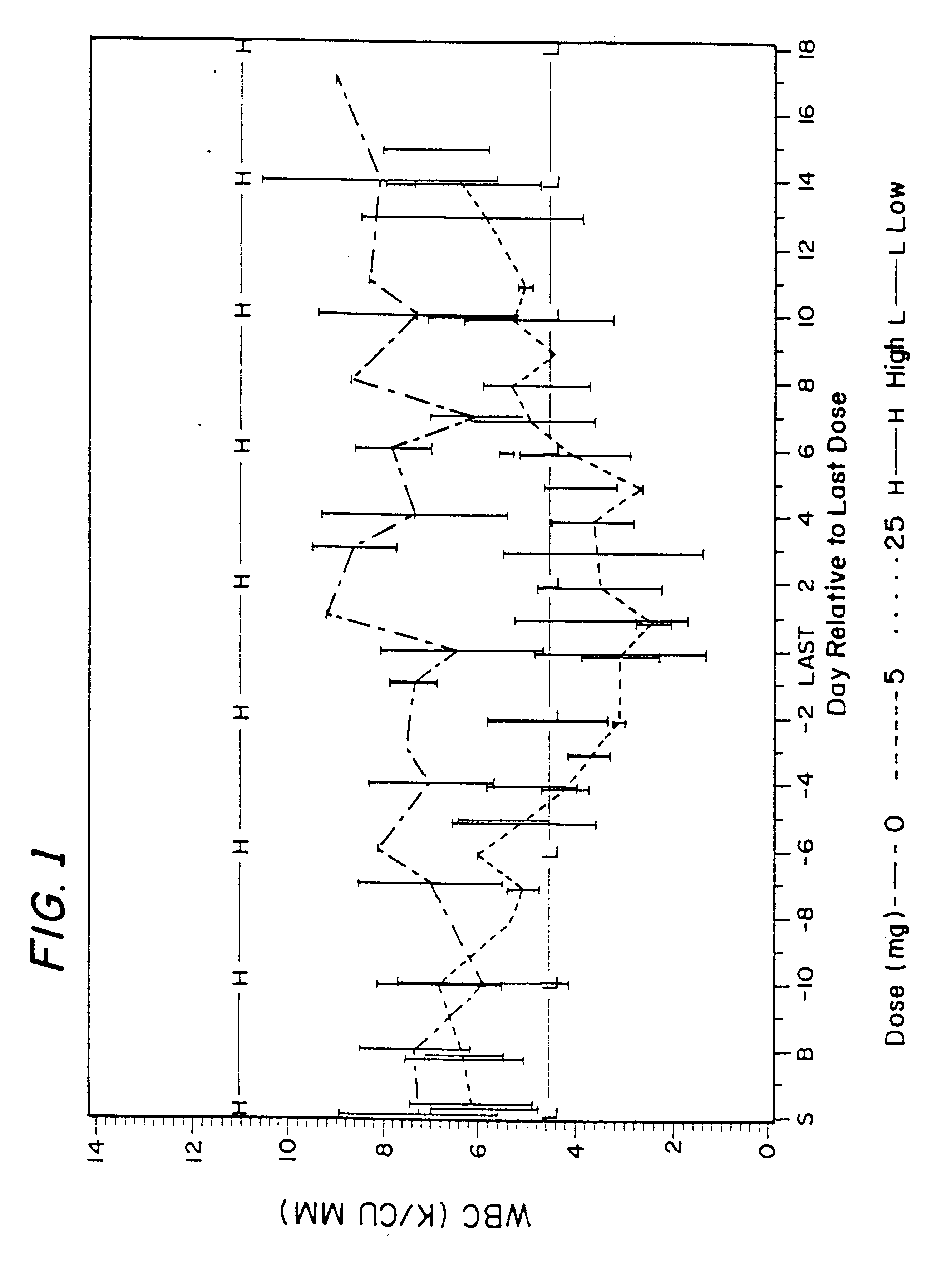
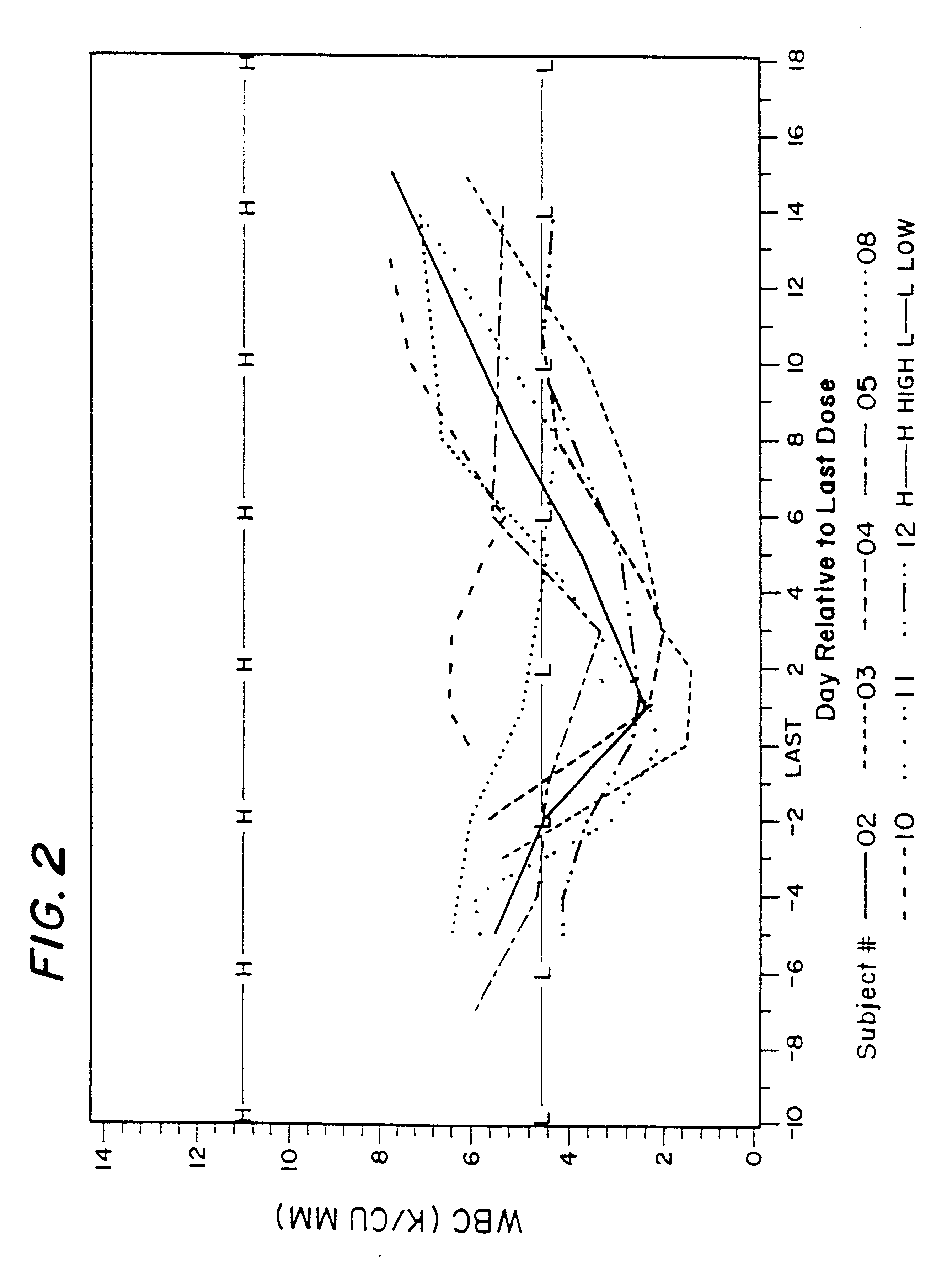
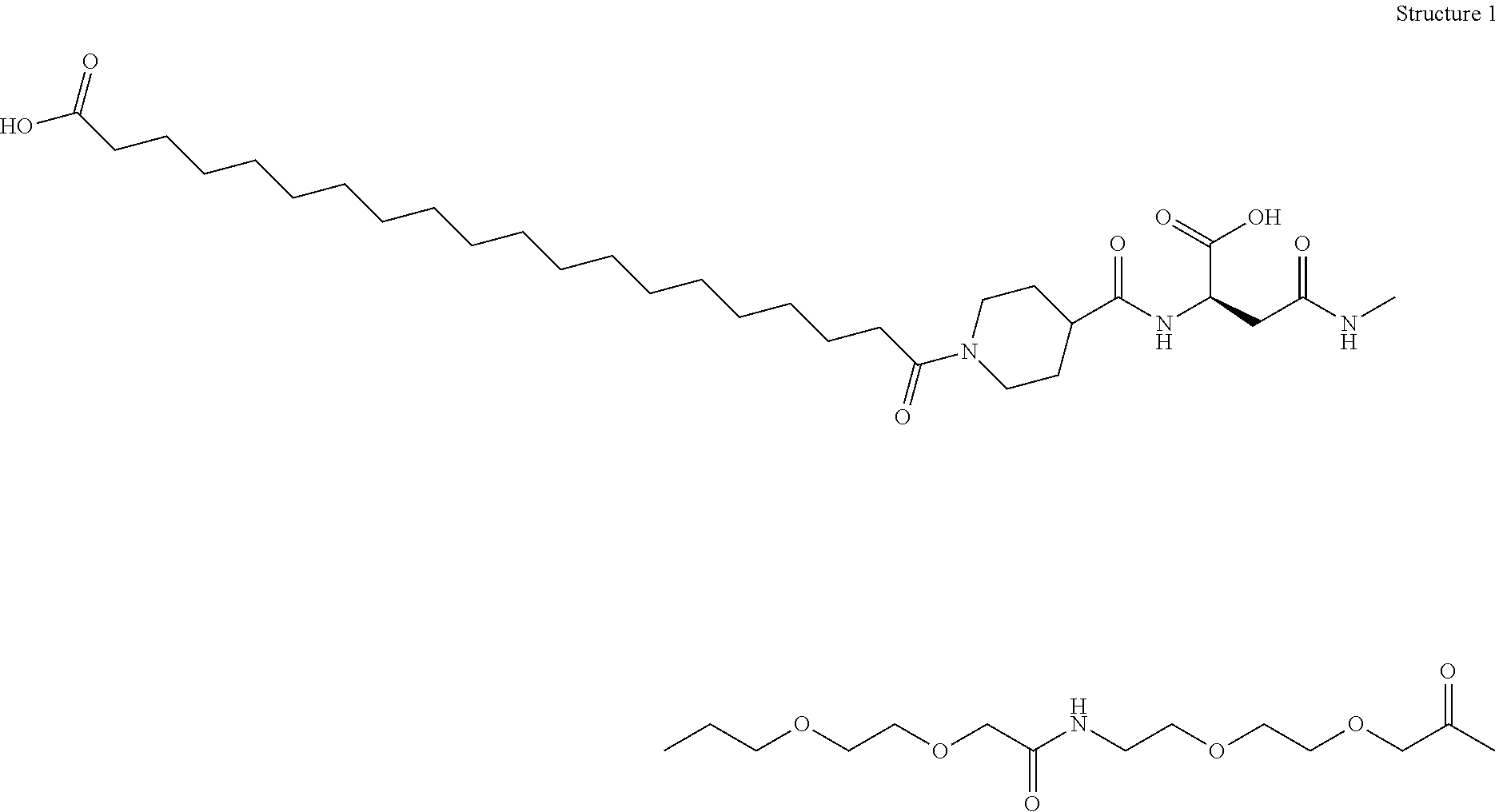
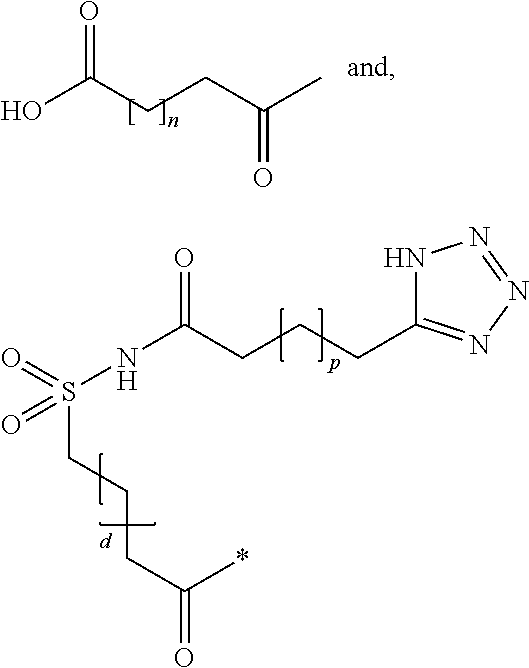
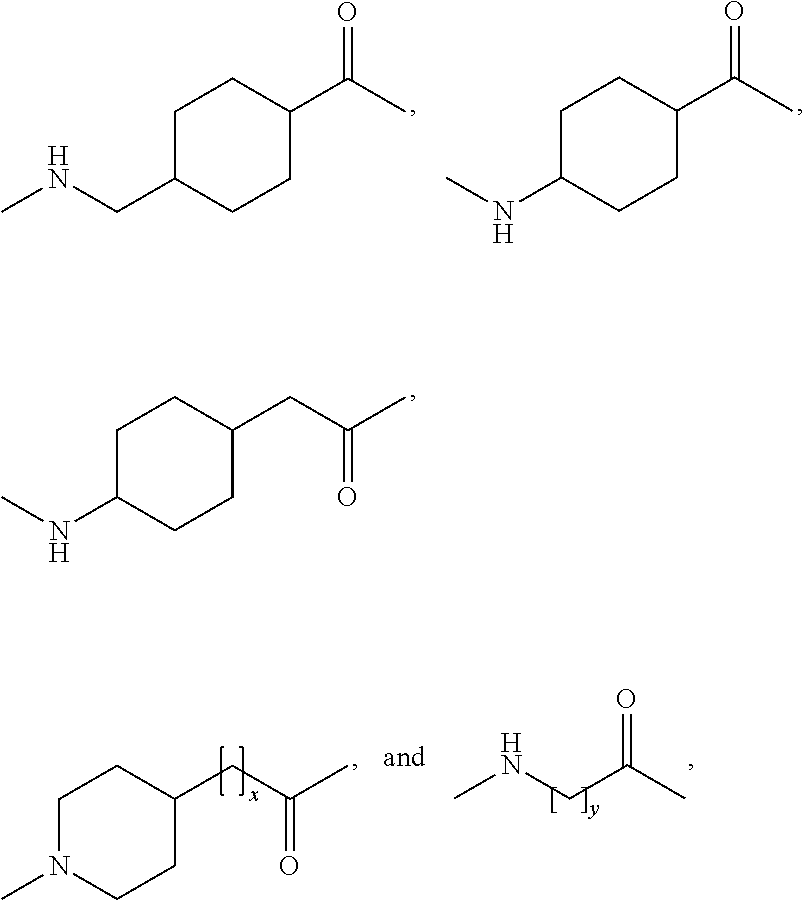
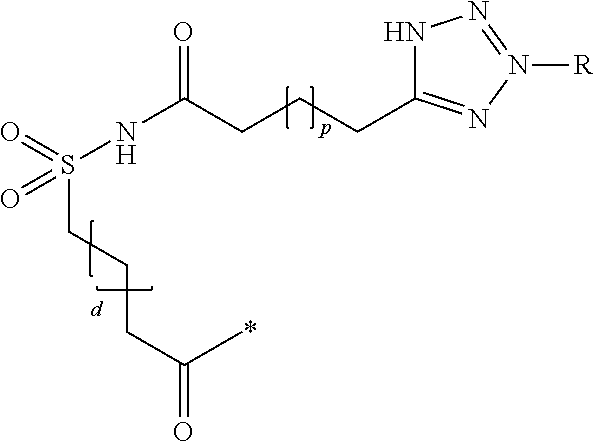
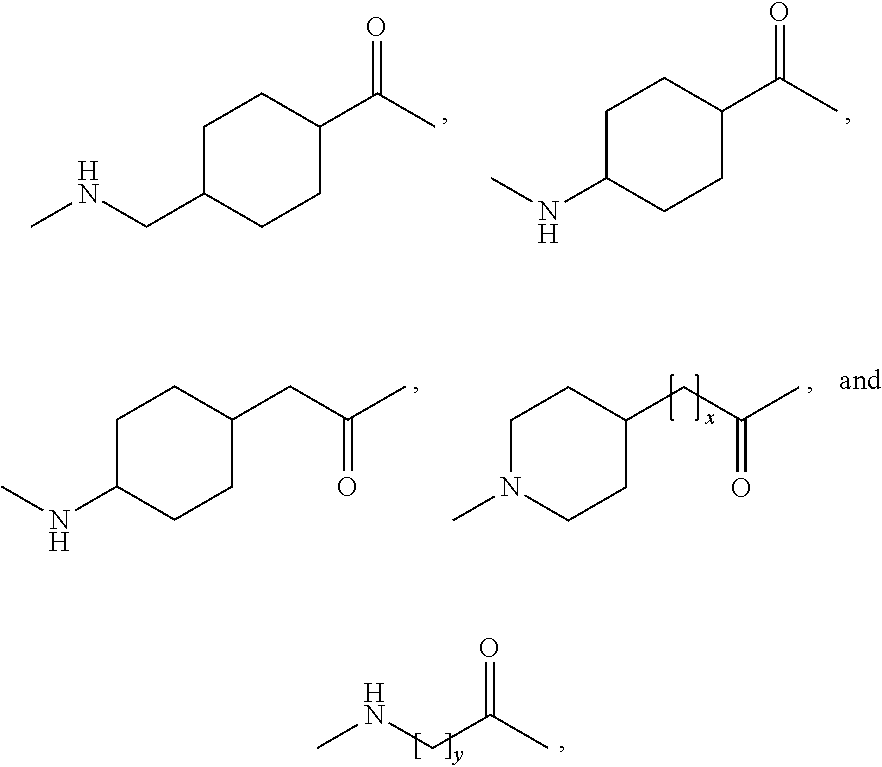
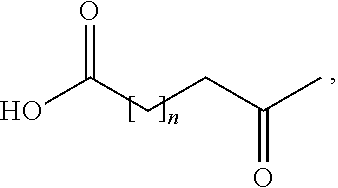
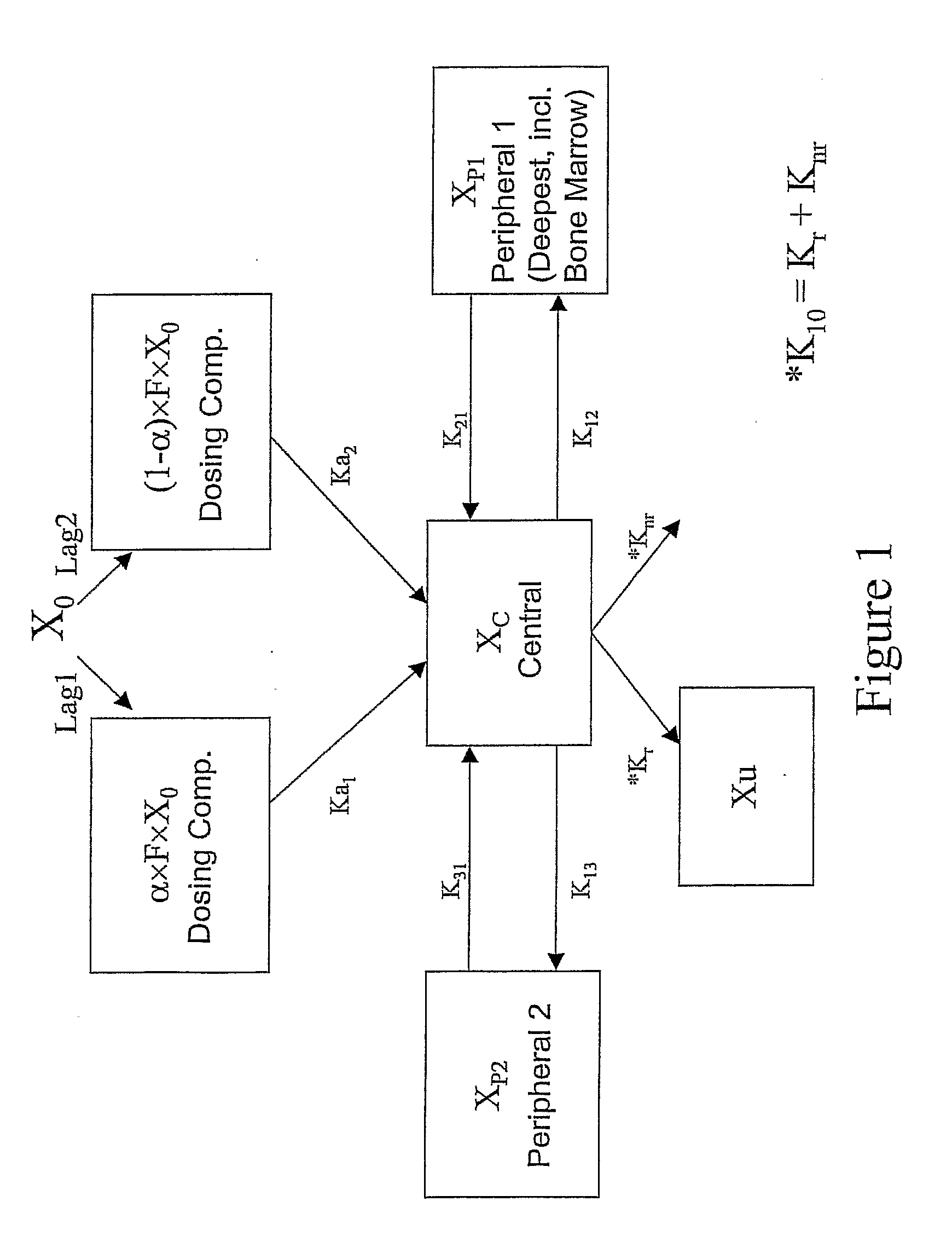
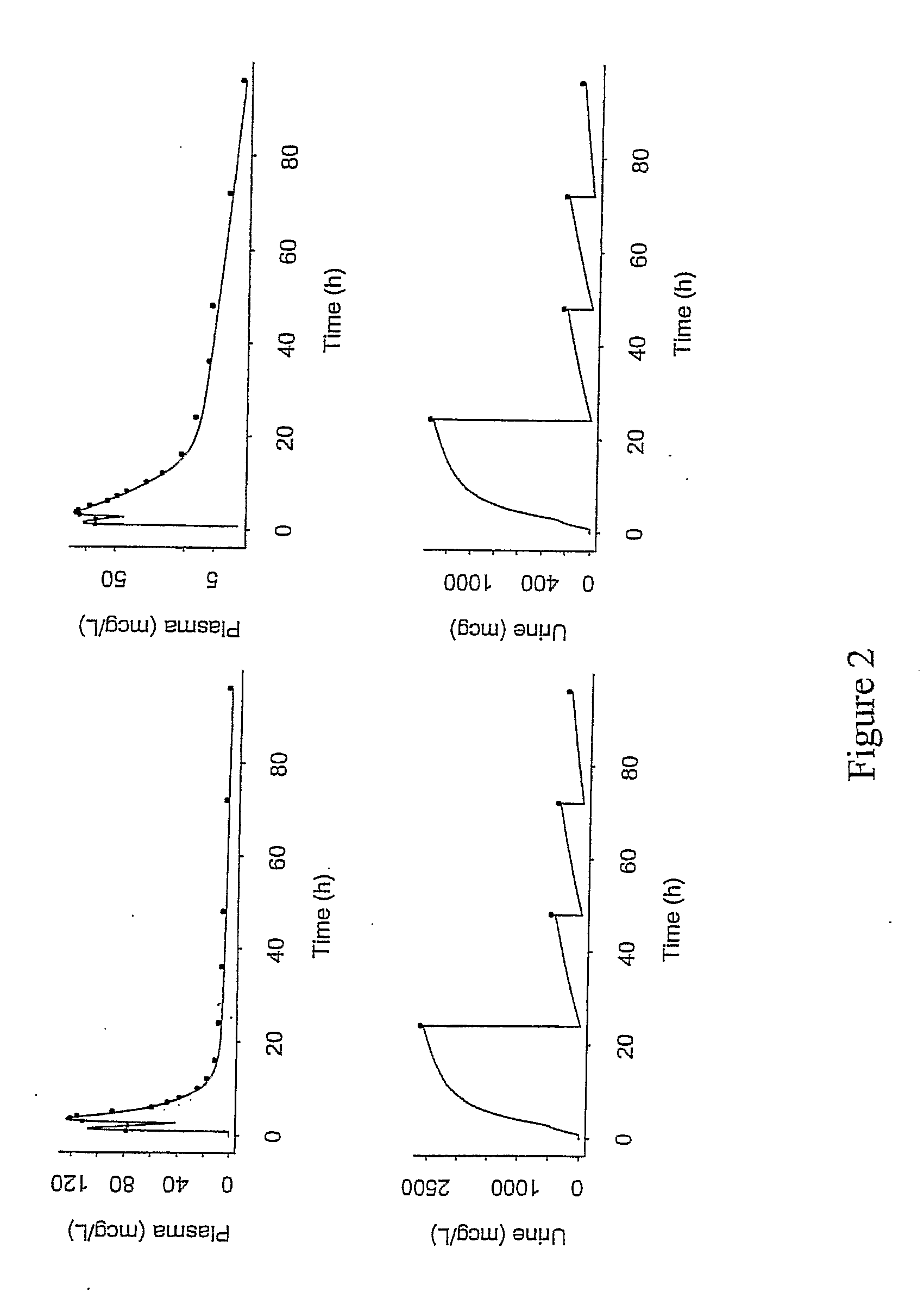
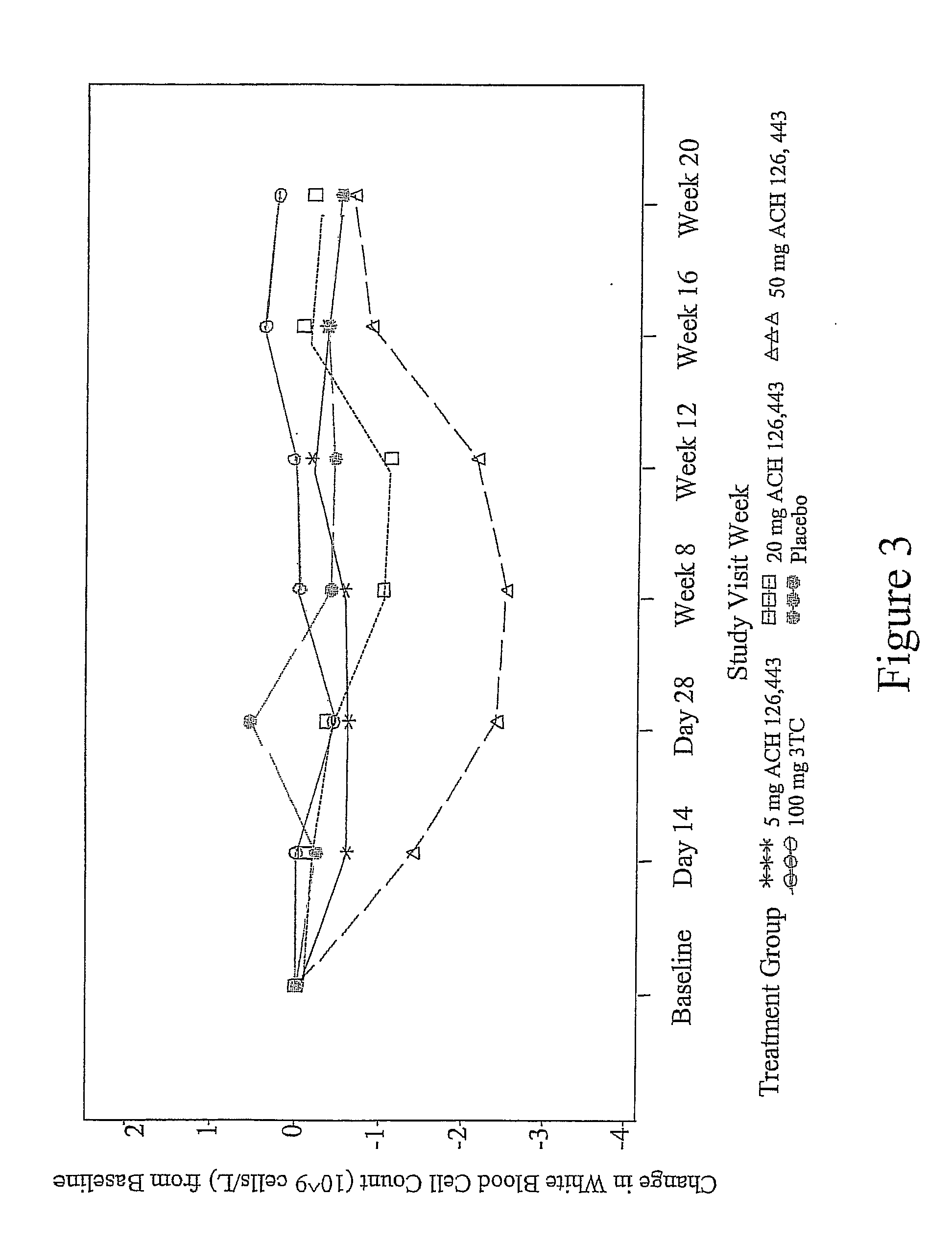
Patents
Literature
41 results about "Once weekly" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
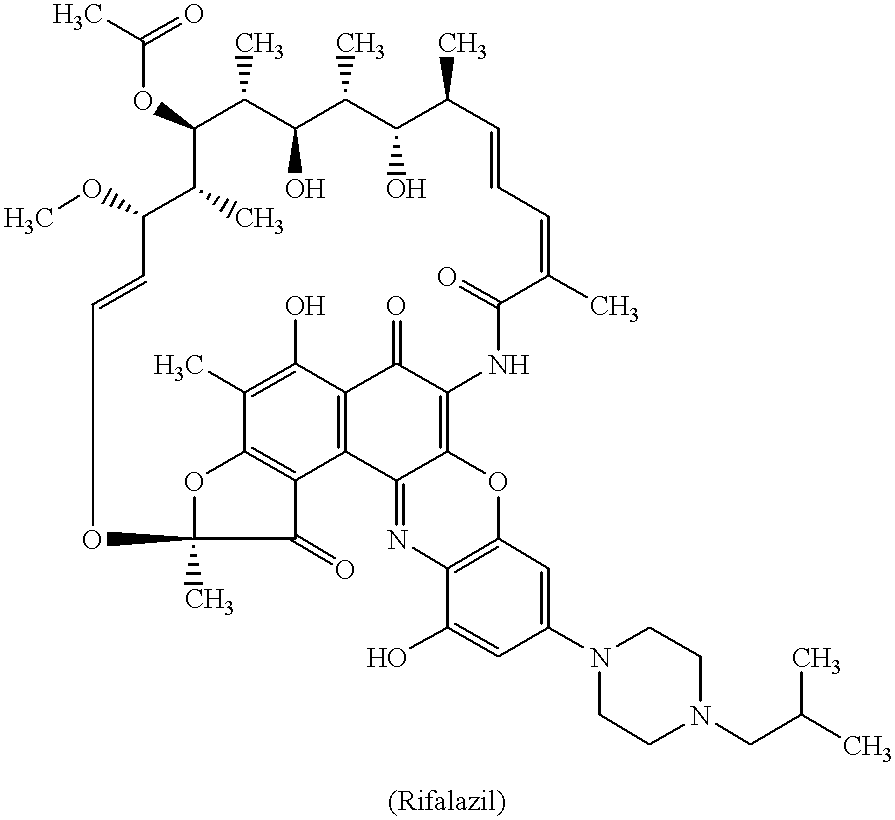
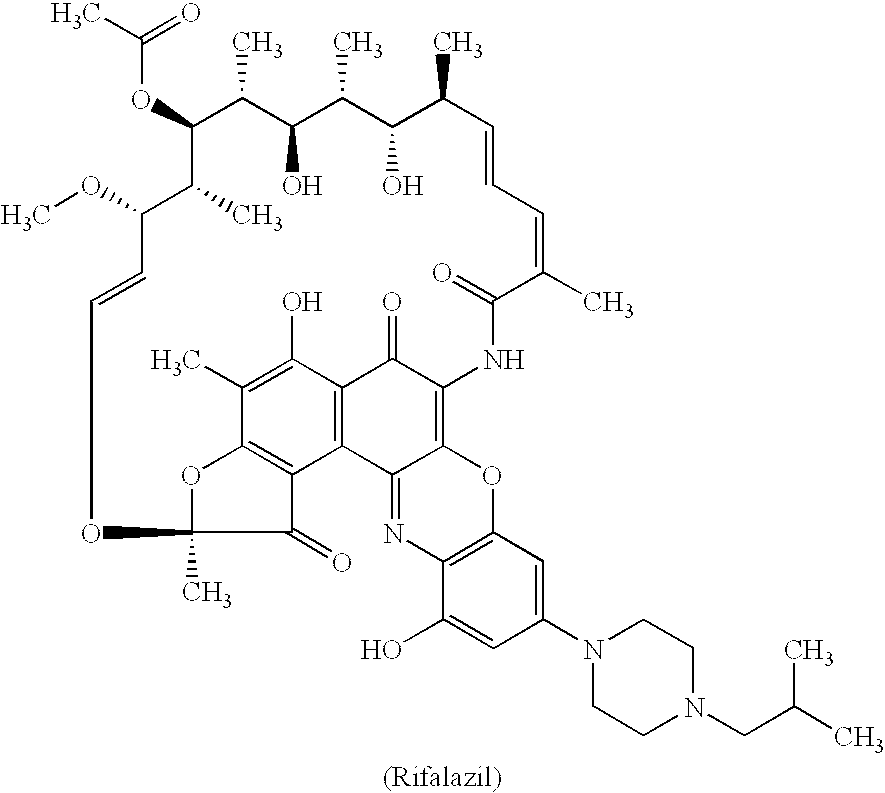
Method for treatment of bacterial infections with once or twice-weekly administered rifalazil
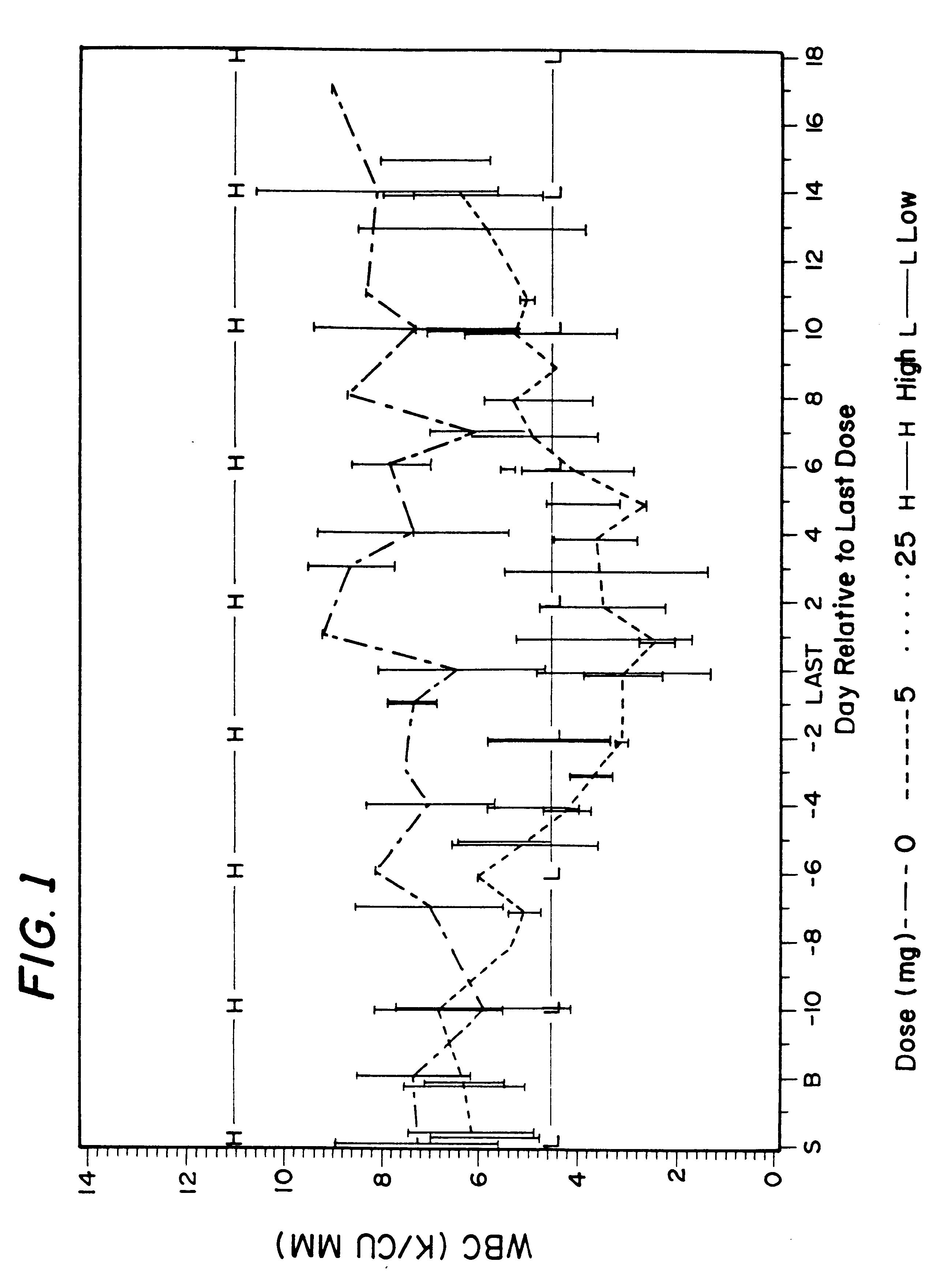
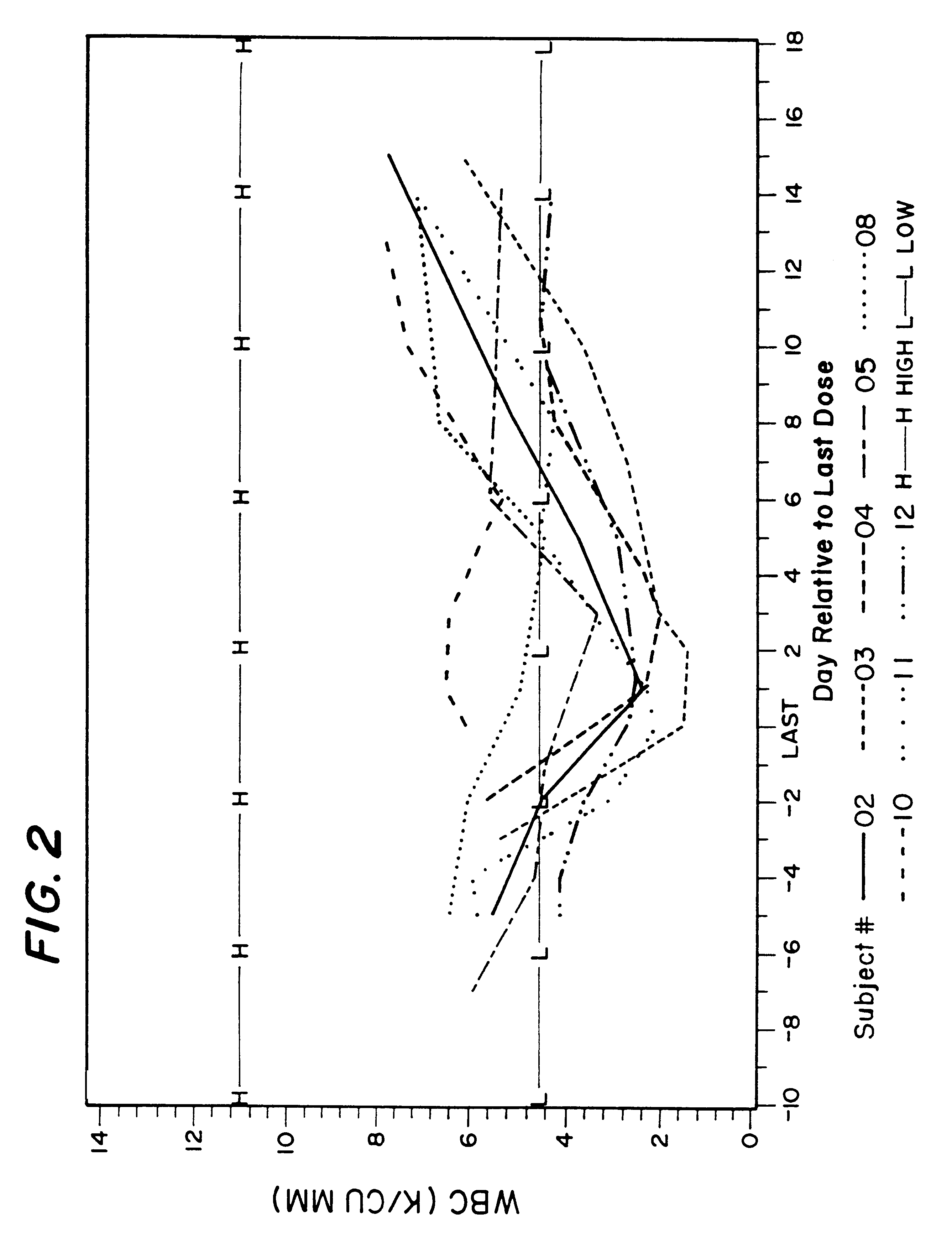
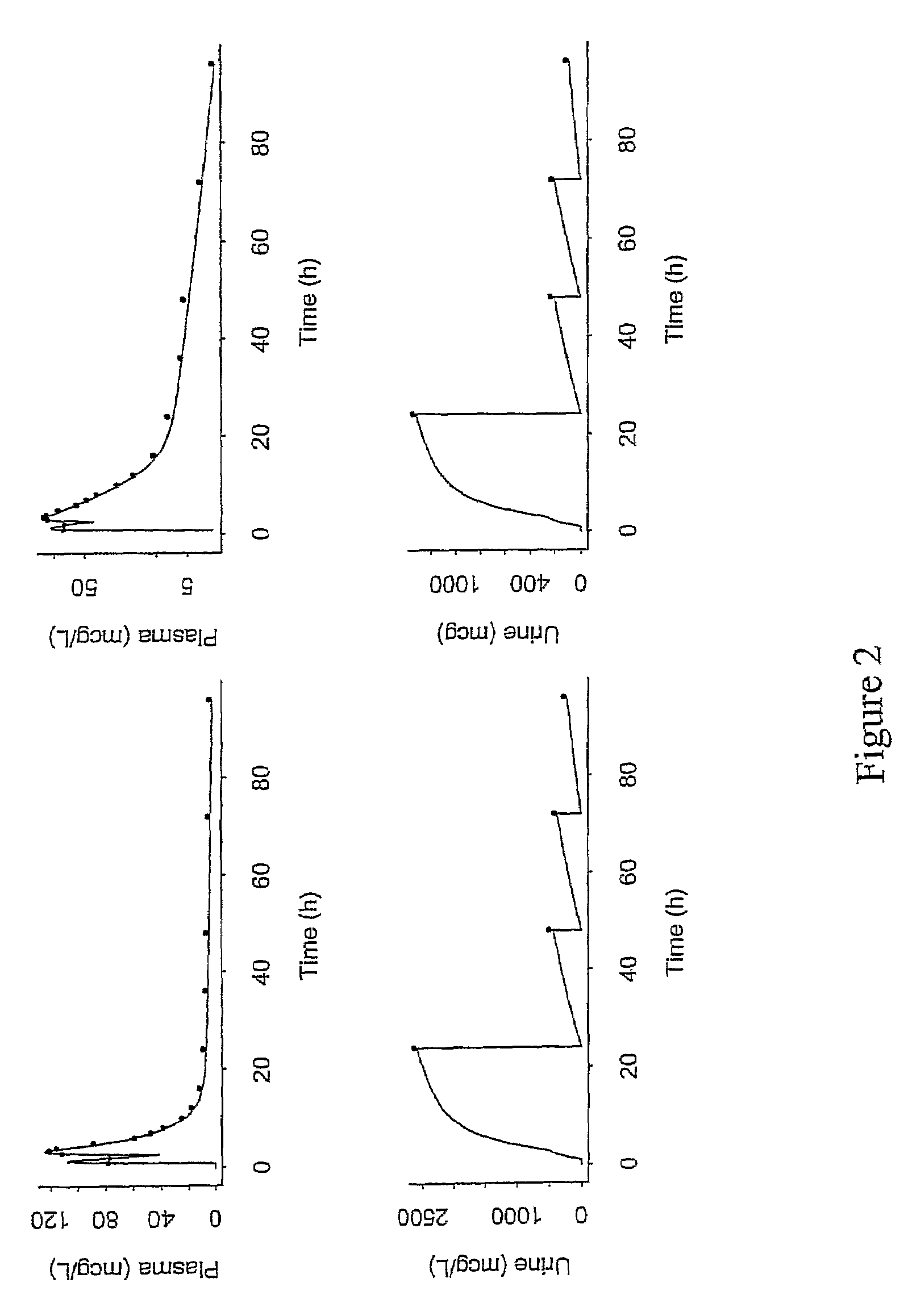
A method for treatment of bacterial infections with rifalazil administered once-weekly or twice-weekly. A method for treatment of tuberculosis caused by Mycobacterium tuberculosis, infections caused by Mycobacterium avium complex, infections caused by Chlamydia pneumoniae and infections caused by Helicobacter pylori by administering to a patient suffering from the bacterial infection 1-100 mg of rifalazil once or twice a week. In this dose regimen, the treatment is fast, efficacious and eliminates undesirable secondary symptoms observed with daily doses of 1-50 mg of rifalazil.
Owner:KANEKA CORP
Method for treatment of bacterial infections with once or twice-weekly administered rifalazil
A method for treatment of bacterial infections with rifalazil administered once-weekly or twice-weekly. A method for treatment of tuberculosis caused by Mycobacterium tuberculosis, infections caused by Mycobacterium avium complex, infections caused by Chlamydia pneumoniae and infections caused by Helicobacter pylori by administering to a patient suffering from the bacterial infection 1-100 mg of rifalazil once or twice a week. In this dose regimen, the treatment is fast, efficacious and eliminates undesirable secondary symptoms observed with daily doses of 1-50 mg of rifalazil.
