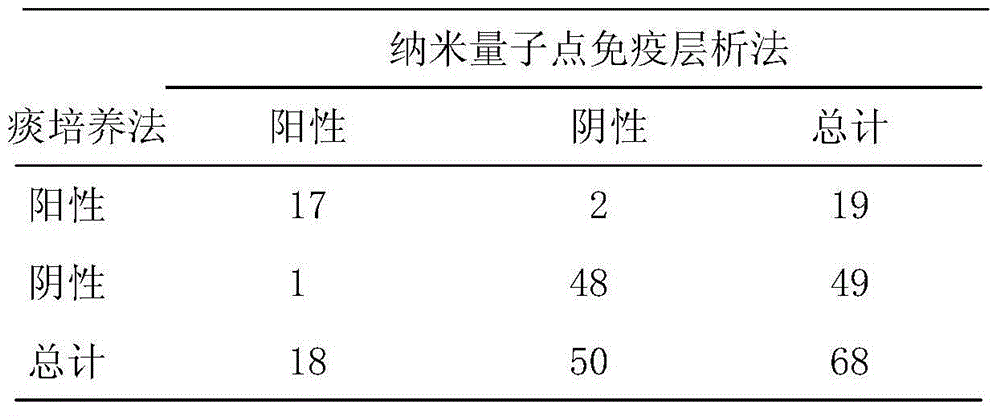
Chlamydia pneumoniae quantum dot immunochromatographic assay detection card and preparing method and application thereof
The invention provides a chlamydia pneumoniae quantum dot immunochromatographic assay detection card and a preparing method and application thereof. The detection card comprises a bottom board, a sample pad, a water absorbing pad, a combining pad and a detection layer. Chlamydia pneumoniae resisting nanometer probes labeled with quantum dots envelop combining pad. The detection layer is formed by a solid-phase nitrocellulose membrane with a detection line and a quality control line. Anti-mouse chlamydia pneumoniae 98KD membrane protein polyclonal antibodies envelop the detection line. Anti-rabbit IgG is encapsulated in the quality control line. The detection layer is bonded to the bottom board. The combining pad and the water absorbing pad are arranged on the upper portions of the two ends of the detection layer respectively, partially overlapped with the detection layer and then bonded to the detection layer and the bottom board. The sample pad is arranged on the combining pad, partially overlapped with the combining pad and then bonded to the combining pad and the bottom board. The chlamydia pneumoniae quantum dot immunochromatographic assay detection card has the advantages of being easy and convenient to operate, fast in detection, capable of achieving quantification, high in sensitivity and the like.
Owner:湖北诺美华抗体药物技术有限公司
Primer probe group and kit for combined detection of mycoplasma pneumoniae and chlamydia pneumoniae based on fluorescence RMA method
PendingCN112592992AChange defectsIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesPolyethylene oxideReverse transcriptase
The invention belongs to the technical field of detection of mycoplasma pneumoniae and chlamydia pneumoniae, and particularly relates to a primer probe group, a kit and a detection method for combineddetection of mycoplasma pneumoniae and chlamydia pneumoniae based on a fluorescence RMA method. The kit comprises a detection tube containing an amplification reaction reagent, a buffer solution, magnesium acetate, standard positive plasmids and sterile double distilled water. The amplification reaction reagent is prepared from primers and probes of MP and Cpn, M-MLV reverse transcriptase, escherichia coli RecA protein, UvsY protein, single-stranded binding protein GP32, Bst polymerase, exonuclease III, polyethylene oxide, trehalose, mannitol, ATP, dNTPs, creatine kinase and creatine phosphate. The standard positive plasmids are recombinant plasmids containing MP and Cpn amplified gene sequences, and are used for positive control of MP and Cpn nucleic acid detection.
Owner:济南国益生物科技有限公司
Immunogenic compositions for pneumonia chlamydia
The invention relates to polypeptides for use as an autotransporter antigen. The invention further relates to methods and uses of a polypeptide for an autotransporter function in preparation of a medicament for the prevention or treatment of a Chlamydia pneumoniae infection or for the preparation of an assay for the diagnosis of a Chlamydia pneumoniae infection in an individual. Also, a method is provided for raising an immune response in an individual by administering to the individual a polypeptide for use as an autotransporter antigen.