Owner:KANEKA CORP
Glucagon-Like Peptide-1 derivatives and their pharmaceutical use
The invention relates to protracted Glucagon-Like Peptide-1 (GLP-1) derivatives and therapeutic uses thereof. The GLP-1 derivative of the invention comprises a modified GLP-1(7-37) sequence having a total of 2-12 amino acid modifications, including Glu22 and Arg26, and being derivatised with an albumin binding residue or pegylated in position 18, 20, 23, 30, 31, 34, 36, 37, or 39. These compounds are useful in the treatment or prevention of diabetes type 2 and related diseases. The compounds are potent, stable, have long half-lives, a high affinity of binding to albumin, and / or a high affinity of binding to the extracellular domain of the GLP-1 receptor (GLP-1R), all of which is of potential relevance for the overall aim of achieving long-acting, stable and active GLP-1 derivatives with a potential for once weekly administration.
Owner:NOVO NORDISK AS
Peptides Derivatized with A-B-C-D- and their Therapeutical Use
The invention relates to protracted peptide derivatives such as Glucagon-Like Peptide-1 (GLP-1), exendin-4, and analogues thereof, as well as therapeutic uses thereof. The peptide derivative of the invention comprises a peptide wherein at least one amino acid residue is derivatized with A-B—C—, or A-B—C-D-. These compounds are useful in the treatment or prevention of diabetes type 2 and related diseases. The compounds are potent, have a low ratio of binding affinity to the GLP-1 receptor in the presence of high / low albumin concentrations, have long half-lives, and have a high affinity of binding to albumin, all of which is of potential relevance for the overall aim of achieving long-acting, stable and active GLP-1 derivatives with a potential for once weekly administration.
Owner:NOVO NORDISK AS
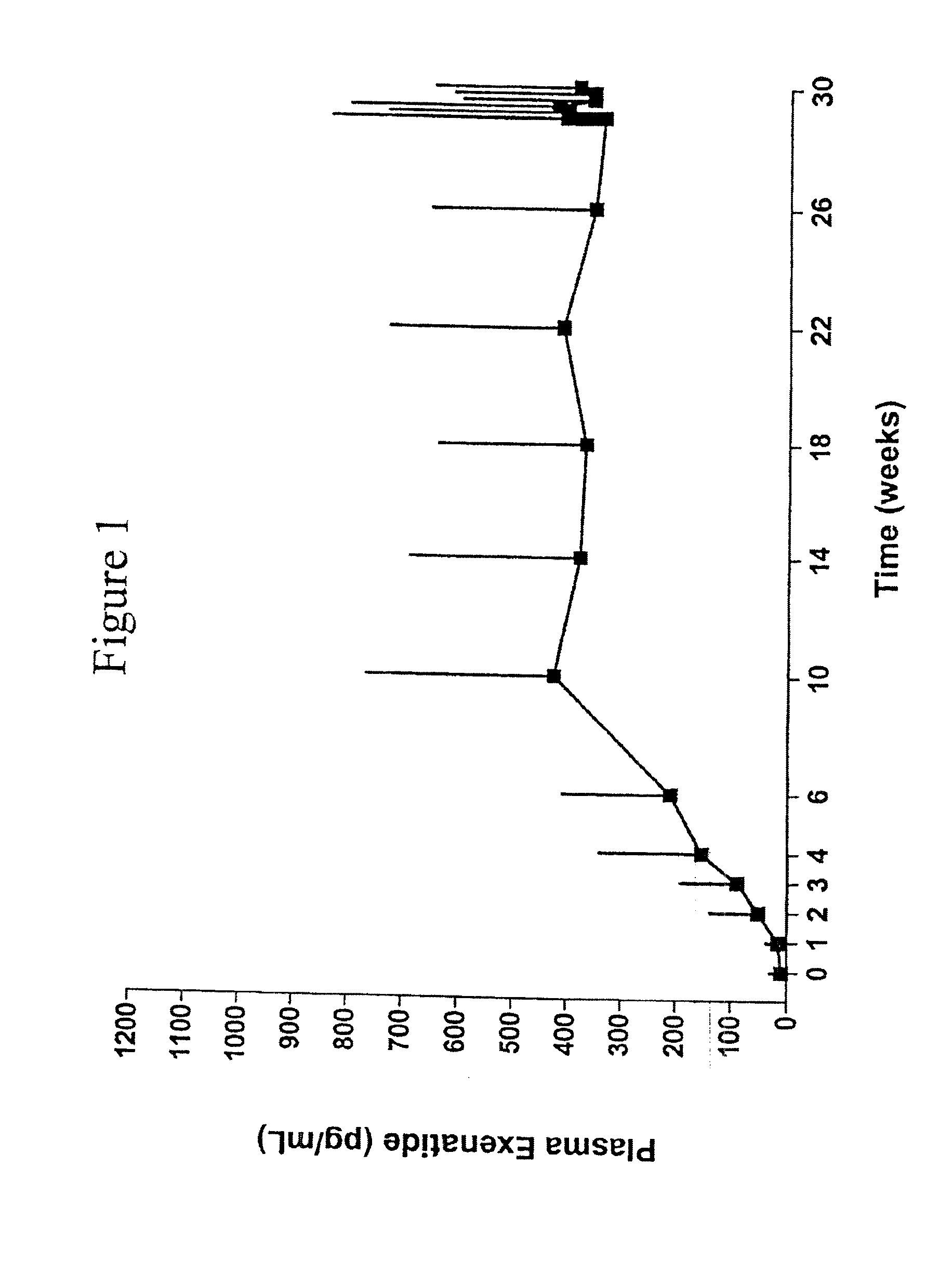
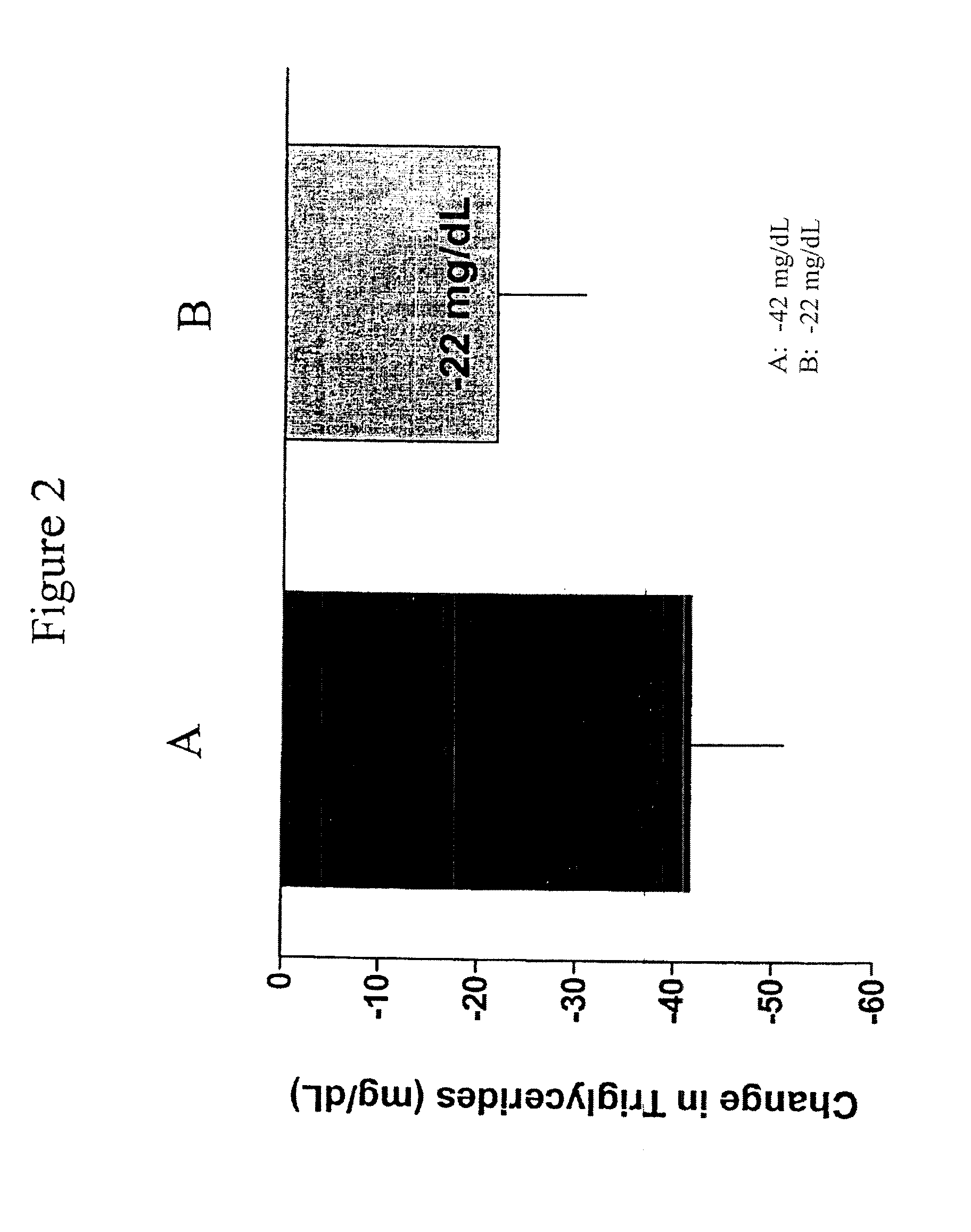
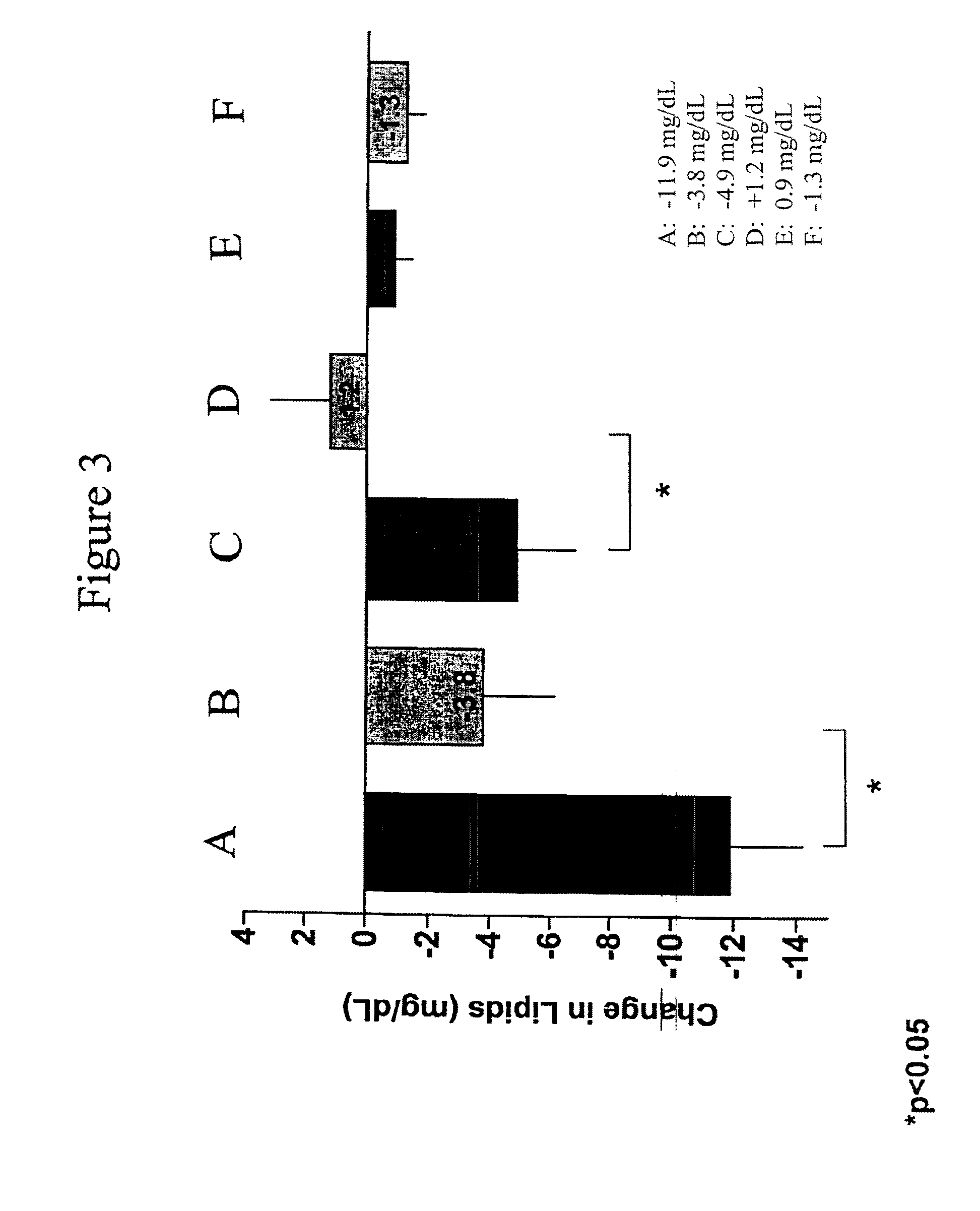
Exendins to lower cholesterol and triglycerides
InactiveUS20110263496A1Lower cholesterol levelsStable statePeptide/protein ingredientsMetabolism disorderDyslipidemiaSucrose
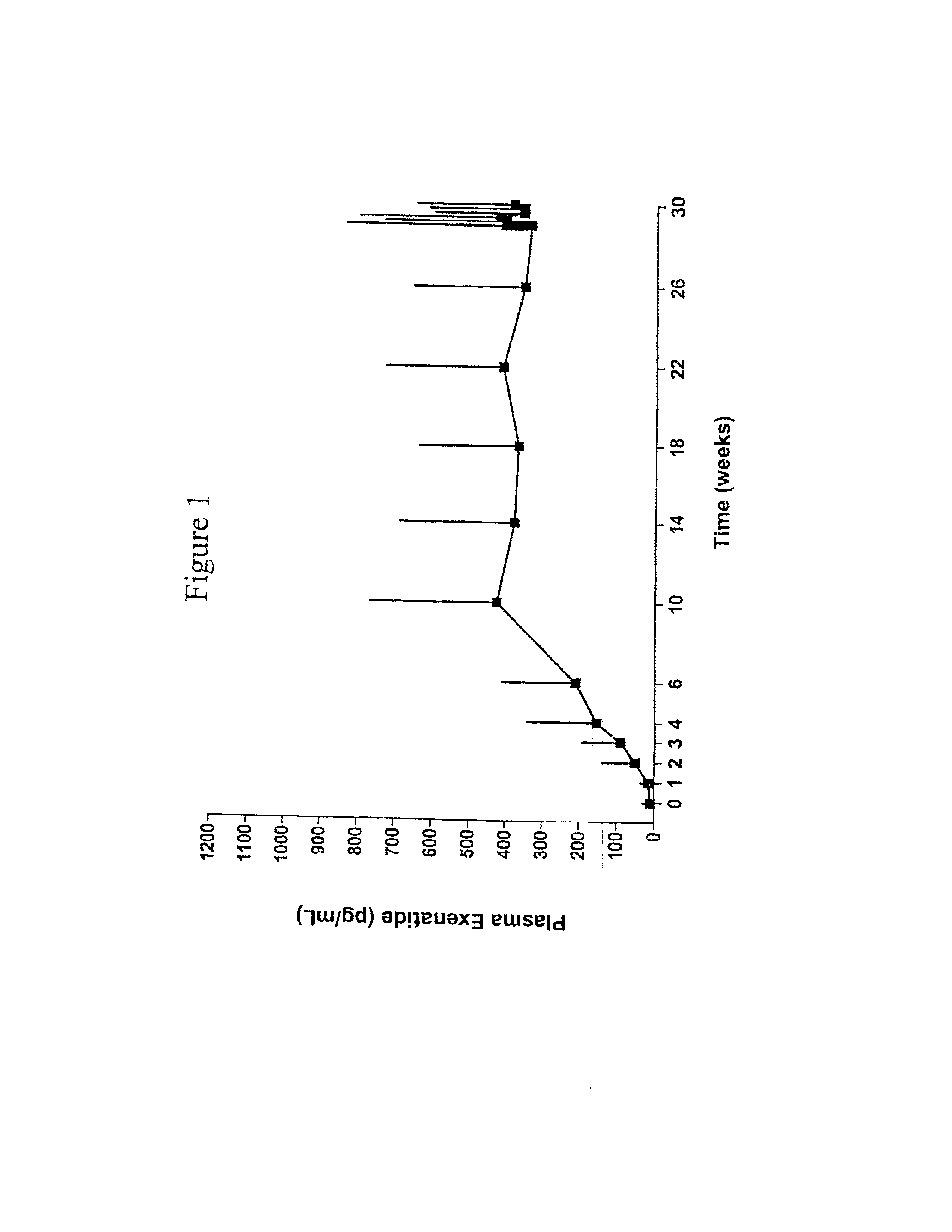
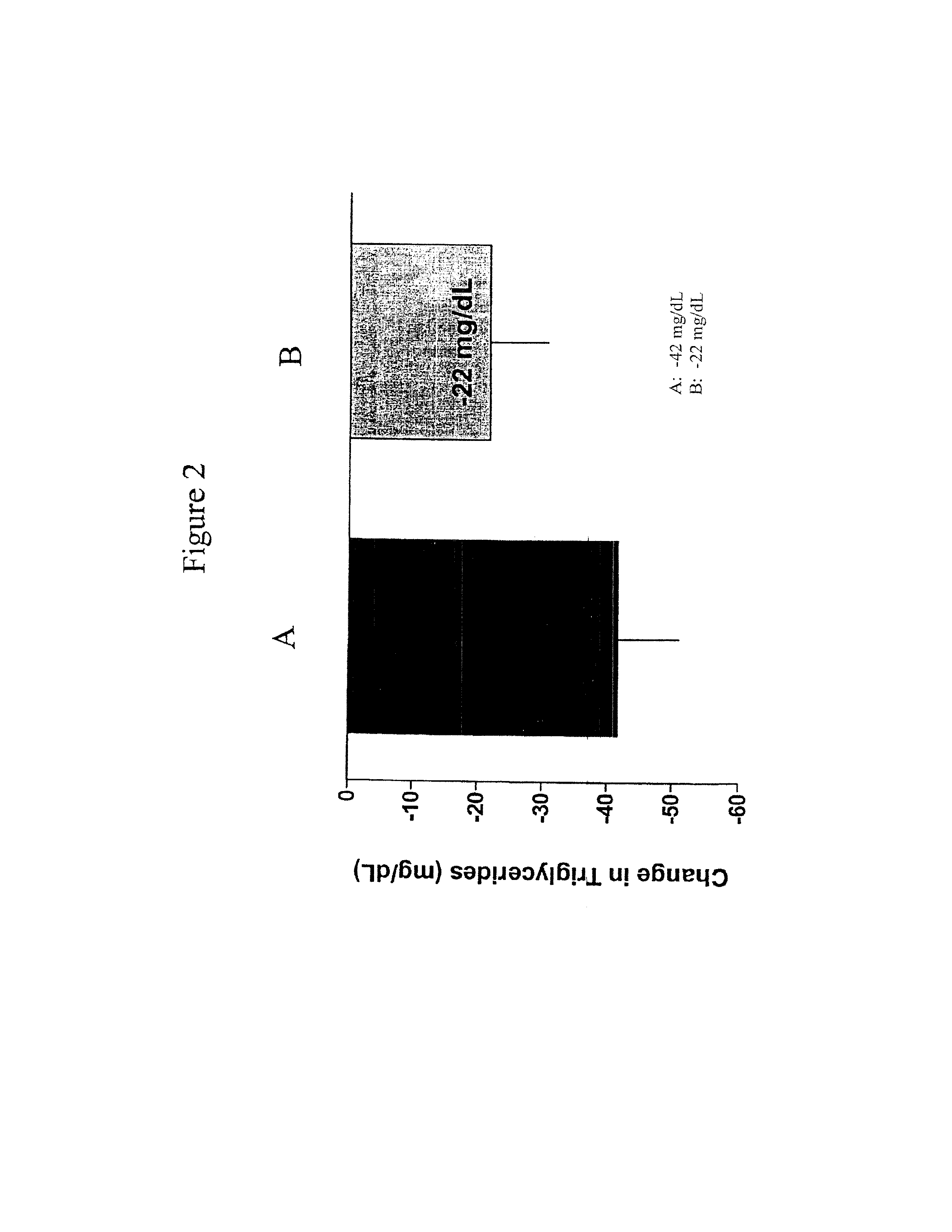
Provided herein are pharmaceutical formulations containing exendins, exendin agonists, or exendin analog agonists that are administered at therapeutic plasma concentration levels over a sustained period of time to lower total cholesterol levels; to lower LDL-cholesterol levels; to lower triglyceride levels; to treat dyslipidemia; to treat and slow the progression of atherosclerosis; and to treat, prevent, and reduce the risk of heart attacks and strokes in patients. In the pharmaceutical formulations and methods of the invention, the exendin may be exendin-4, an exendin-4 agonist, or an exendin-4 analog agonist. The pharmaceutical formulations may be polymer-based pharmaceutical formulations that may be administered once weekly. An exemplary pharmaceutical formulation comprises 5% (w / w) of exenatide, about 2% (w / w) of sucrose, and about 93% (w / w) of a poly(lactide-co-glycolide) polymer, wherein the poly(lactide-co-glycolide) polymer is in the form of microshperes encapsulating the exenatide.
Owner:ASTRAZENECA PHARMA LP
Glucagon-like peptide-1 derivatives and their pharmaceutical use
The invention relates to protracted Glucagon-Like Peptide-1 (GLP-1) derivatives and therapeutic uses thereof. The GLP-1 derivative of the invention comprises a modified GLP-1(7-37) sequence having a total of 2-12 amino acid modifications, including Glu22 and Arg26, and being derivatised with an albumin binding residue or pegylated in position 18, 20, 23, 30, 31, 34, 36, 37, or 39. These compounds are useful in the treatment or prevention of diabetes type 2 and related diseases. The compounds are potent, stable, have long half-lives, a high affinity of binding to albumin, and / or a high affinity of binding to the extracellular domain of the GLP-1 receptor (GLP-1R), all of which is of potential relevance for the overall aim of achieving long-acting, stable and active GLP-1 derivatives with a potential for once weekly administration.
Owner:NOVO NORDISK AS