Owner:CHIRON CORP
Pneumonia pathogenic bacteria rapid identification gene chip
ActiveCN107287311BNucleotide librariesMicrobiological testing/measurementPseudomonasAerobacter cloacae
The invention discloses a rapid-recognition gene chip for pathogenic bacteria of pneumonia. The gene chip can detect 15 pathogenic bacteria including streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, acinetobacter baumannii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae, and clinically common and difficult-to-culture pathogenic bacteria are contained. In a preparation process of the gene chip, design, screening and verification of probes are performed by adopting 16S rDNA and a specific gene sequence corresponding to each of the pathogenic bacteria, and types of the bacteria in a to-be-detected sample are identified from levels of genus and species simultaneously and respectively. The gene chip has the advantages that the defect that clinical detection of the pathogenic bacteria of pneumonia is not timely and comprehensive at present is overcome, and one novel detection way is provided for early diagnosis and early treatment of patients with pneumonia.
Owner:GENERAL HOSPITAL OF PLA +1
Method for detecting artery plaque stability
InactiveCN112442543AEasy to copyGood Economic Value ProspectsMicrobiological testing/measurementMicroorganism based processesDiseaseAssay
The invention relates to the field of laboratory medicine and cardiovascular and cerebrovascular diseases. The invention relates to a method for assaying human peripheral blood, in particular to a method for detecting artery plaque stability. According to the method, the stability of an artery plaque is judged by detecting chlamydia pneumoniae infection in the peripheral blood and in the artery plaque by inspecting a specific nucleotide sequence of a chlamydia pneumoniae genome in the peripheral blood. The method specifically comprises the following three aspects that the chlamydia pneumoniaeinfection in the peripheral blood is used as a new blood risk factor for arterial plaque occurrence and development and related diseases; according to the novel method for detecting the artery plaquestability, the inflammatory response of the artery plaque is typed and staged according to the infection condition and the infection degree of the chlamydia pneumoniae in the artery plaque of an individual; and according to the detected infection condition of the chlamydia pneumoniae in human peripheral blood and artery plaques, a method for implementing the application in a medication scheme forstabilizing the artery plaques and reducing inflammatory response is determined. The method has good social value and economic value.
Owner:孙余华
Antigenic polypeptide of chlamydia pneumoniae
Antigen polypeptide of Chlamydia pneumoniae, which includes polypeptide A containing the sequence of at least 5 consecutive amino acid residues in the polypeptide of SEQ ID NO: 1; DNA encoding the polypeptide; a recombinant vector containing the DNA; a transformant containing the vector; Methods for producing anti-Chlamydia pneumoniae antibodies using antigenic polypeptides as antigens; methods for detecting and analyzing the anti-Chlamydia pneumoniae antibodies; applications of antigenic polypeptides; fusion proteins composed of dihydrofolate reductase and Chlamydia pneumoniae antigenic polypeptides, wherein SEQ ID The polypeptide of NO: 14 is combined with polypeptide A containing the sequence of at least 5 consecutive amino acid residues in the polypeptide of SEQ ID NO: 1; DNA encoding the fusion protein; recombinant vector containing the DNA; transformation containing the vector Methods for producing anti-Chlamydia pneumoniae antibodies using fusion proteins as antigens; methods for detecting and analyzing anti-Chlamydia pneumoniae antibodies using fusion proteins as antigens; application of fusion proteins; probes and primers for detecting and analyzing Chlamydia pneumoniae genes; application of the Methods for detecting and analyzing Chlamydia pneumoniae genes with probes or primers, and applications of the probes or primers.
Owner:HITACHI CHEM CO LTD
Indirect ELISA detection kit and detection method for detecting chlamydia pneumoniae antibody
InactiveCN111122860AStrong specificityIncreased sensitivityMaterial analysisAntibody conjugateSerum dilution
The invention discloses an indirect ELISA detection kit for detecting a chlamydia pneumoniae antibody and a detection method. The detection kit comprises a chlamydia pneumoniae recombinant antigen ELISA coated ELISA strip, a serum dilution plate, a diluent, a positive control solution, a negative control solution, an enzyme-labeled secondary antibody conjugate, a washing solution, a TMB developingsolution and a stop solution. The stop solution is 2 M concentrated sulfuric acid. The sequence of the CP recombinant antigen is SEQ ID NO. 1. The optimal antigen coating concentration and serum dilution are optimized through a square matrix, the dilution of the enzyme-labeled secondary antibody is determined, and the type and closing time of the confining liquid and the antibody incubation timeare determined. The kit has the advantages of strong specificity, high sensitivity, good stability, high flux and the like when being used for detecting the CP antibody, can well detect the CP antibody in human serum, and has certain guiding significance for clinical diagnosis.
Owner:南京拂晓生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com