Methods of sustaining dietary ketosis and its effects on lipid profile
ActiveUS20170172969A1Rapid and sustained elevationGood for healthMetabolism disorderOrganic chemistry methodsLipid formationDisease
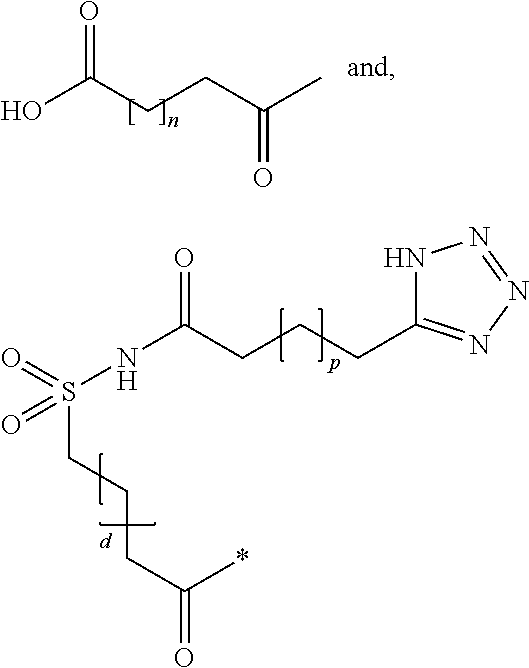
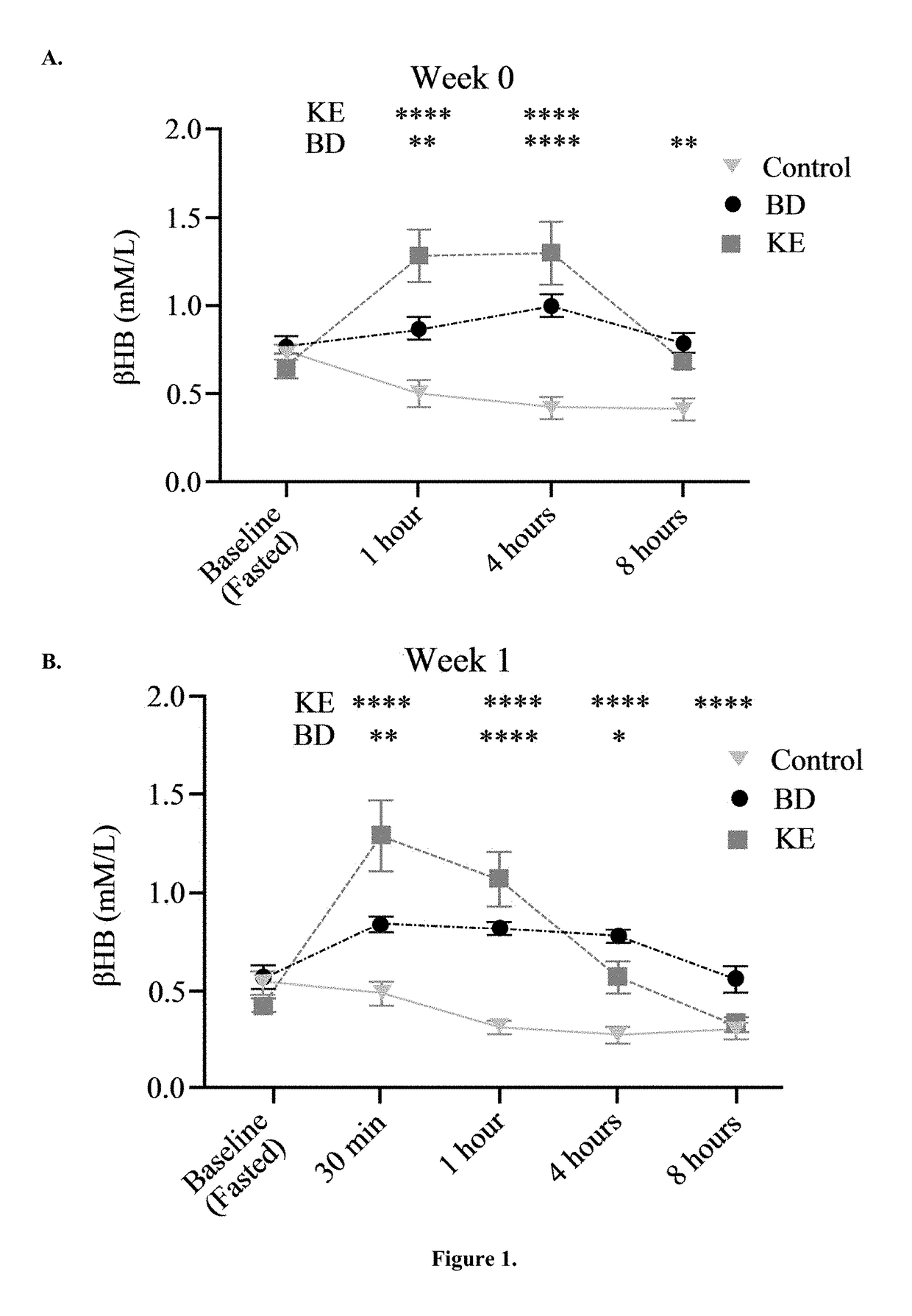
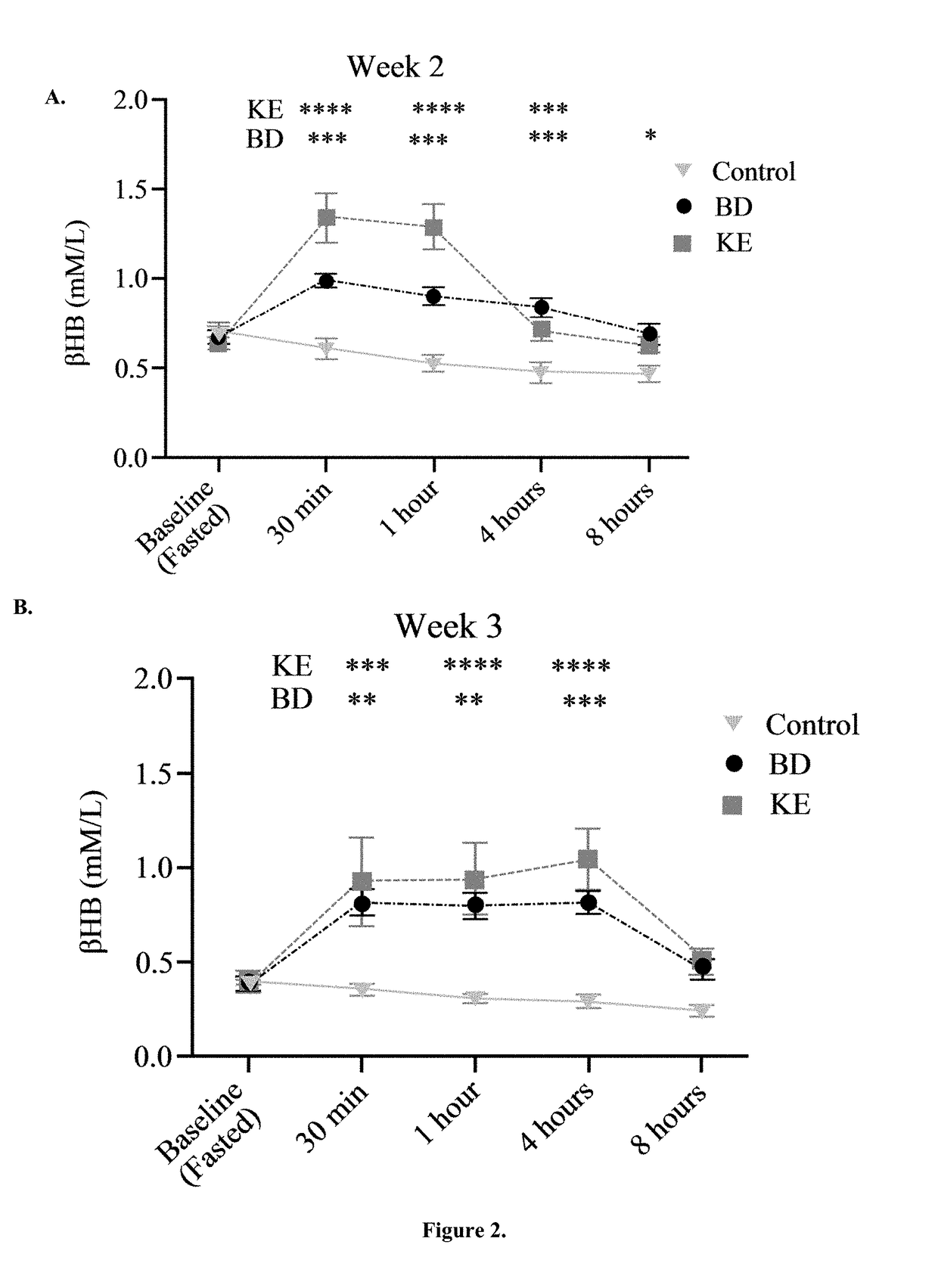
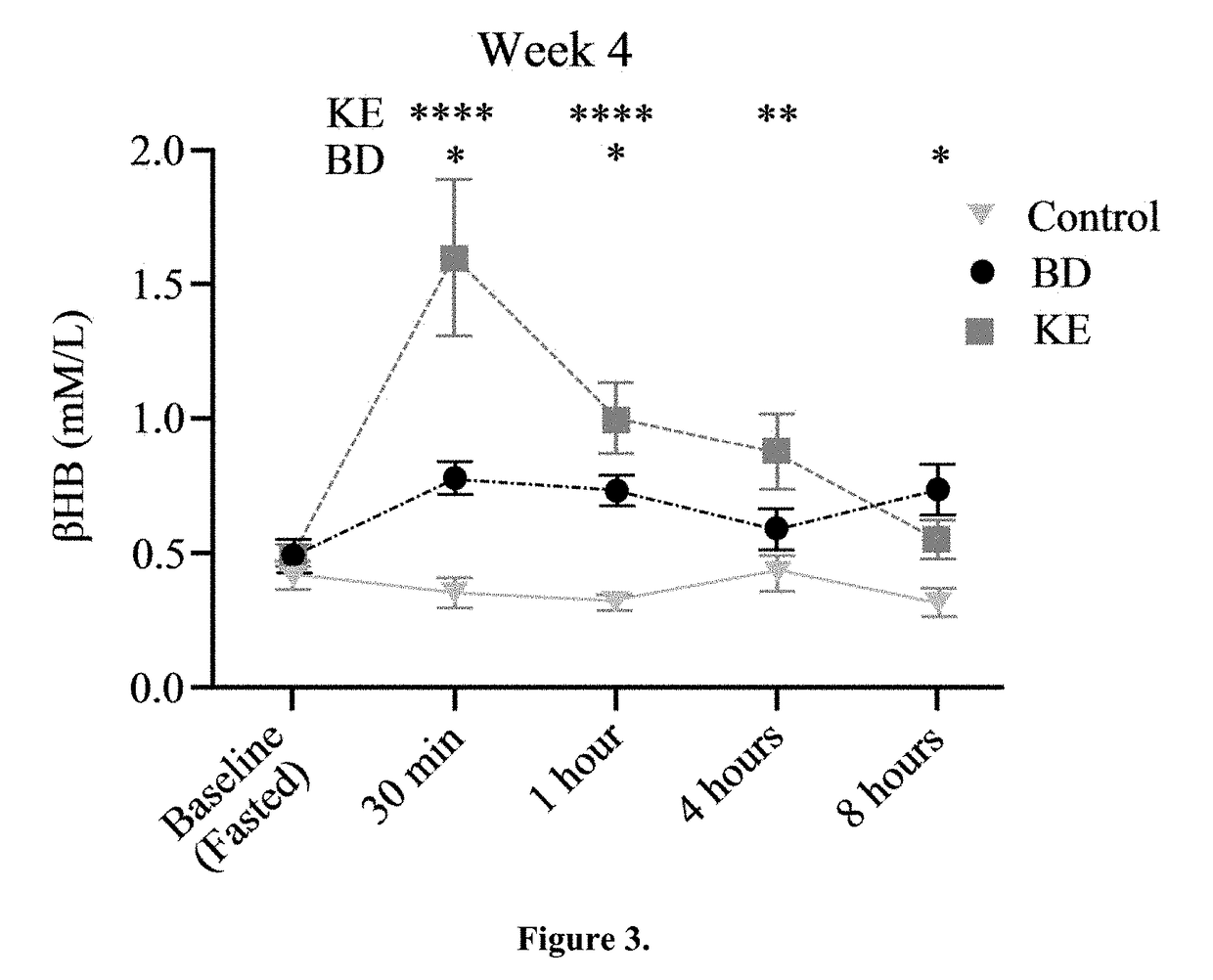
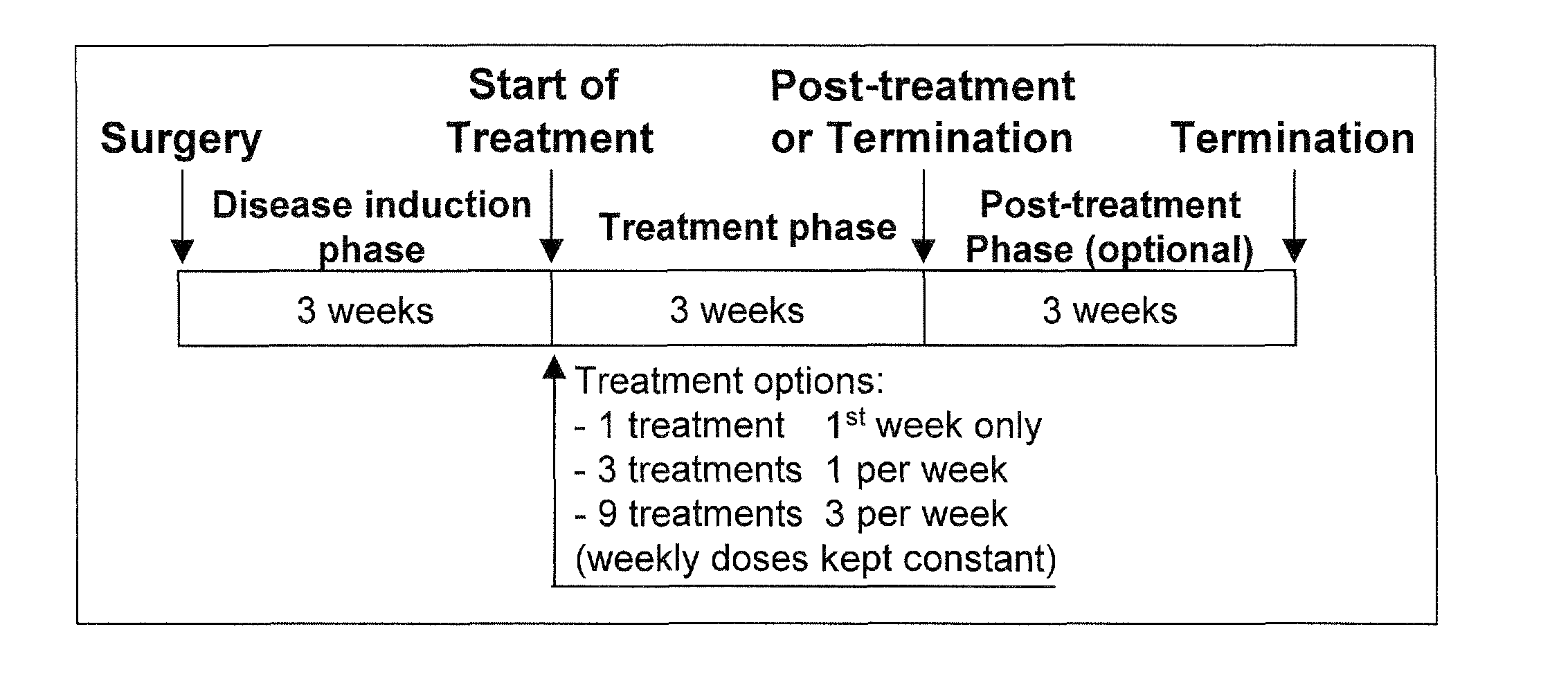
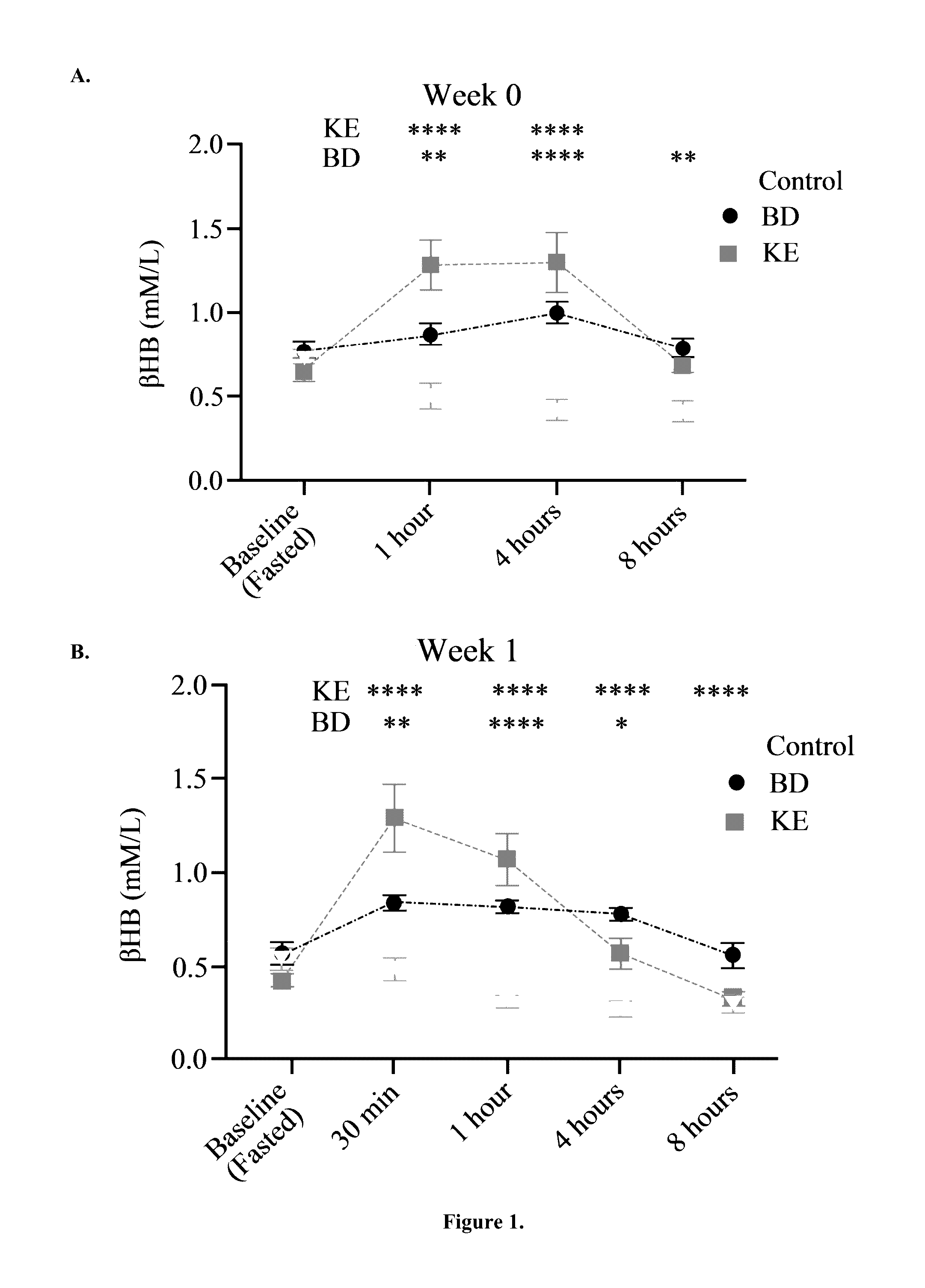
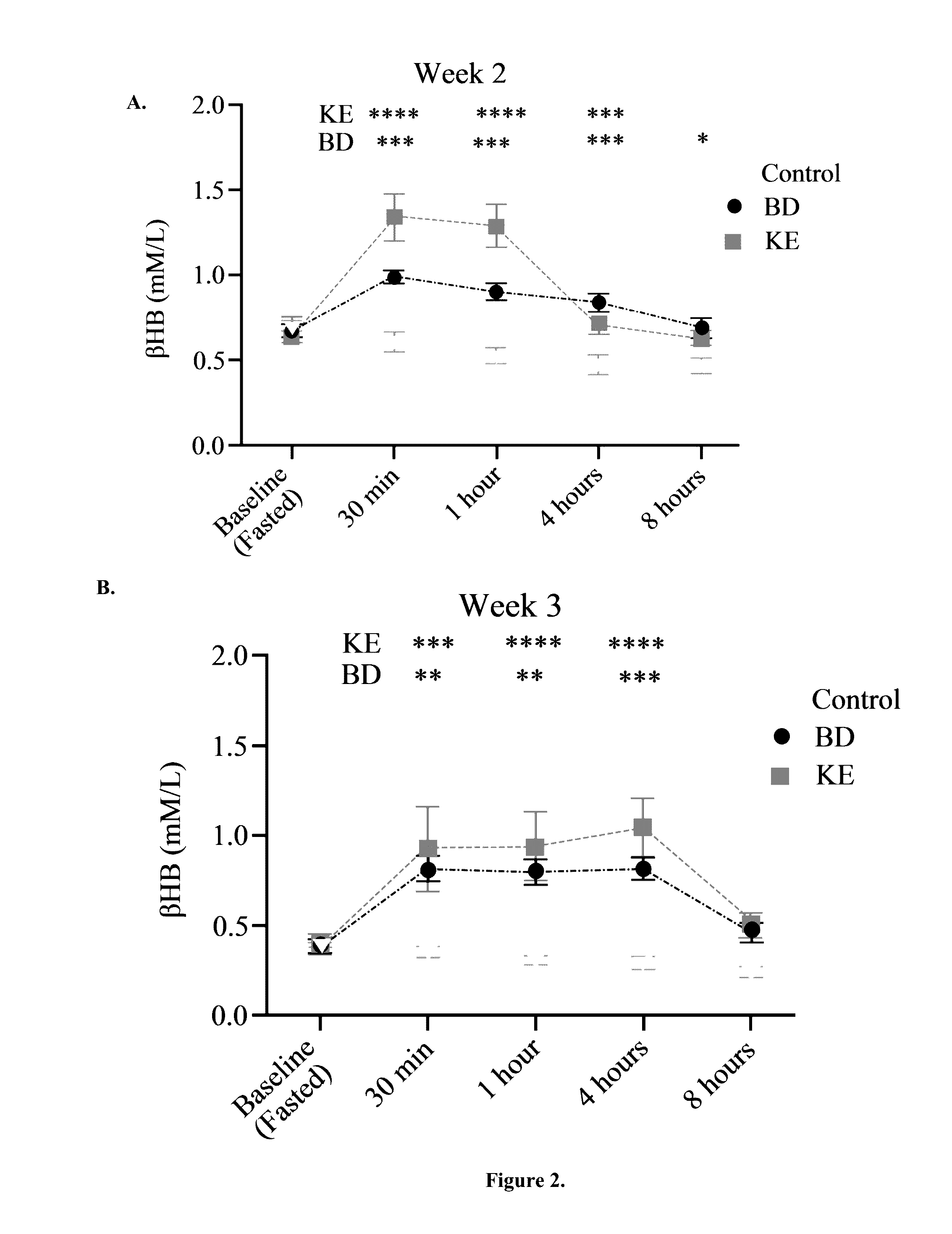
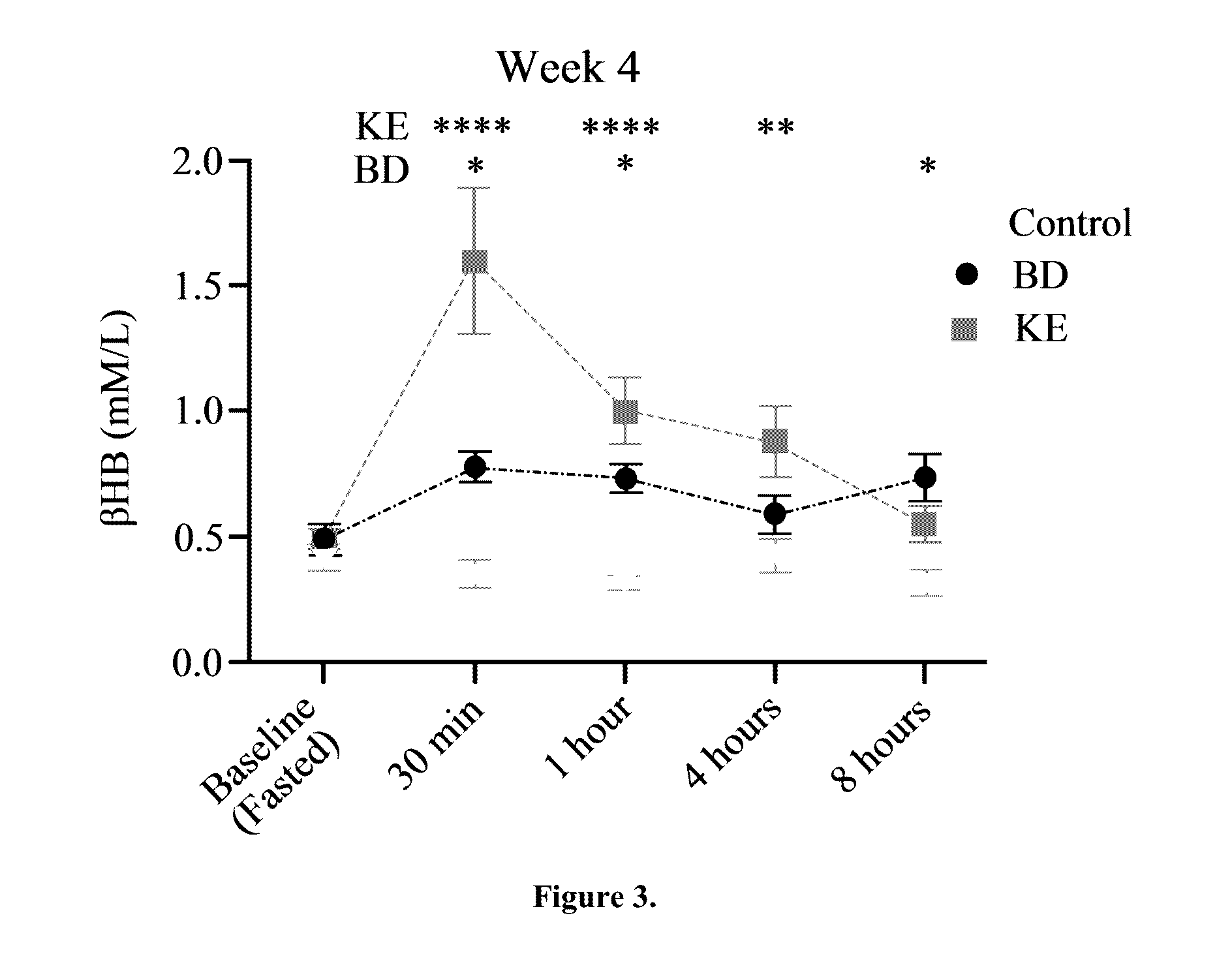
The ketogenic diet (KD) has therapeutic implications in many disease states. It was hypothesized ketone precursor supplementation would elevate blood ketone levels to therapeutic ranges (2-7 mM) without need for dietary restriction. The effects of ketogenic agents were tested on blood glucose, ketones, and lipids with a 28-day dose escalation study in male Sprague-Dawley rats: R,S-1,3-Butandiol (BD), acetoacetate ketone ester (KE), and control (H2O) (n≧8). Days 1-28, rats received a daily 5 g / kg intragastric gavage, based on previous toxicology studies. Once weekly, whole blood samples (10 μl) were acquired for analysis of glucose and βHB at 0, 0.5, 1, 4, 8, and 12 hours after test substance administration, or until βHB returned to baseline. At day 1 and 28, 10 μL of whole blood were collected to measure triglycerides, total cholesterol, and HDL concentration. Significant elevation of blood ketone was observed with a significant inverse relationship with blood glucose for the duration of the experiment. There were no significant changes in the lipid panel for any of the substances. There were significant reductions in body weight when animals were treated with either BD or KE as compared to control.
Owner:UNIV OF SOUTH FLORIDA
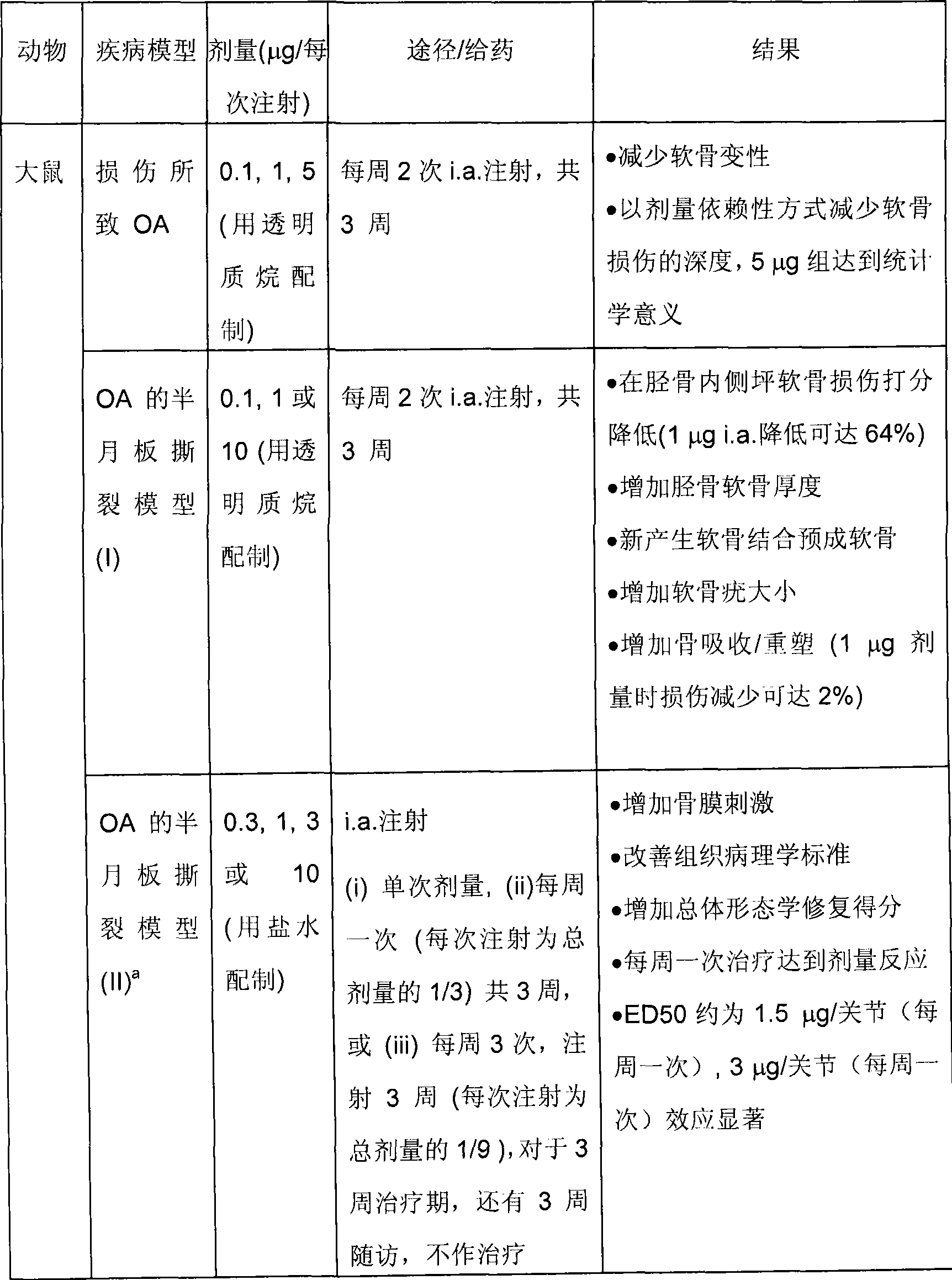
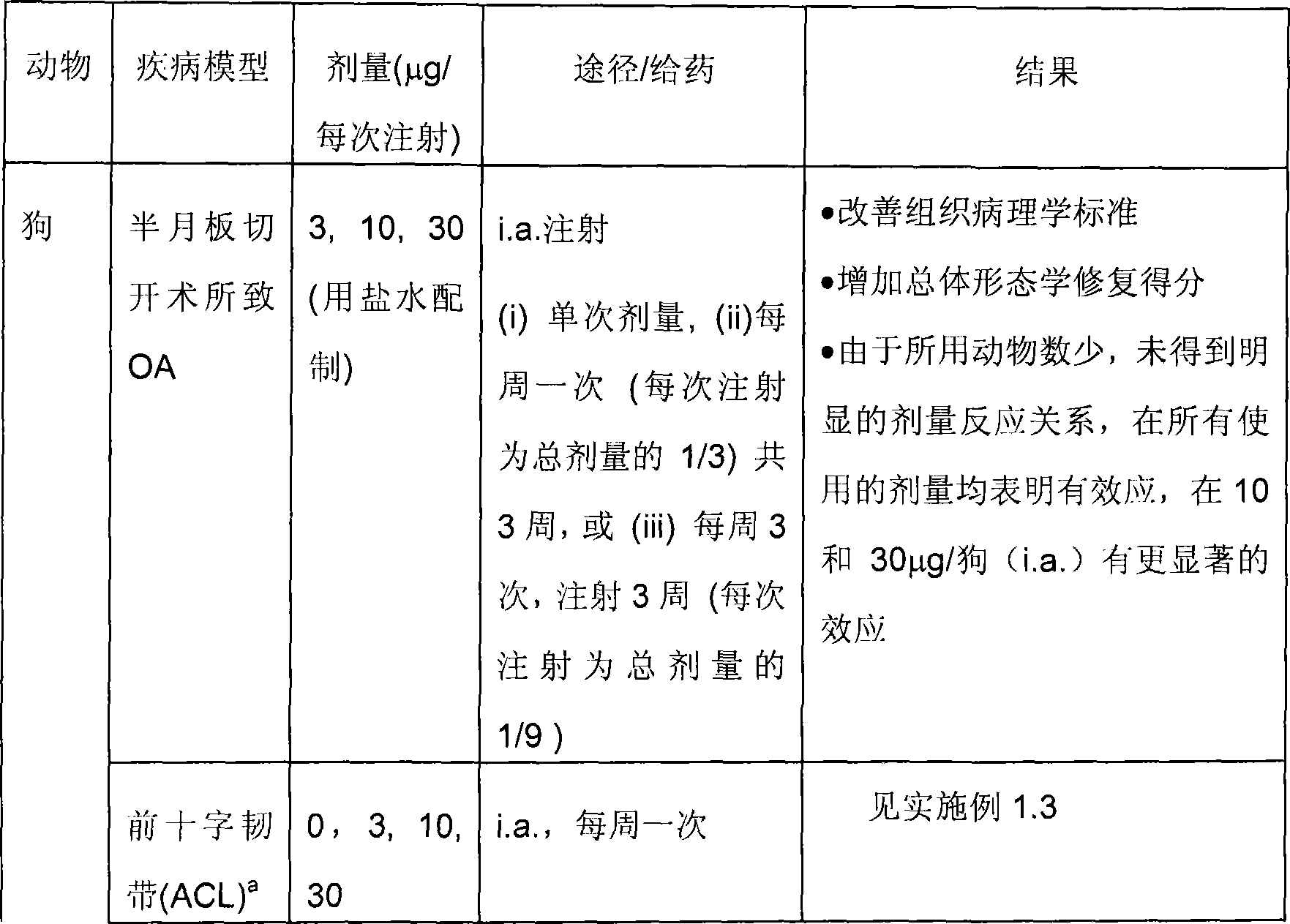
Treatment of cartilage disorders with fgf-18
This invention concerns the treatment of cartilage disorder and osteoarthritis in particular. More specifically, it relates to the use of FGF-18 in treatment regimens and for the manufacture of a medicament for the treatment of patients having a cartilage disorder such as osteoarthritis, such as for example knee osteoarthritis or secondary hip osteoarthritis. Specifically provided is a preferred treatment scheme comprising once weekly administration of an FGF-18 compound per treatment cycle.
Owner:ARES TRADING SA
Truncated glp-1 derivaties and their therapeutical use
InactiveUS20100292133A1Nervous disorderPeptide/protein ingredientsAmino acid substitutionExtracellular Structure
The invention relates to truncated GLP-1 analogues, in particular a GLP-1 analogue which is a modified GLP-1(7-35) (SEQ ID No 1) having: i) a total of 2, 3, 4, 5 6, 7, 8, or 9 amino acid substitutions as compared to GLP-1(7-35), including a) a Glu residue at a position equivalent to position 22 of GLP-1(7-35), and b) an Arg residue at a position equivalent to position 26 of GLP-1(7-35); as well as derivatives thereof, and therapeutic uses and compositions. These analogues and derivatives are highly potent, have a good binding affinity to the GLP-1 receptor, also to the extracellular domain of the GLP-1 receptor, which is of potential relevance achieving long-acting, stable GLP-1 compounds with a potential for once weekly administration.
Owner:NOVO NORDISK AS
Peptides derivatized with a-b-c-d- and their therapeutical use
The invention relates to protracted peptide derivatives such as Glucagon-Like Peptide-1 (GLP-1), exendin-4, and analogues thereof, as well as therapeutic uses thereof. The peptide derivative of the invention comprises a peptide wherein at least one amino acid residue is derivatized with A-B-C-, or A-B-C-D-. These compounds are useful in the treatment or prevention of diabetes type 2 and related diseases. The compounds are potent, have a low ratio of binding affinity to the GLP-1 receptor in the presence of high / low albumin concentrations, have long half-lives, and have a high affinity of binding to albumin, all of which is of potential relevance for the overall aim of achieving long-acting, stable and active GLP-1 derivatives with a potential for once weekly administration.
Owner:NOVO NORDISK AS
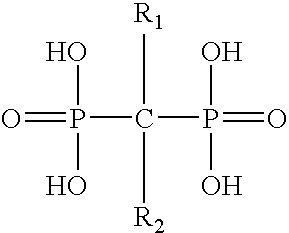
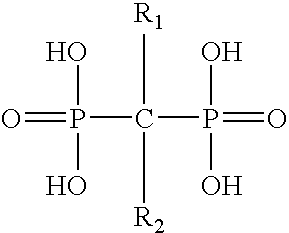
Pharmaceutical formulation for oral delivery of bisphosphates
InactiveUS20050182028A1Minimizing potential esophageal irritationMinimize irritationBiocidePhosphorous compound active ingredientsDosing regimenPharmacy
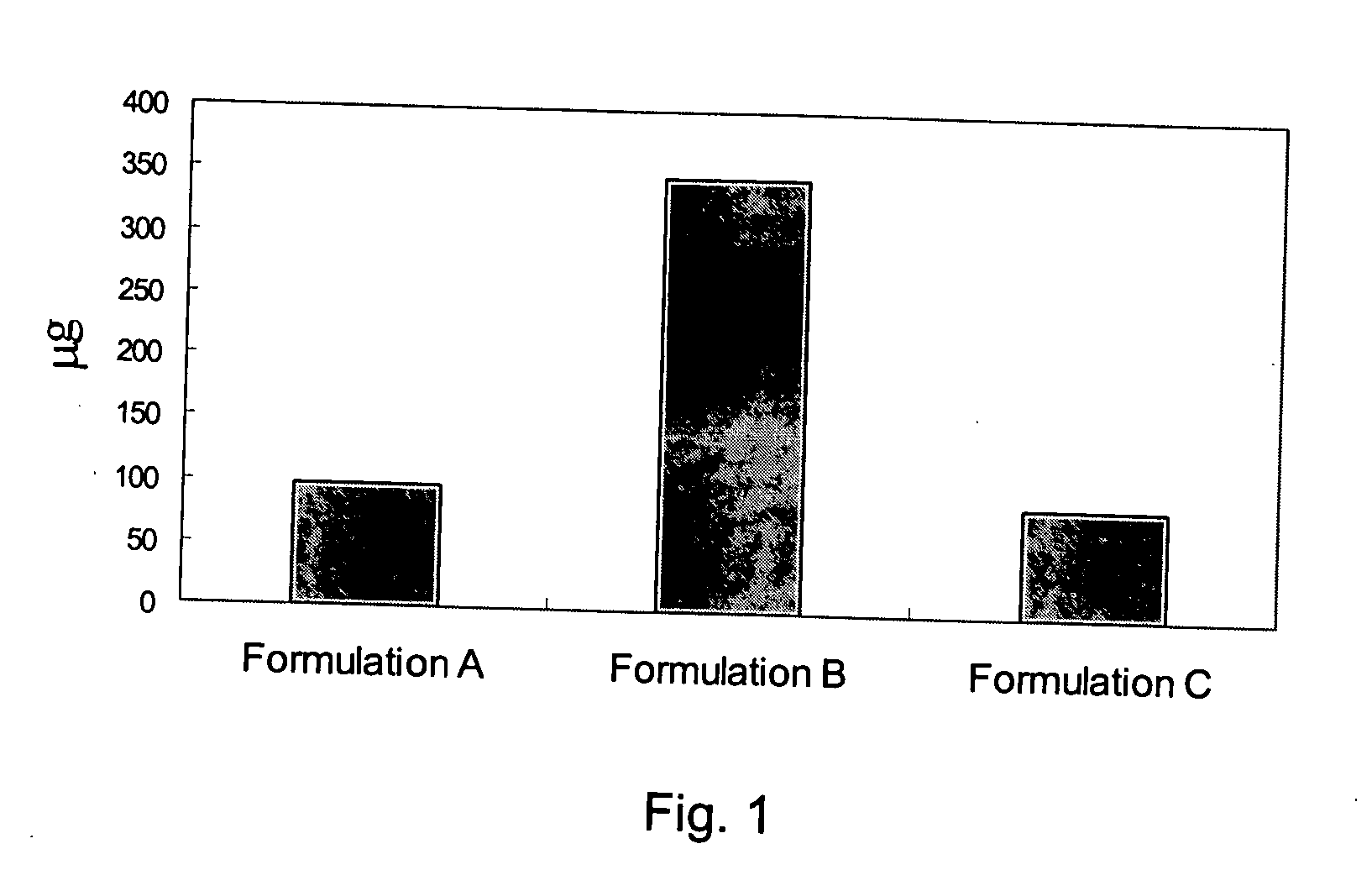
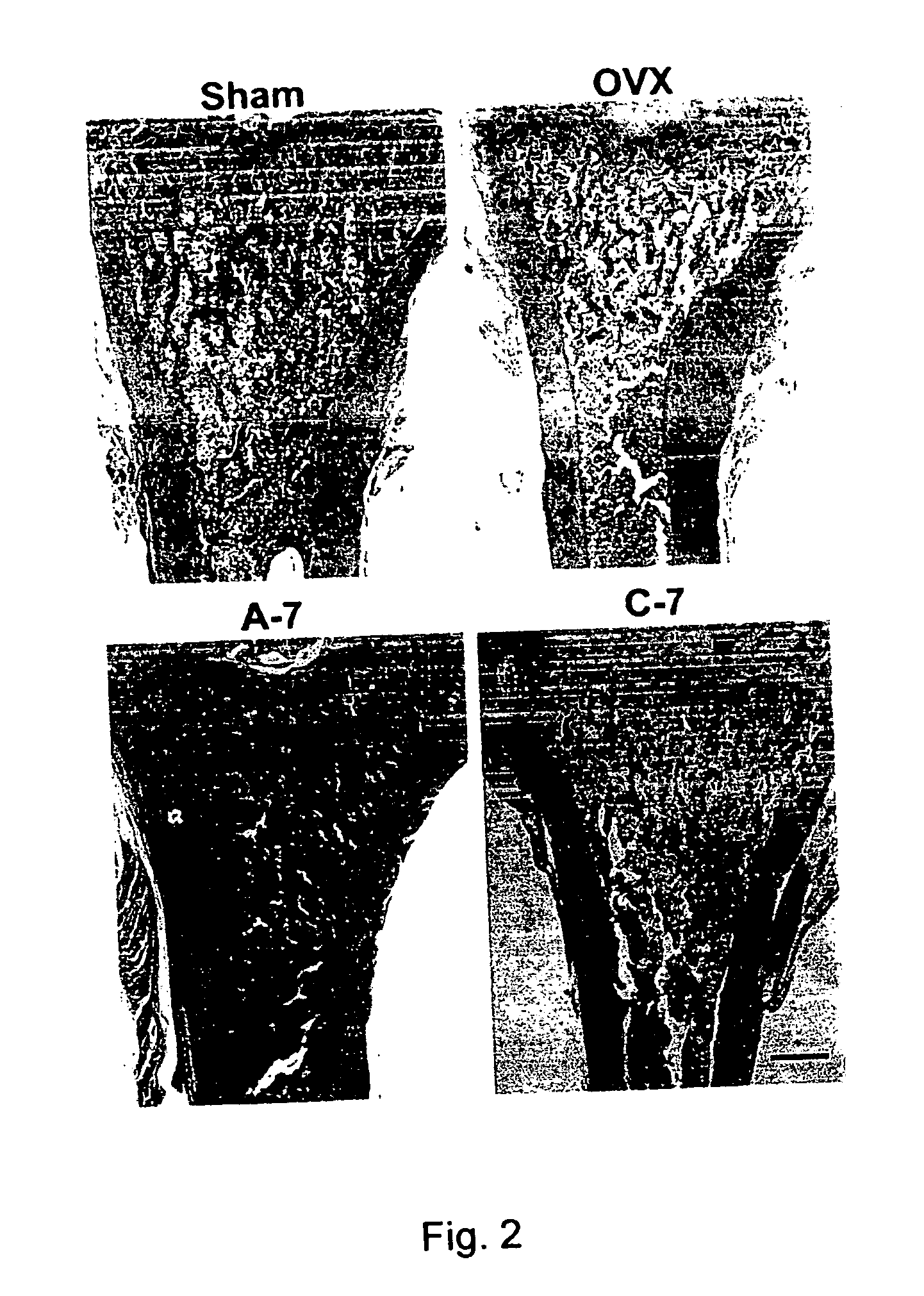
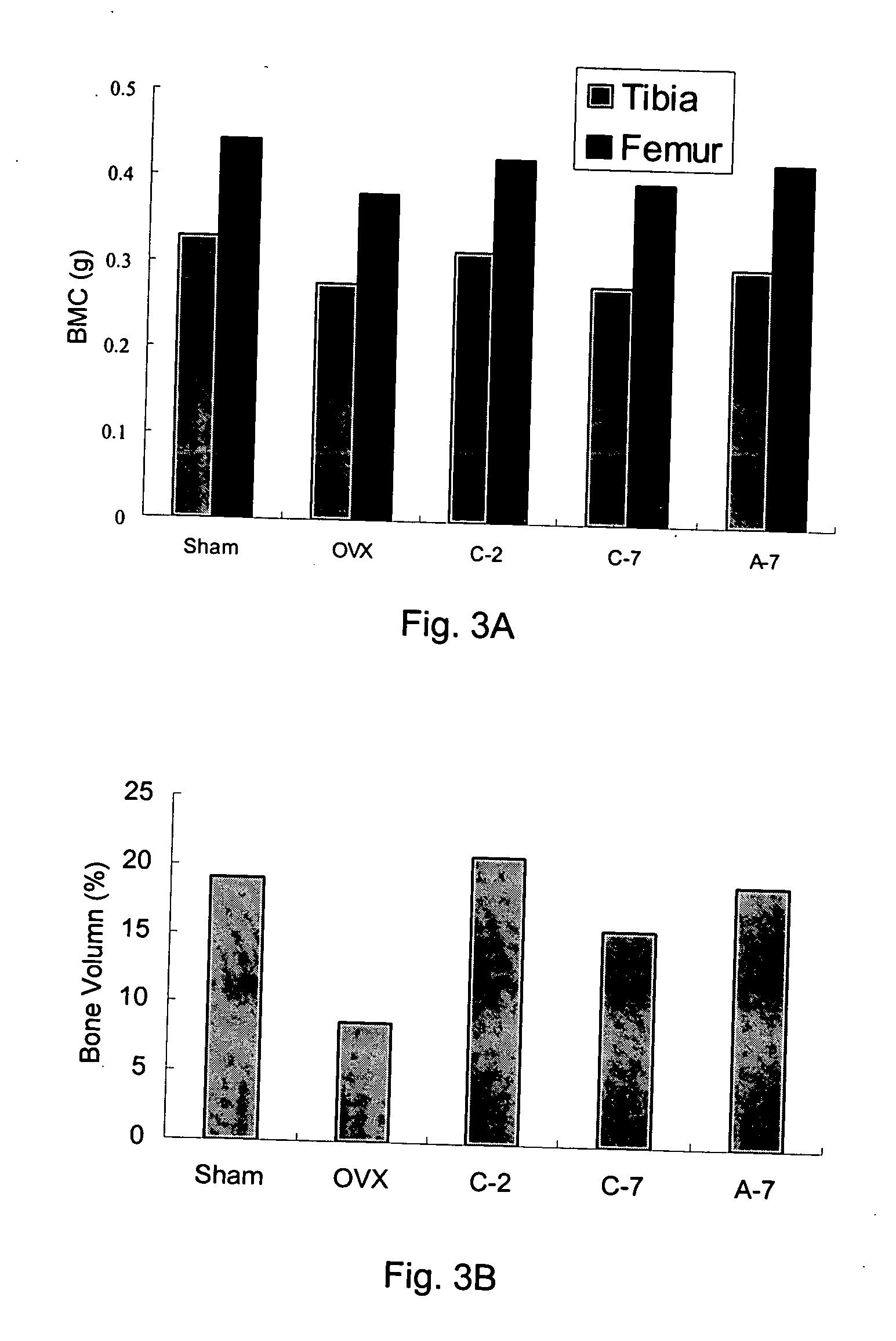
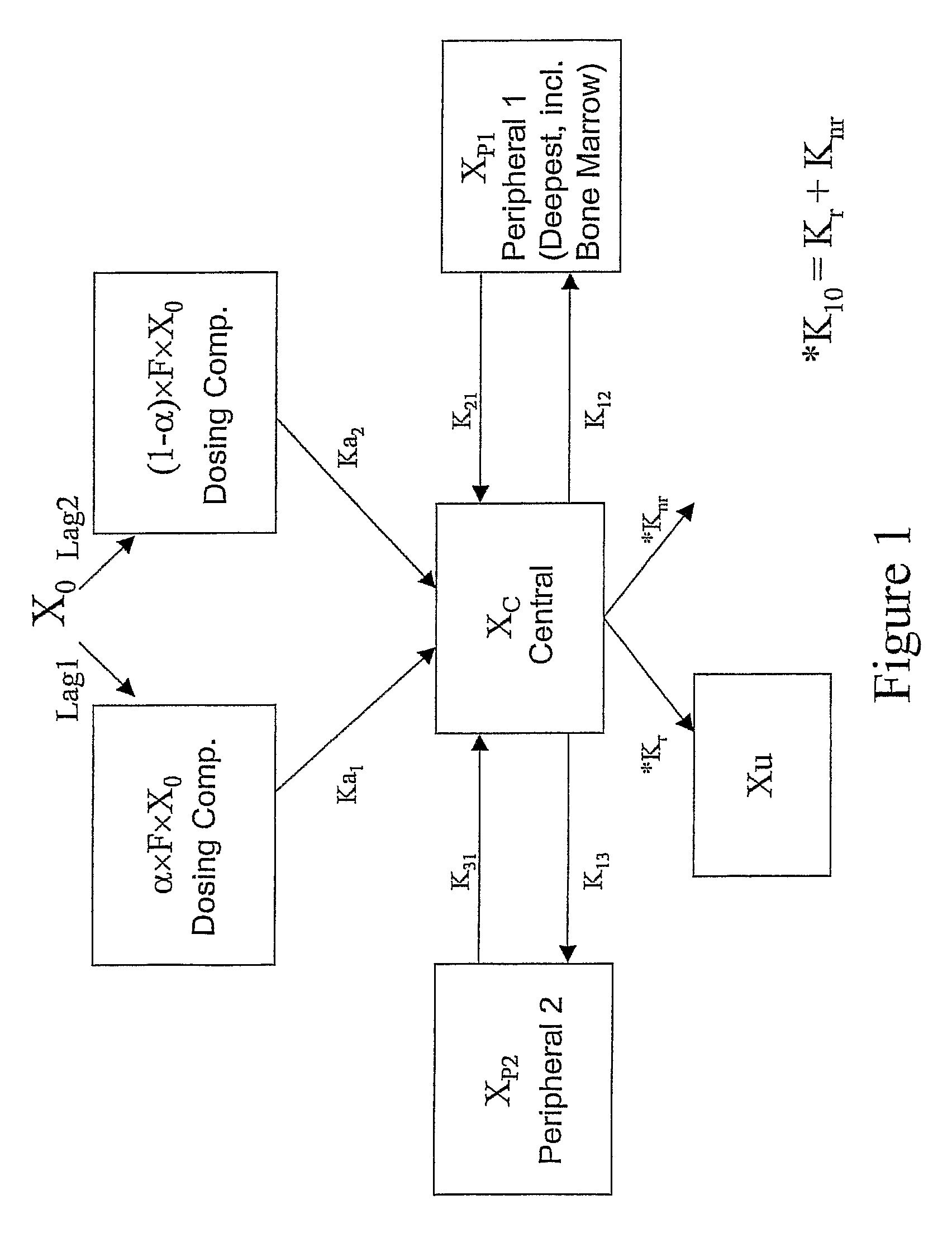
The present invention discloses a method for treating or preventing a bone disorder in a mammal in need thereof comprising orally administering to said mammal a pharmaceutically effective amount of a pharmaceutical composition of at least one bisphosphonate, or a pharmaceutically acceptable salt or esters thereof, and at least one aminoalky methacrylate copolymer, according to a dosing schedule having a dosing interval selected from once-weekly dosing, twice-monthly dosing, once-monthly, once-quarterly and once-annually dosing. The present invention further discloses a method for treating or preventing a bone disorder in a mammal in need thereof comprising continuously orally administering a unit dosage per-day to said mammal in a short time for a long time therapy.
Owner:CHEN CHIH MING JAMES
Low dose therapy for treating viral infections
InactiveUS7741334B2Patient compliance is goodBiocideAntiviralsReverse transcriptaseImmunodeficiency virus
A method of treating viral infections, particularly Hepatitis B (HBV) and Human Immunodeficiency Virus (HIV), by administering a low dose of Elvucitabine to a patient suffering viral infection is provided herein. The Elvucitabine dosages provided herein for effective anti-viral therapy are approximately 10-fold less than the effective dosages of currently marketed reverse transcriptase inhibitors. The Elvucitabine dosage may be given BID, daily, once every 48 hours, or once weekly. Also provided herein are packaged pharmaceutical formulations comprising Elvucitabine and instructions for treating a viral infection by administering a low BID, daily, once / 48 hour, or weekly dosage of Elvucitabine. The low dose Elvucitabine formulations provided herein have the additional benefit of improving patient compliance with anti-viral therapy.
Owner:ACHILLION PHARMA INC
Exendins To Lower Cholesterol And Triglycerides
InactiveUS20130172250A1Stable stateReduce riskPeptide/protein ingredientsMetabolism disorderDyslipidemiaSucrose
Provided herein are pharmaceutical formulations containing exendins, exendin agonists, or exendin analog agonists that are administered at therapeutic plasma concentration levels over a sustained period of time to lower total cholesterol levels; to lower LDL-cholesterol levels; to lower triglyceride levels; to treat dyslipidemia; to treat and slow the progression of atherosclerosis; and to treat, prevent, and reduce the risk of heart attacks and strokes in patients. In the pharmaceutical formulations and methods of the invention, the exendin may be exendin-4, an exendin-4 agonist, or an exendin-4 analog agonist. The pharmaceutical formulations may be polymer-based pharmaceutical formulations that may be administered once weekly. An exemplary pharmaceutical formulation comprises 5% (w / w) of exenatide, about 2% (w / w) of sucrose, and about 93% (w / w) of a poly(lactide-co-glycolide) polymer, wherein the poly(lactide-co-glycolide) polymer is in the form of microshperes encapsulating the exenatide.
Owner:ASTRAZENECA PHARMA LP
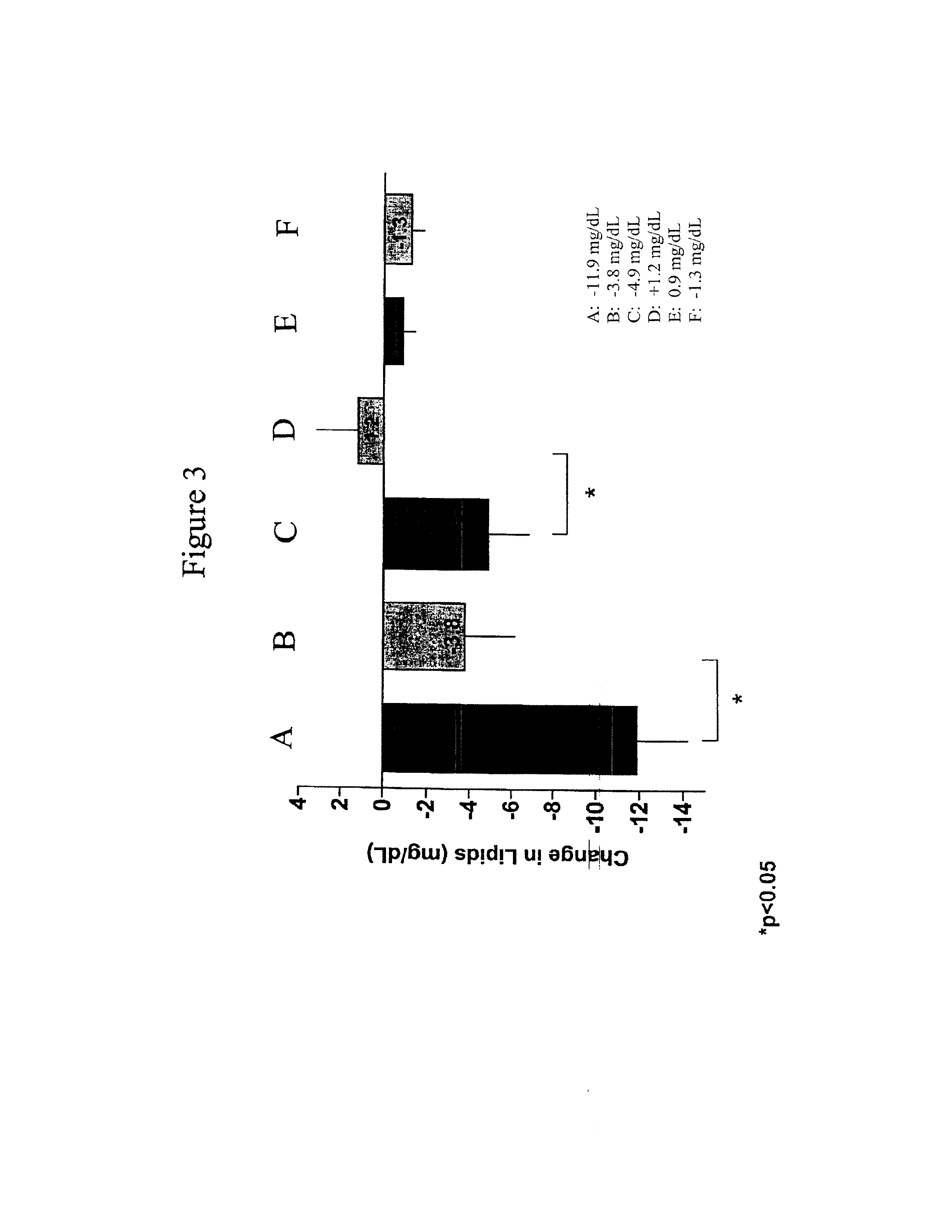
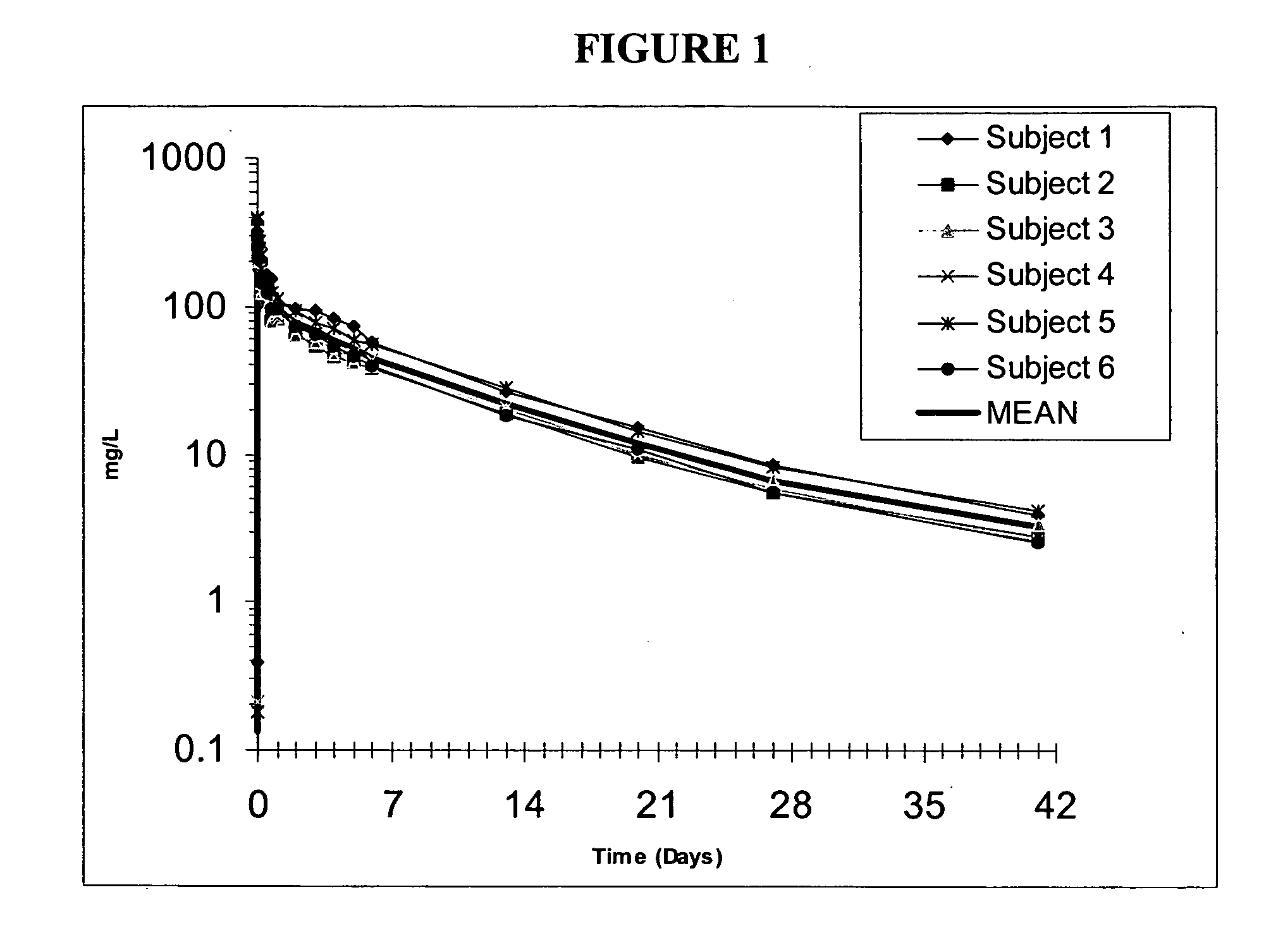
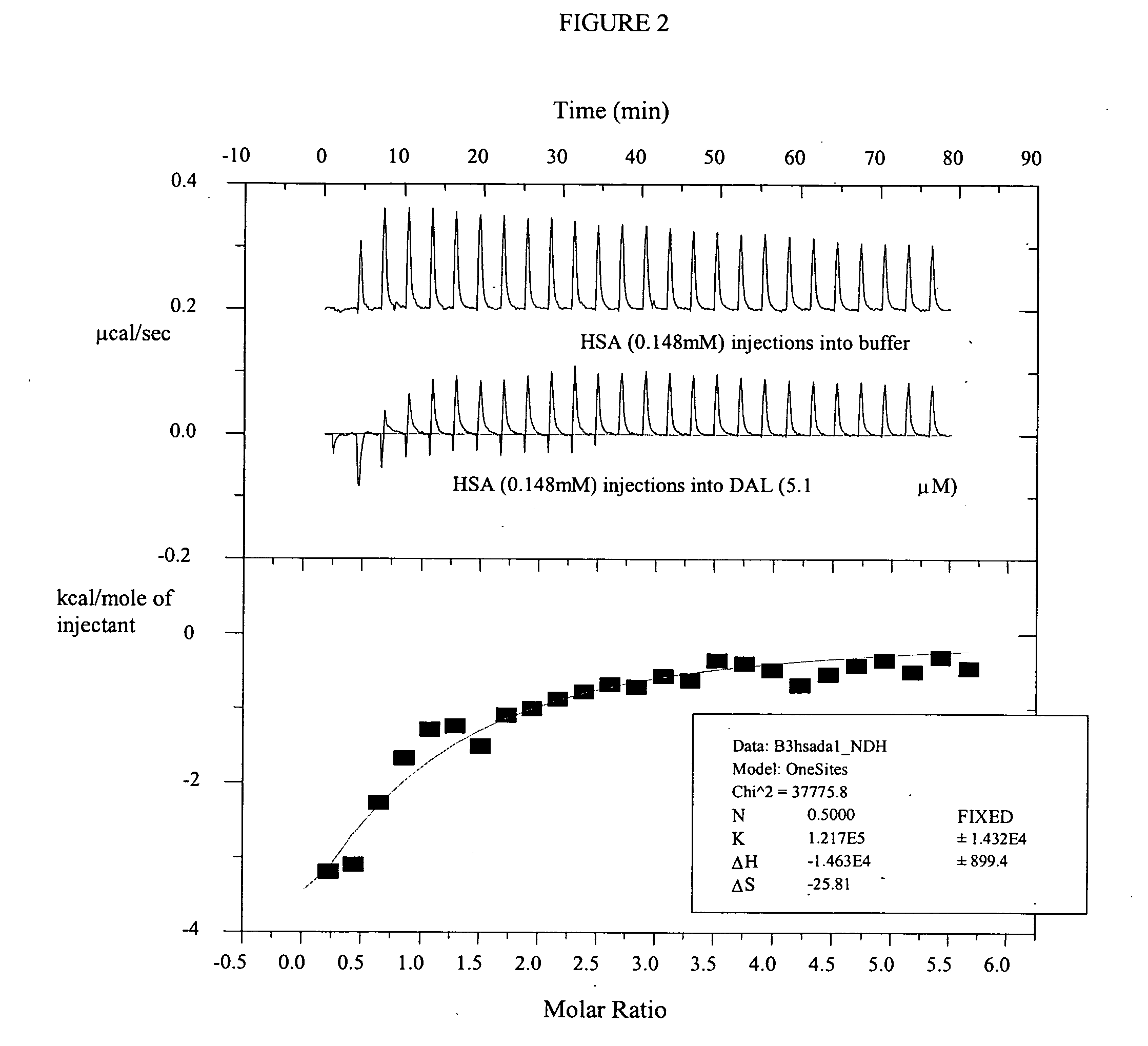
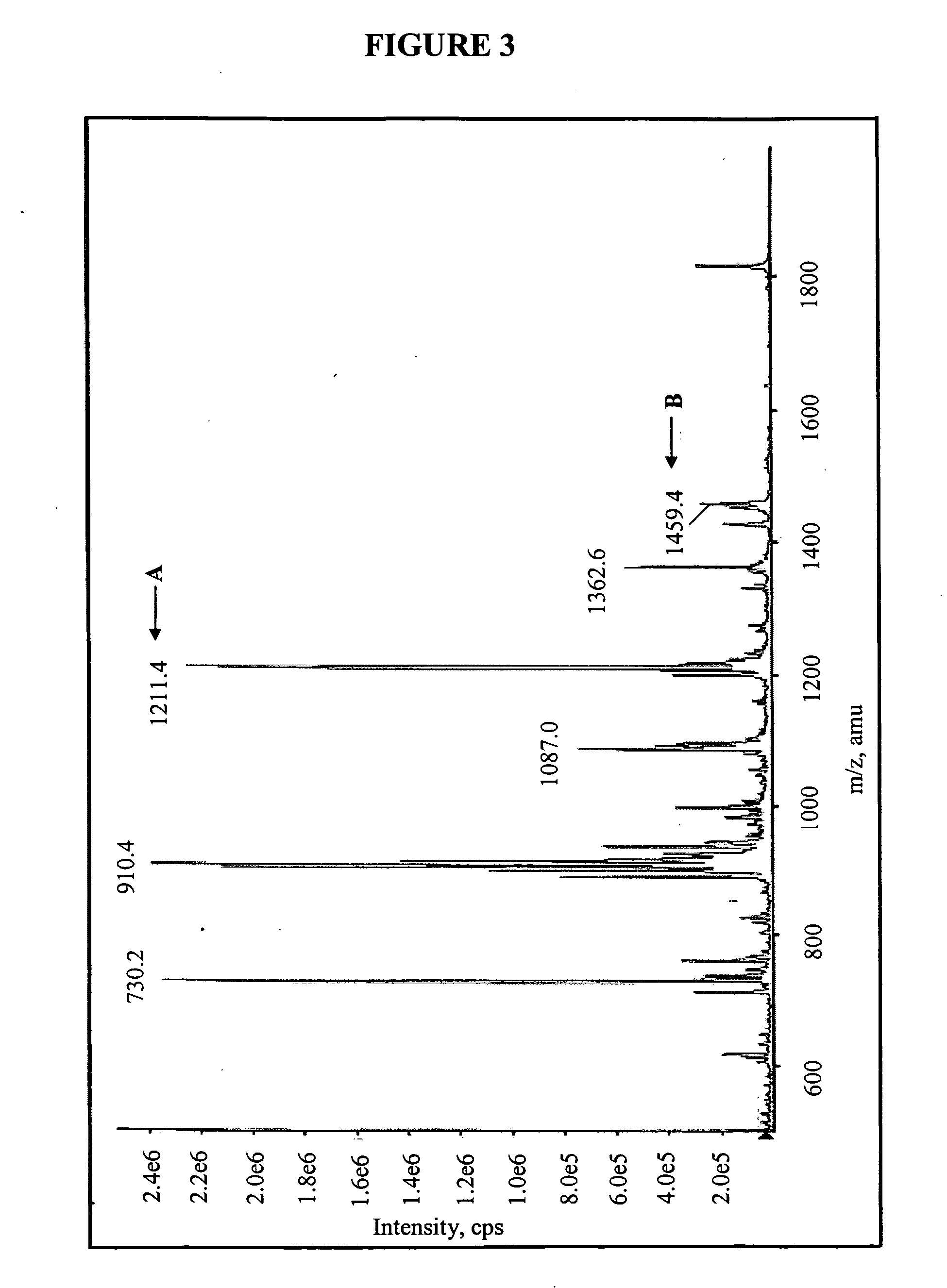
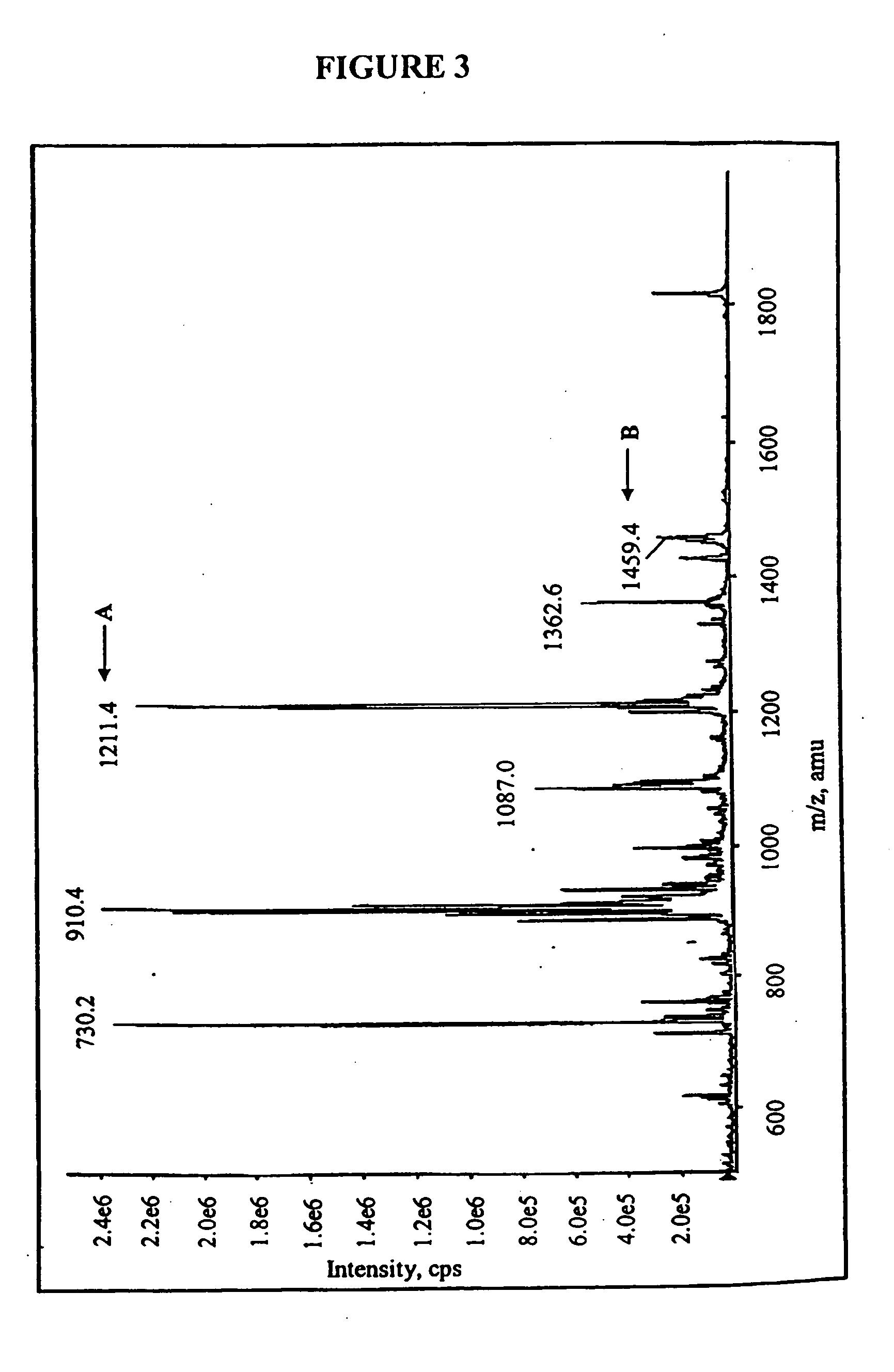
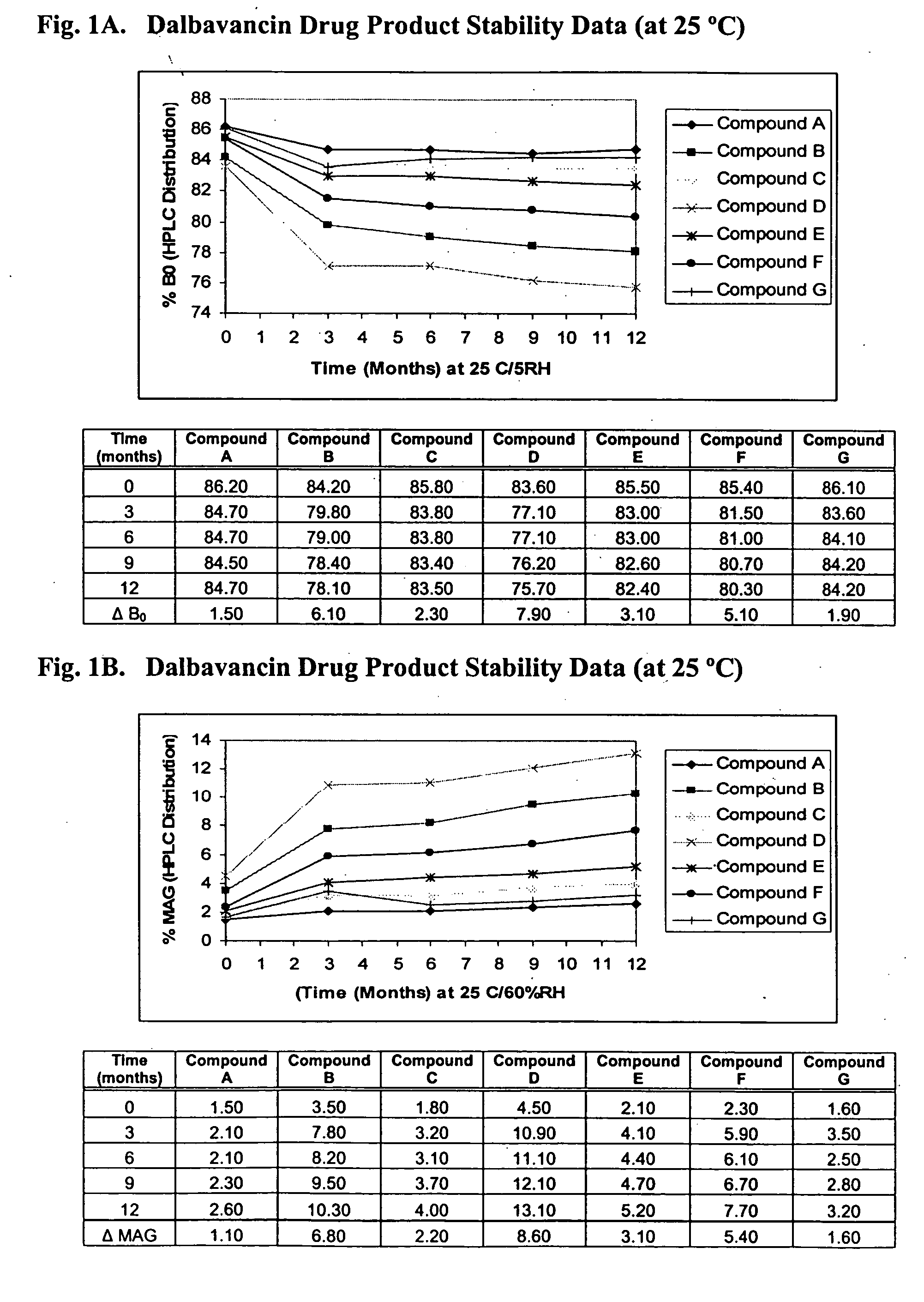
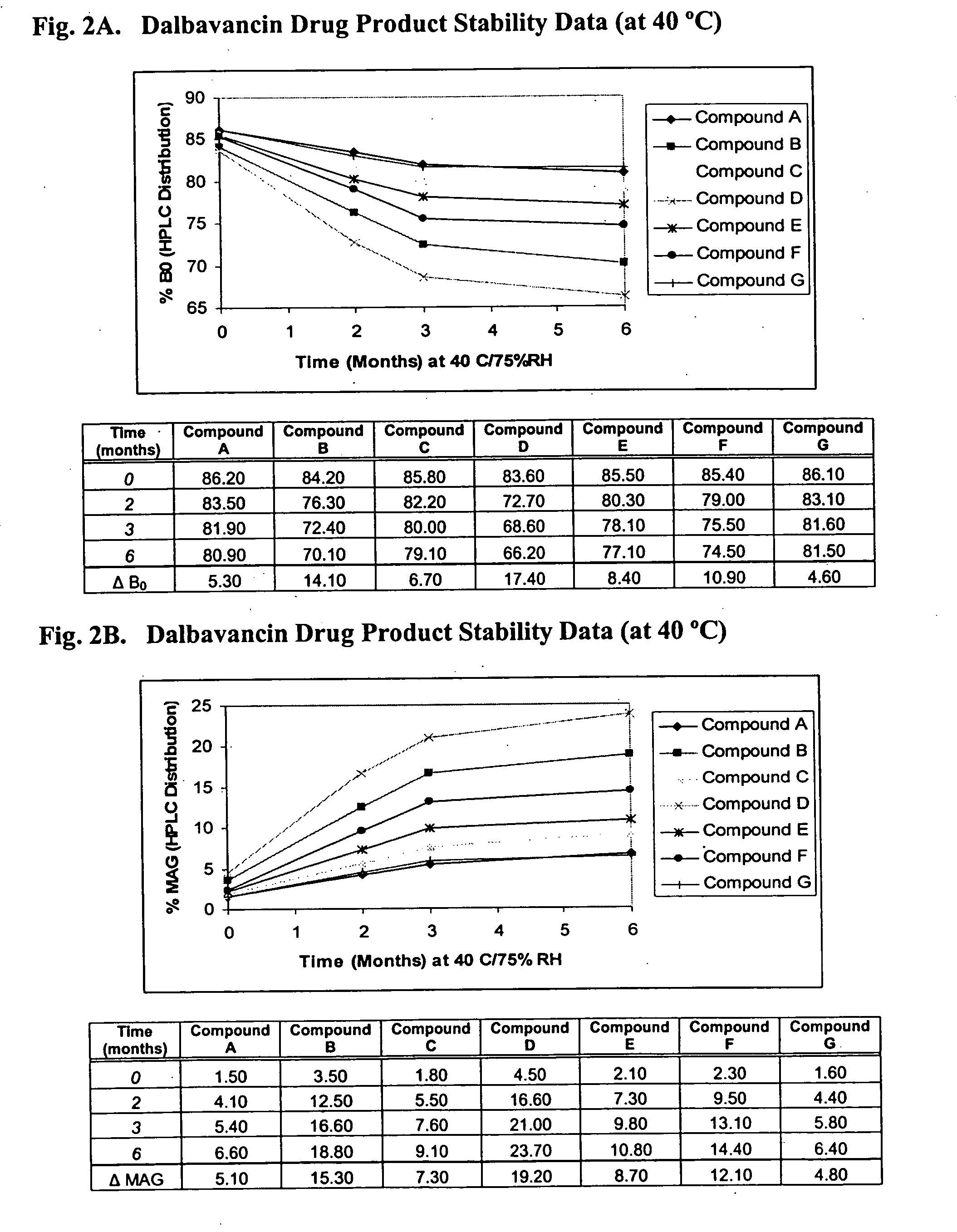
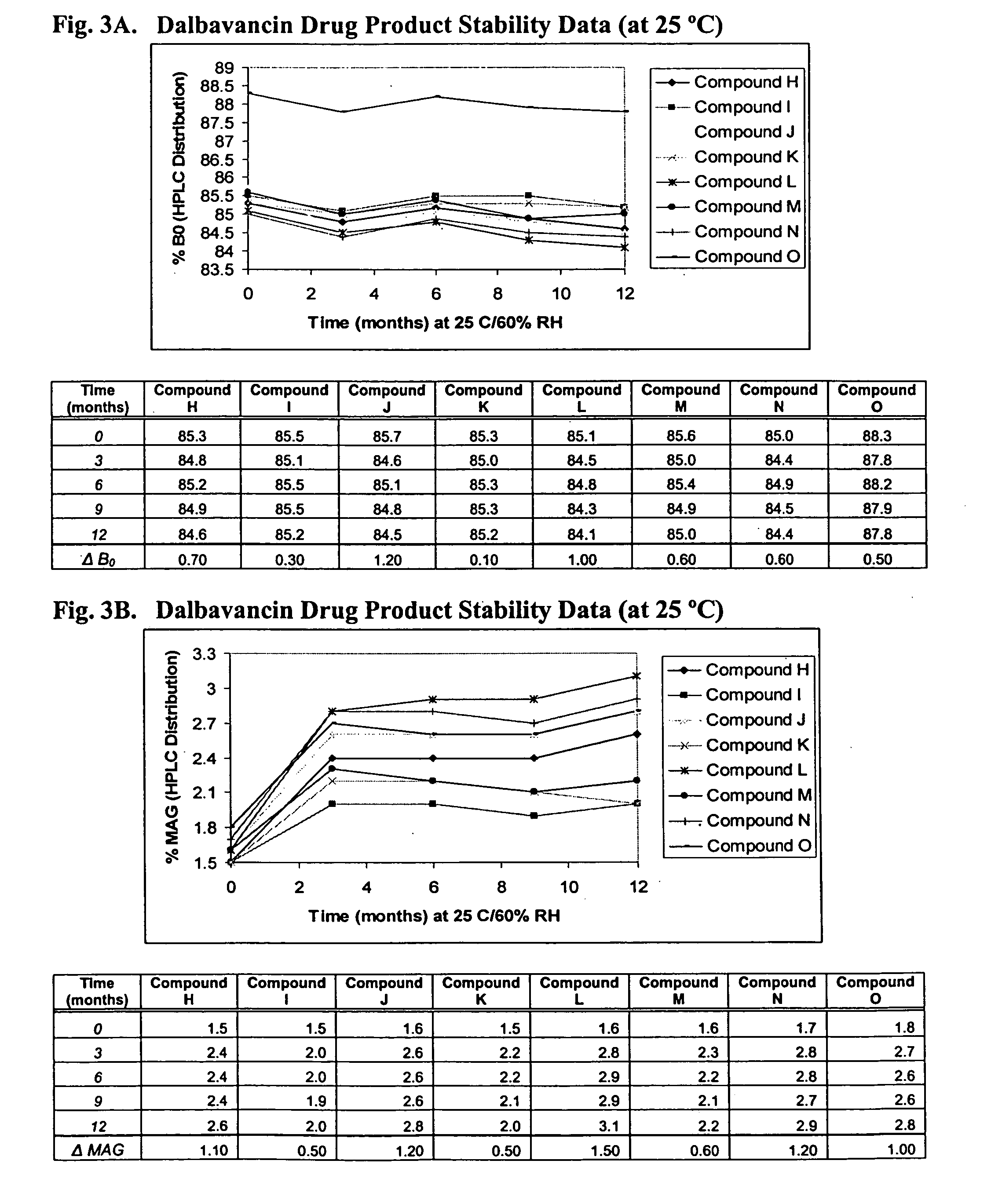
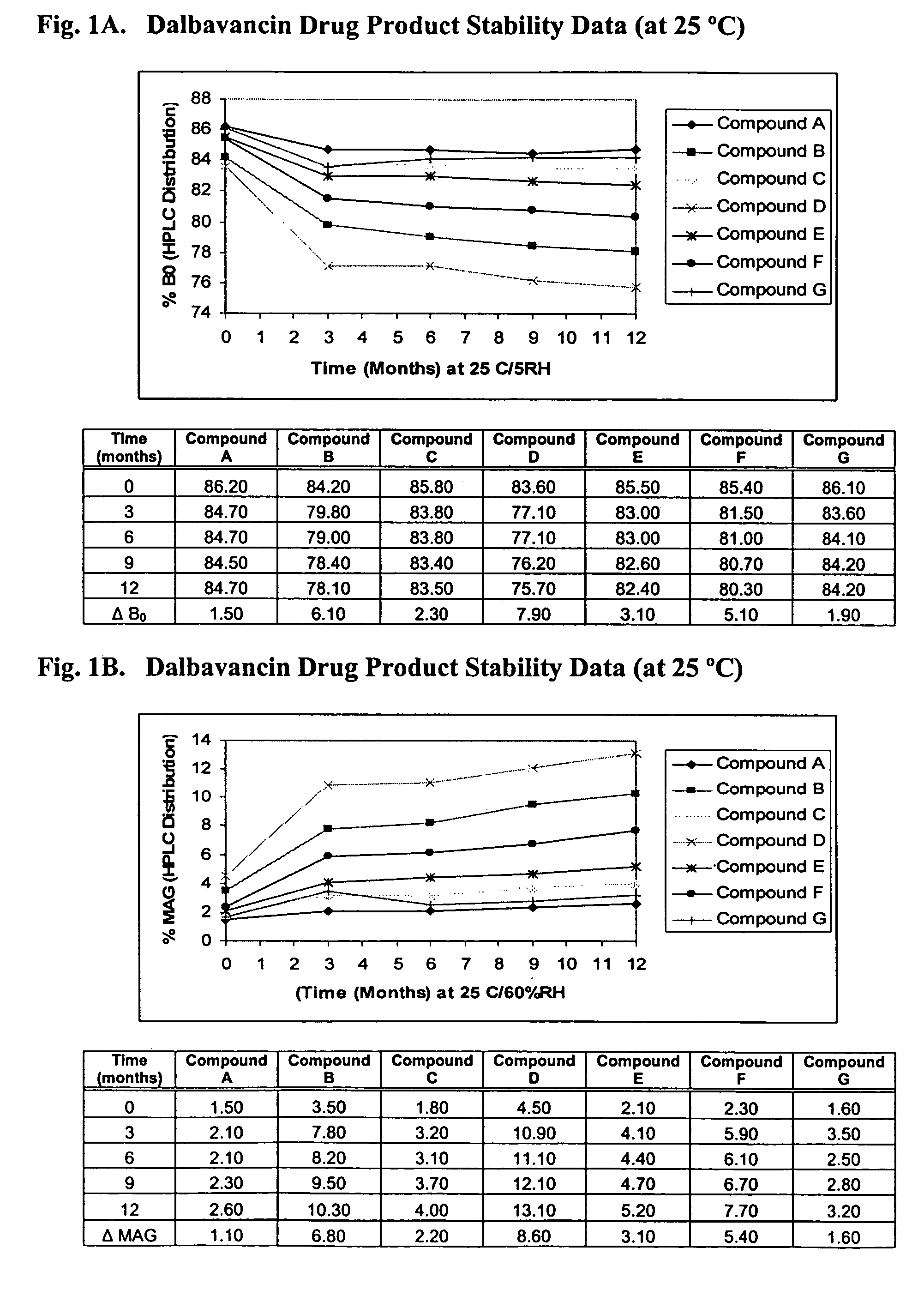
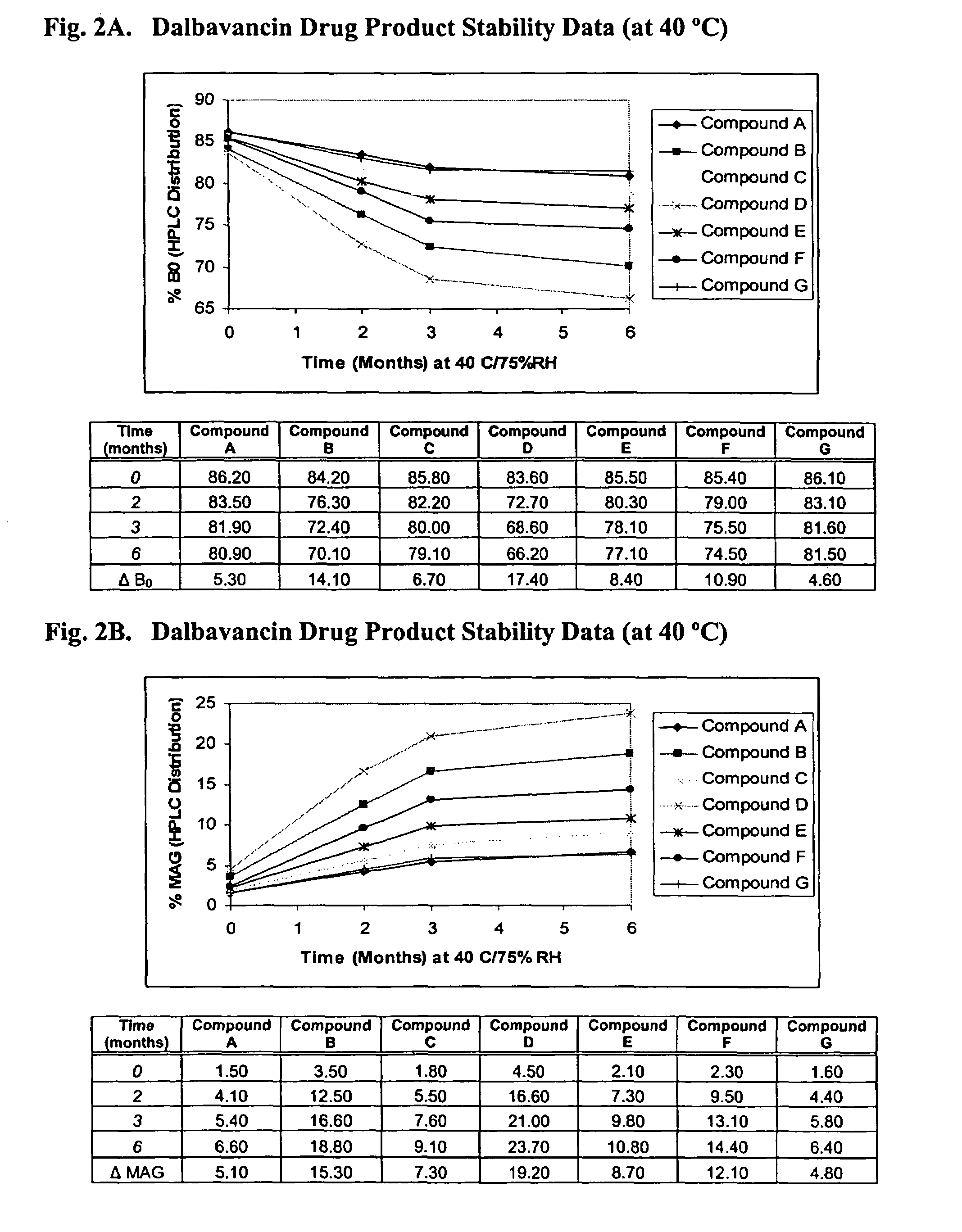
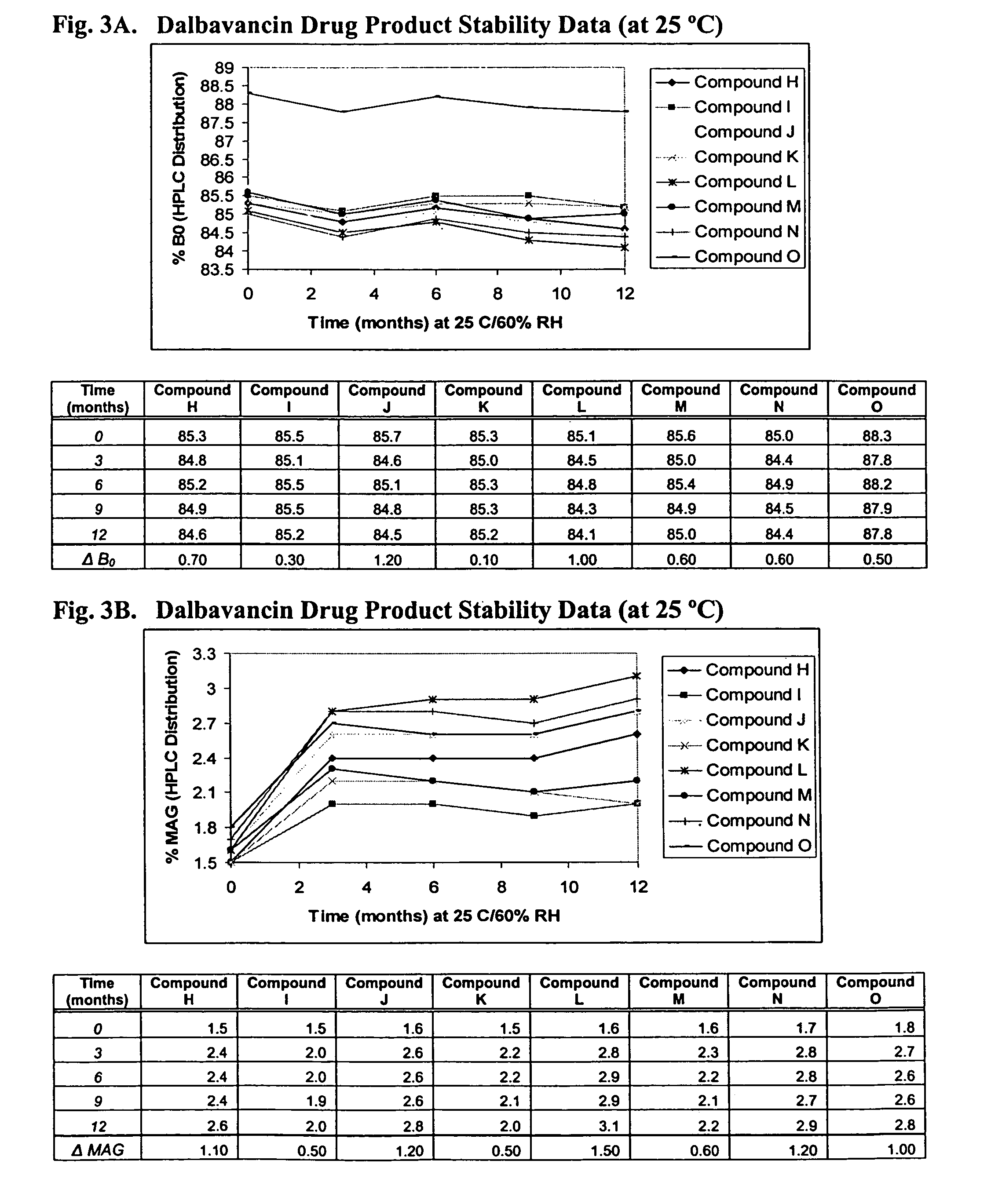
Compositions and methods for treating bacterial infections with protein-dalbavancin complexes
InactiveUS20050004011A1Improve antibacterial propertiesAntibacterial agentsBiocideDalbavancinMedicine
The invention provides methods and compositions for treatment of bacterial infections. Methods of the invention include administration of dalbavancin for treatment of a bacterial infection, in particular a Gram-positive bacterial infection of skin and soft tissue, under conditions where a protein-dalbavancin complex forms, or administering a protein-dalbavancin complex. Dosing regimes include once weekly administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection.
Owner:VICURON PHARM INC
Long acting trial receptor agonists for treatment of autoimmune diseases
ActiveUS20150259397A1Increasing apoptosis of pro-inflammatory immune cellsShorten the progressPeptide/protein ingredientsAntipyreticDiseaseRegulatory T cell
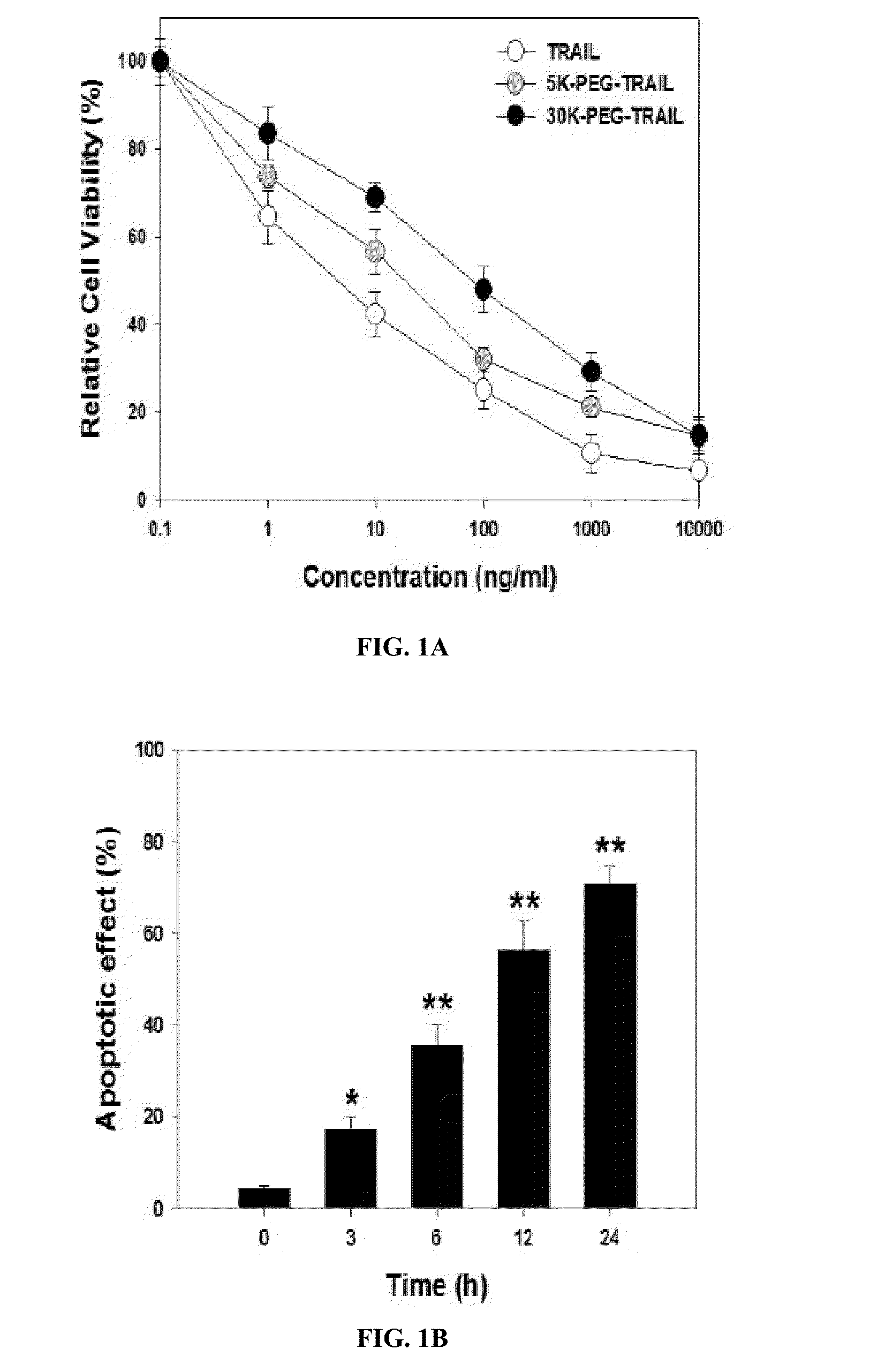
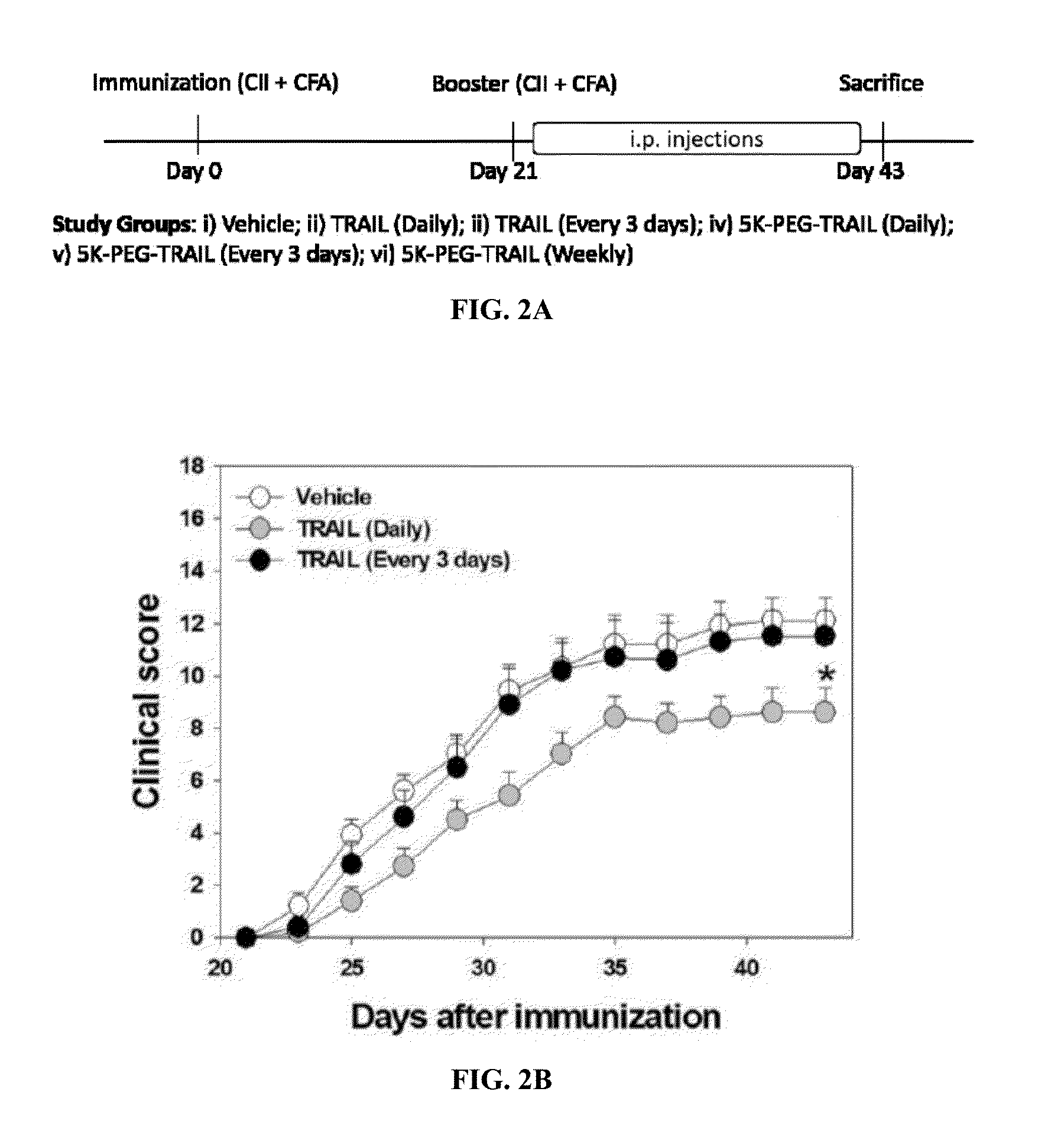
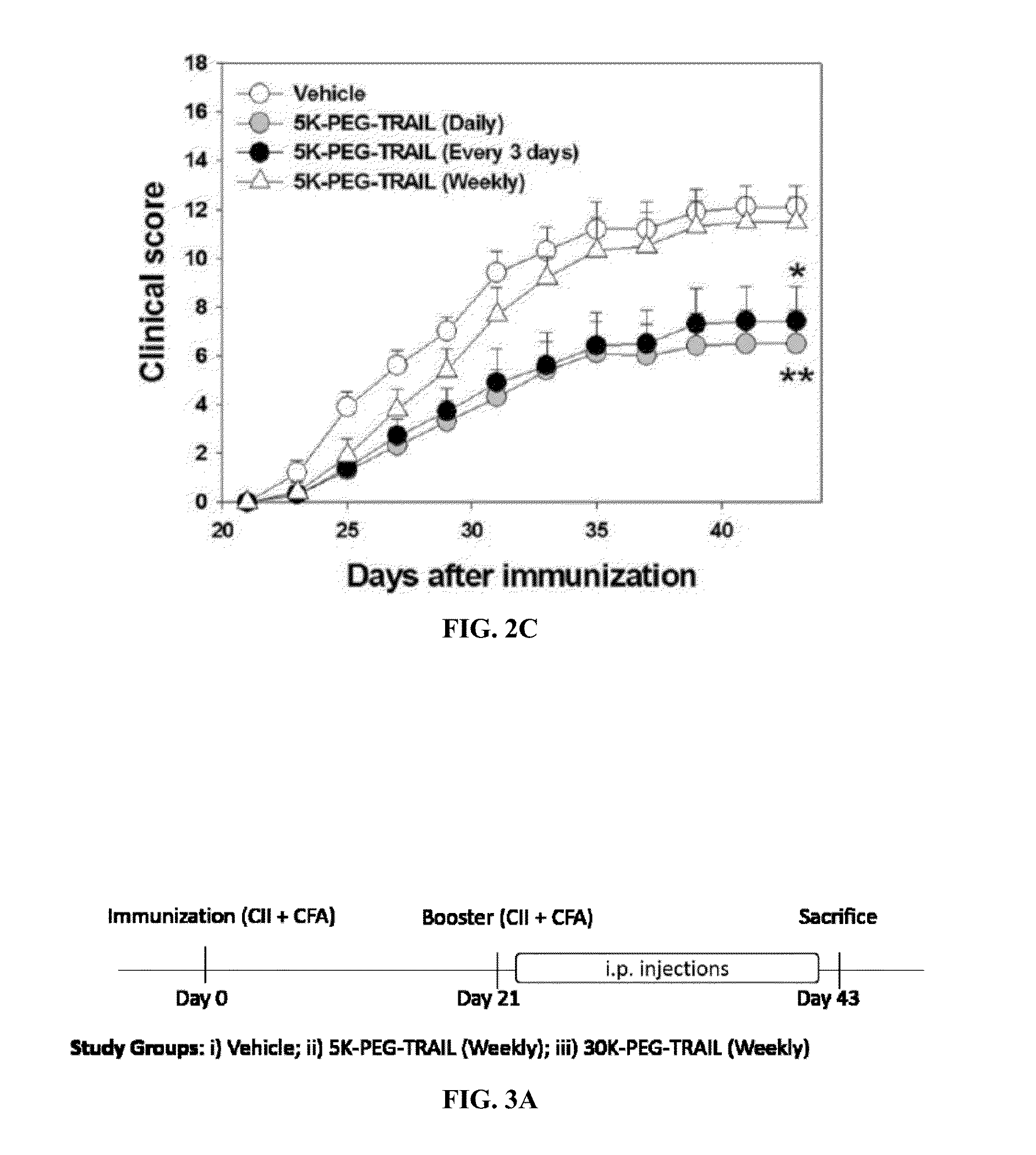
Methods of treating an autoimmune disease such as rheumatoid arthritis, methods of increasing apoptosis of pro-inflammatory immune cells or synoviocytes, methods of increasing the quantity of the anti-inflammatory regulatory T cells, and methods of slowing the progression of inflammation in a subject include systemically administering to the subject a pharmaceutical composition including an effective amount of a TRAIL-conjugate. Preferably, the TRAIL-conjugate is effective for at least 3 days, more preferably at least 7 days, without being part of a nanocomplex that modulates the circulation half-life or release kinetics of the TRAIL-conjugate. Combination therapies including administering a second active agent, most preferably a TNF-α inhibitor, as well as pharmaceutical composition dosage units including a TRAIL-conjugate and a TNF-α inhibitor in an effective amount for a single once weekly dose for treatment of rheumatoid arthritis are also provided.
Owner:D&D PHARMATECH INC
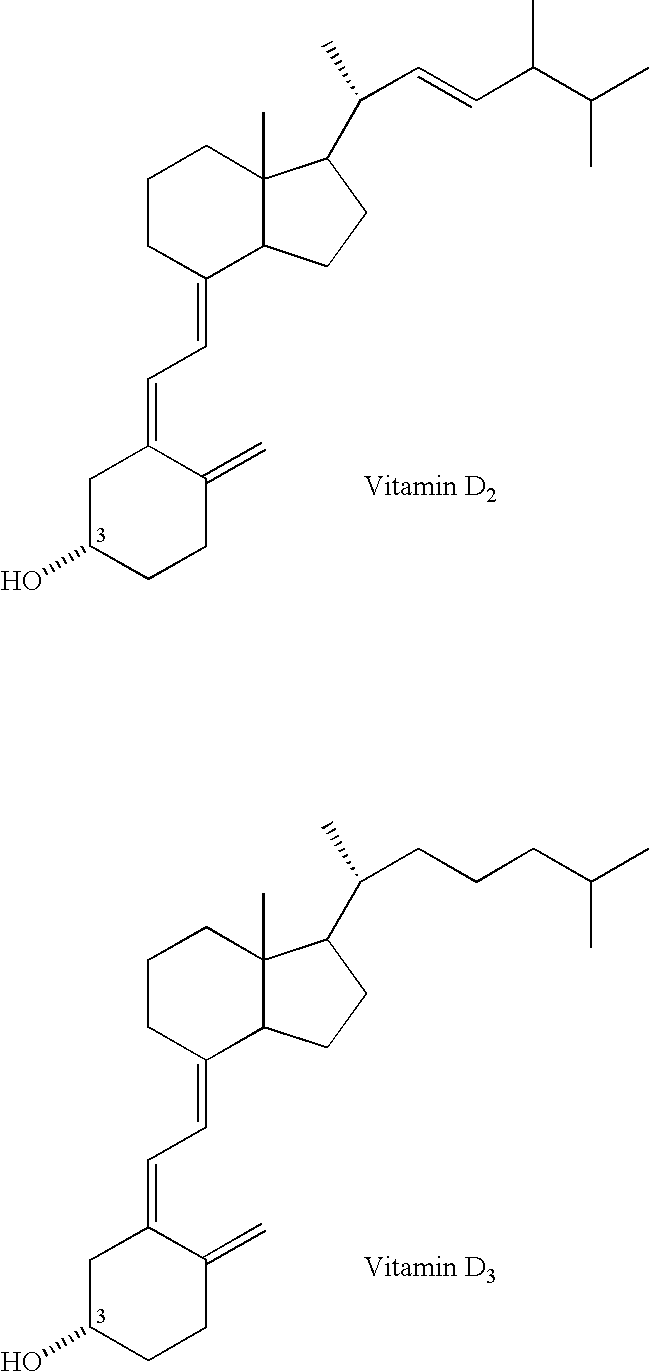
Method for inhibiting bone resorption with an alendronate and vitamin d formulation
Composition and method for preventing or treating abnormal bone resorption in mammals, the composition characterized as containing, a supplementary effective amount of a non-activated metabolite of vitamin D2 and / or D3 and a pharmaceutically effective amount of bisphosphonate to provide vitamin D nutrition during treatment to facilitate normal bone formation and mineralization, while minimizing the occurrence of or potential for the complications associated with vitamin D insufficiency, such as hypocalcemia and osteomalacia. The method of preventing or treating may be further characterized by concomitantly administering the components simultaneously or alternately at dosing intervals selected from once-weekly, twice-weekly, bi-weekly, monthly, and bimonthly.
Owner:DAIFOTIS ANASTASIA G +2
Treatment of cartilage disorders
This invention concerns the treatment of cartilage disorder and osteoarthritis in particular. More specifically, it relates to the use of FGF-18 in treatment regimens and for the manufacture of a medicament for the treatment of patients having a cartilage disorder such as osteoarthritis, such as for example knee osteoarthritis or secondary hip osteoarthritis. Specifically provided is a preferred treatment scheme comprising once weekly administration of an FGF-18 compound per treatment cycle.
Owner:ARES TRADING SA
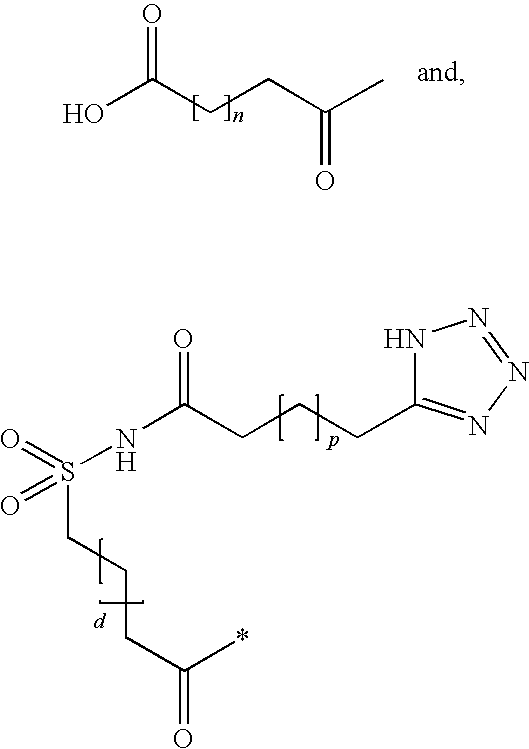
Novel trifluoromethylsulfonamide gamma secretase inhibitor
ActiveUS20110275719A1Improved pharmacokinetic propertiesReduce processing stepsBiocideNervous disorderDiseaseGastrointestinal toxicity
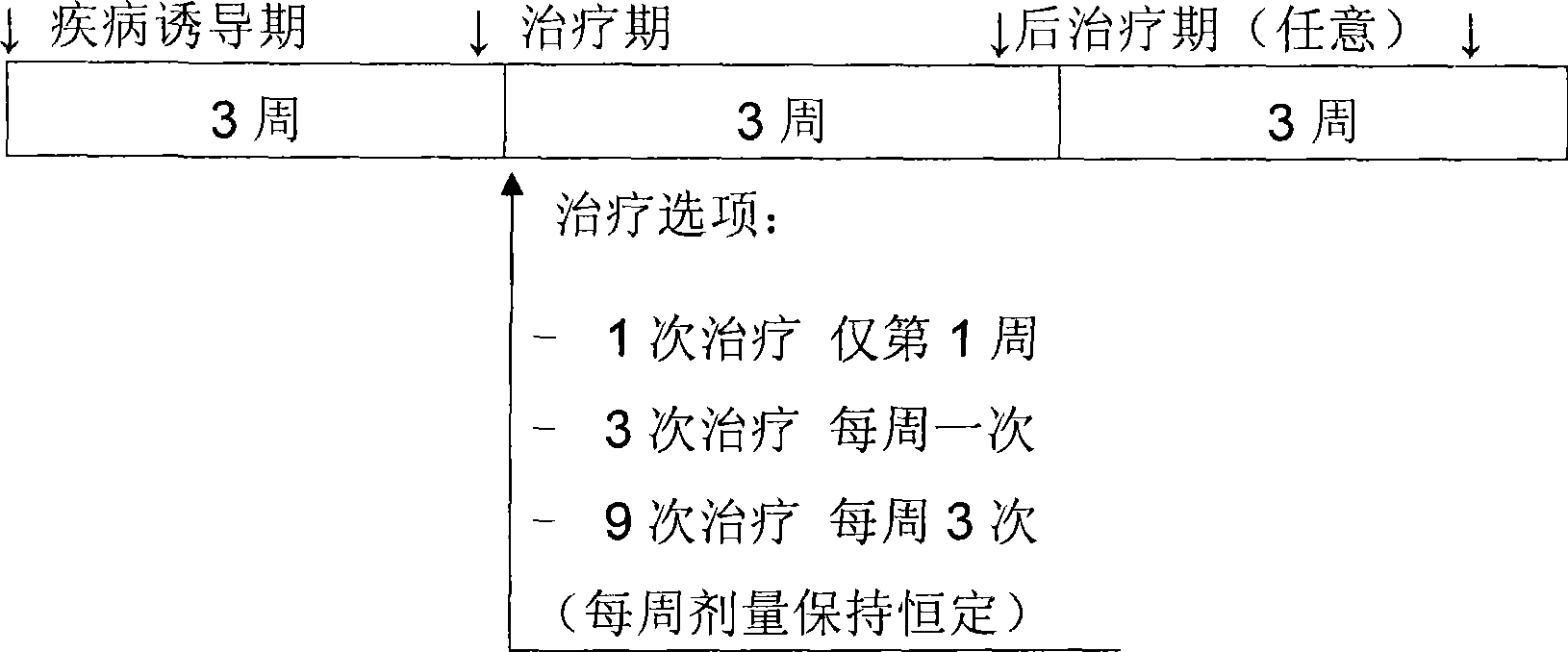
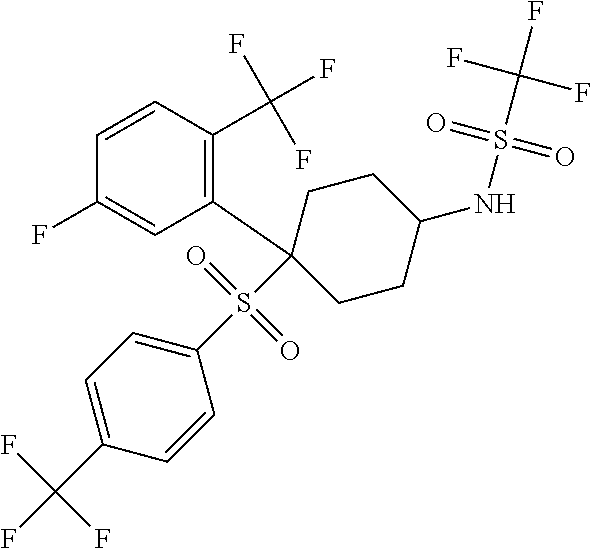
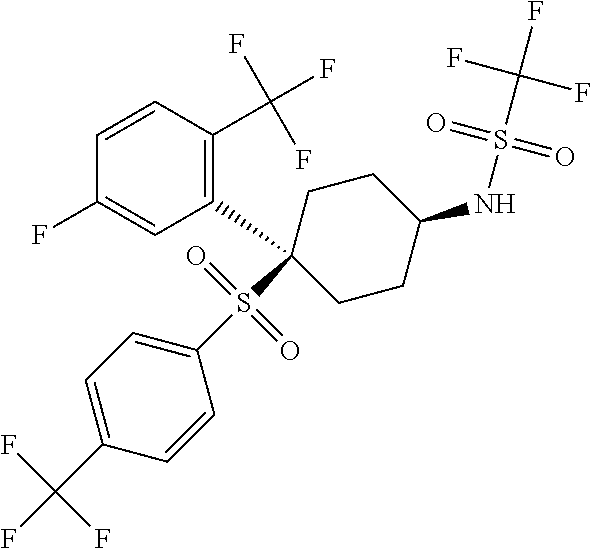
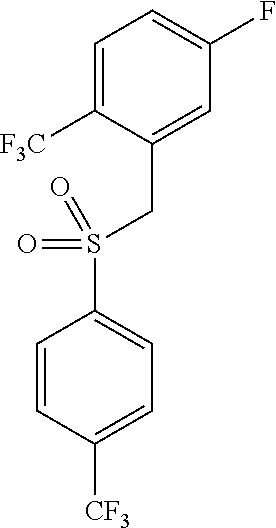
The present invention is directed to a novel trifluoromethylsulfonamide derivative which inhibits the processing of APP by the putative γ-secretase and thus is useful in the treatment or prevention of Alzheimer's disease. This compound possesses favorable pharmacokinetic properties in higher species (rhesus) and thus can be dosed on an intermittent dosing regiment (e.g., once weekly). When dosed on such a regiment the compound exhibits significant and continuous Aβ lowering without the manifestation of Notch associated gastrointestinal toxicity for extended periods, e.g., 7 days. Pharmaceutical compositions and methods of use are also included.
Owner:MERCK SHARP & DOHME LLC
Truncated GLP-1 Derivatives and Their Therapeutical Use
InactiveUS20140296131A1Nervous disorderPeptide/protein ingredientsAmino acid substitutionExtracellular Structure
The invention relates to truncated GLP-1 analogues, in particular a GLP-1 analogue which is a modified GLP-1(7-35) (SEQ ID No 1) having: i) a total of 2, 3, 4, 5 6, 7, 8, or 9 amino acid substitutions as compared to GLP-1(7-35), including a) a Glu residue at a position equivalent to position 22 of GLP-1(7-35), and b) an Arg residue at a position equivalent to position 26 of GLP-1(7-35); as well as derivatives thereof, and therapeutic uses and compositions. These analogues and derivatives are highly potent, have a good binding affinity to the GLP-1 receptor, also to the extracellular domain of the GLP-1 receptor, which is of potential relevance achieving long-acting, stable GLP-1 compounds with a potential for once weekly administration.
Owner:NOVO NORDISK AS
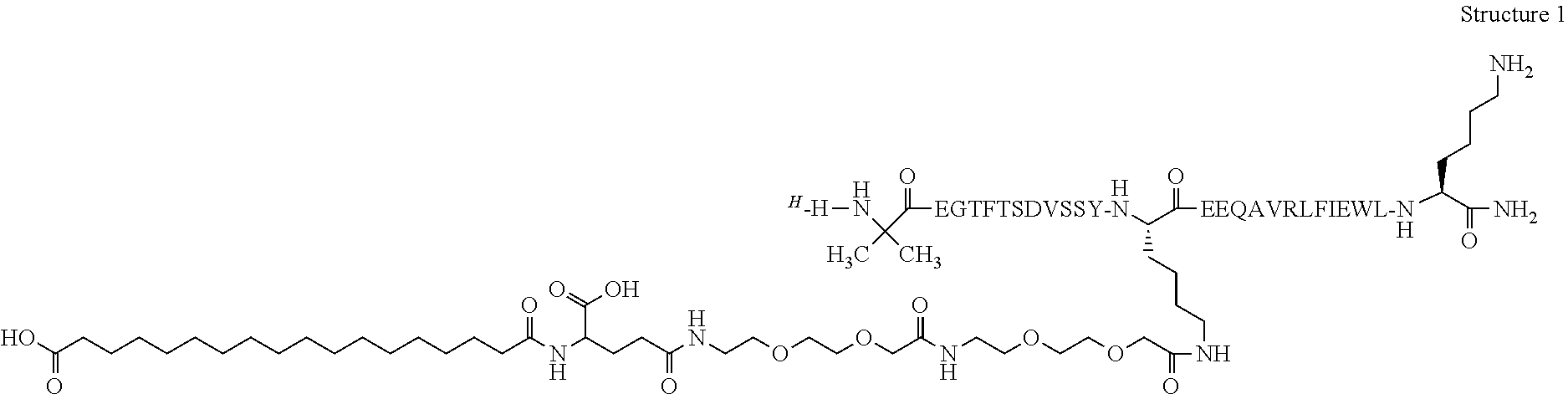
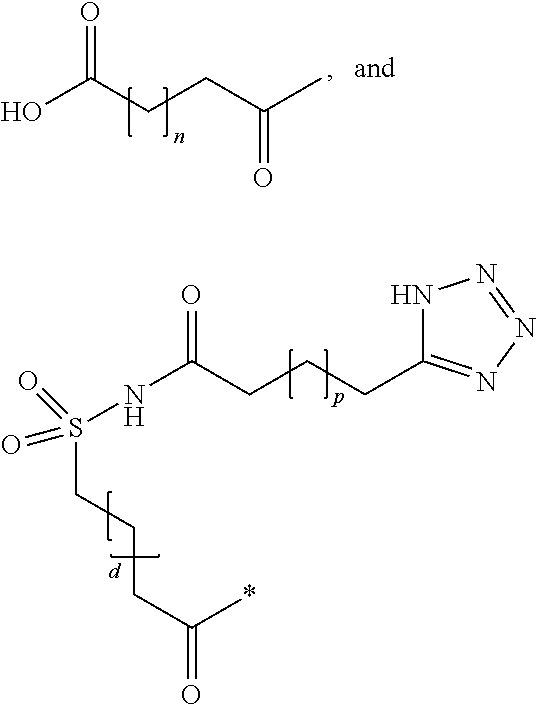
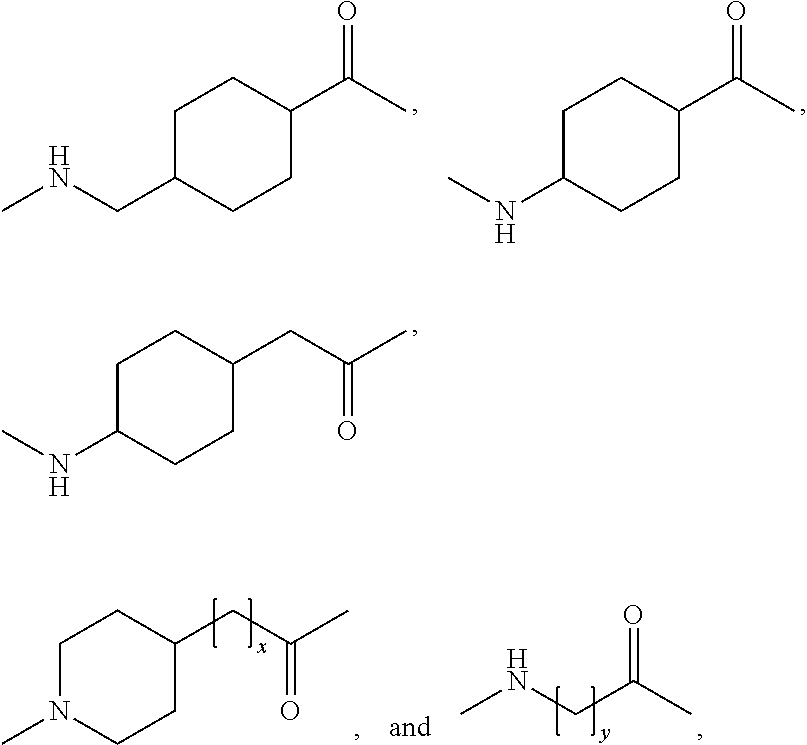
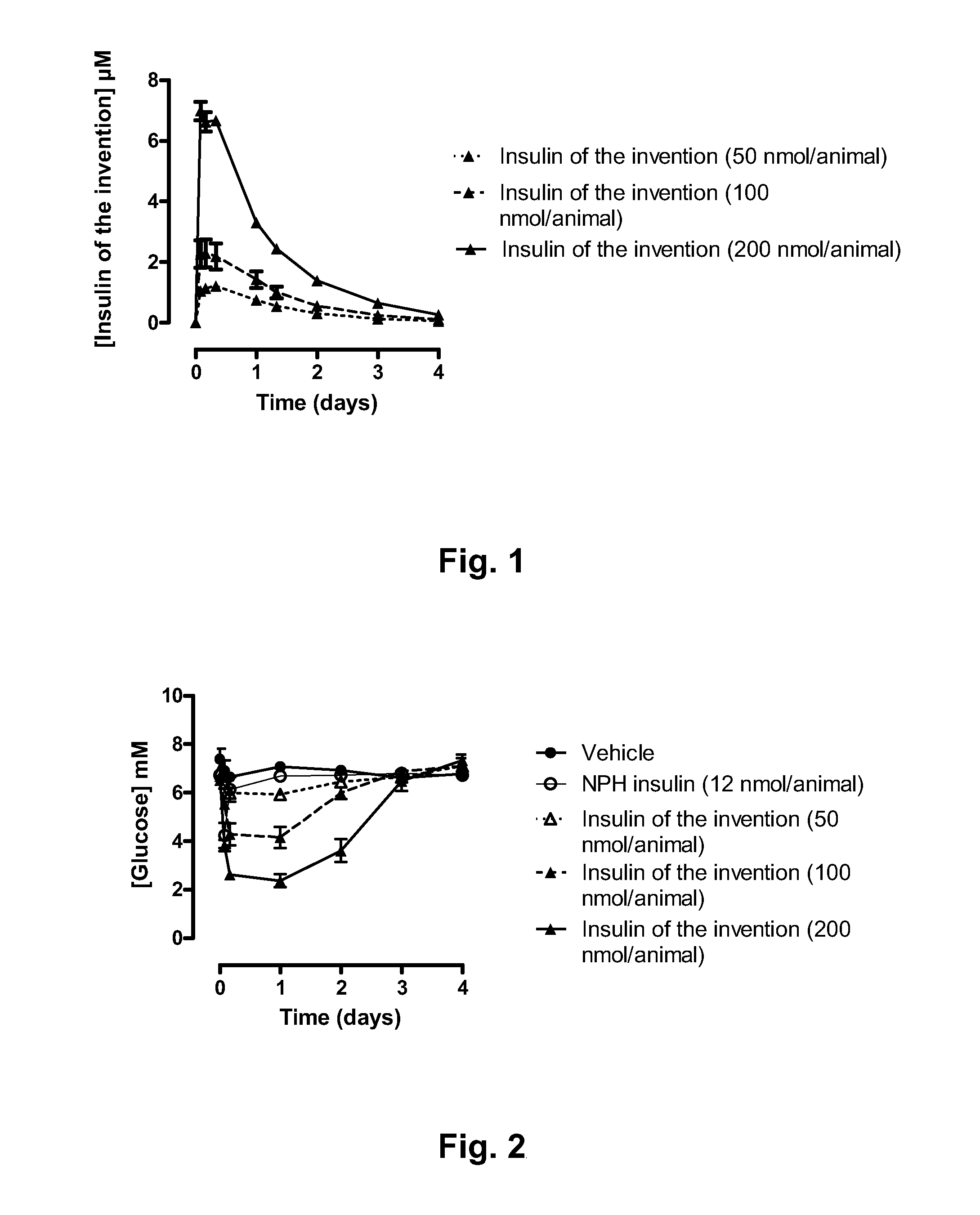
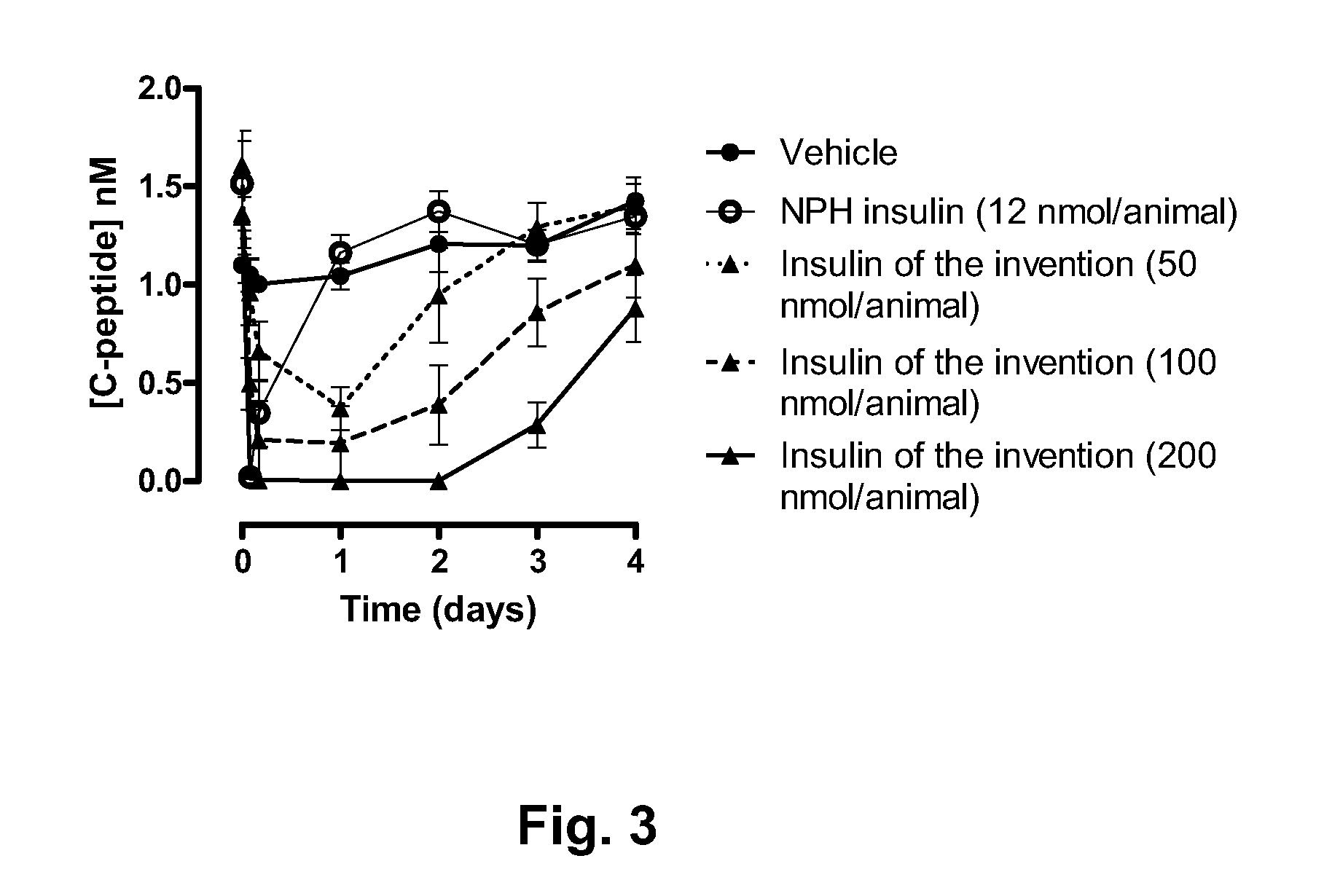
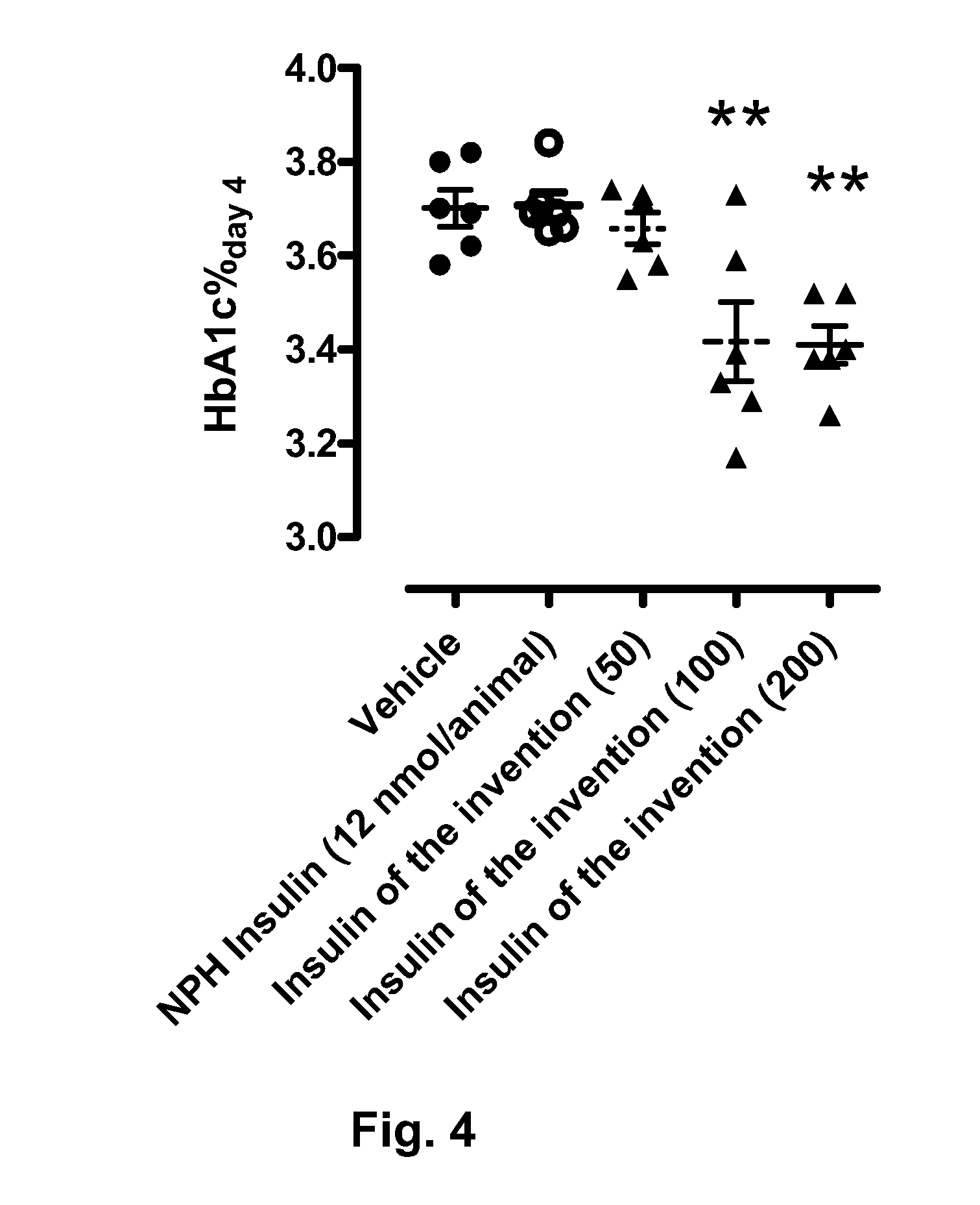
Novel use of insulin derivatives
InactiveUS20150111820A1Improve conveniencePeptide/protein ingredientsMetabolism disorderOnce weeklyDiabetic patient
This invention relates to insulin derivatives having long duration of action. The insulin derivatives have been found to be useful for the treatment of diabetes, and in particular for less frequent administration to the diabetic patients, in particular as seldom as about once weekly.
Owner:NOVO NORDISK AS
Methods of administering dalbavancin for treatment of skin and soft tissue infections
InactiveUS20050032721A1Reduce adverse side effectsAntibacterial agentsBiocideDalbavancinSoft tissue infection
The invention provides methods and compositions for treatment of bacterial infections. Methods of the invention include administration of dalbavancin for treatment of a bacterial infection, in particular an uncomplicated Gram-positive bacterial infection of skin and soft tissue. Dosing regimes include once weekly administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection.
Owner:CAVALERI MARCO +6
Dalbavancin compositions for treatment of bacterial infections
ActiveUS20050004050A1Reduce adverse side effectsAntibacterial agentsBiocideDalbavancinGram-positive bacterial infections
The invention provides methods and compositions for treatment of bacterial infections. Methods of the invention include administration of dalbavancin formulations for treatment of a bacterial infection, in particular a Gram-positive bacterial infection of skin and soft tissue. Dosing regimes include once weekly administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection.
Owner:VICURON PHARM INC
Treatment of cartilage disorders with FGF-18
This invention concerns the treatment of cartilage disorder and osteoarthritis in particular. More specifically, it relates to the use of FGF-18 in treatment regimens of patients having a cartilage disorder such as osteoarthritis, such as for example knee osteoarthritis or secondary hip osteoarthritis. Specifically provided is a preferred treatment scheme comprising once weekly administration of an FGF-18 compound per treatment cycle.
Owner:ARES TRADING SA
Methods of sustaining dietary ketosis and its effects on lipid profile
InactiveUS20160317487A1Rapid and sustained elevationGood for healthMetabolism disorderOrganic chemistry methodsDiseaseLipid formation
Owner:UNIV OF SOUTH FLORIDA
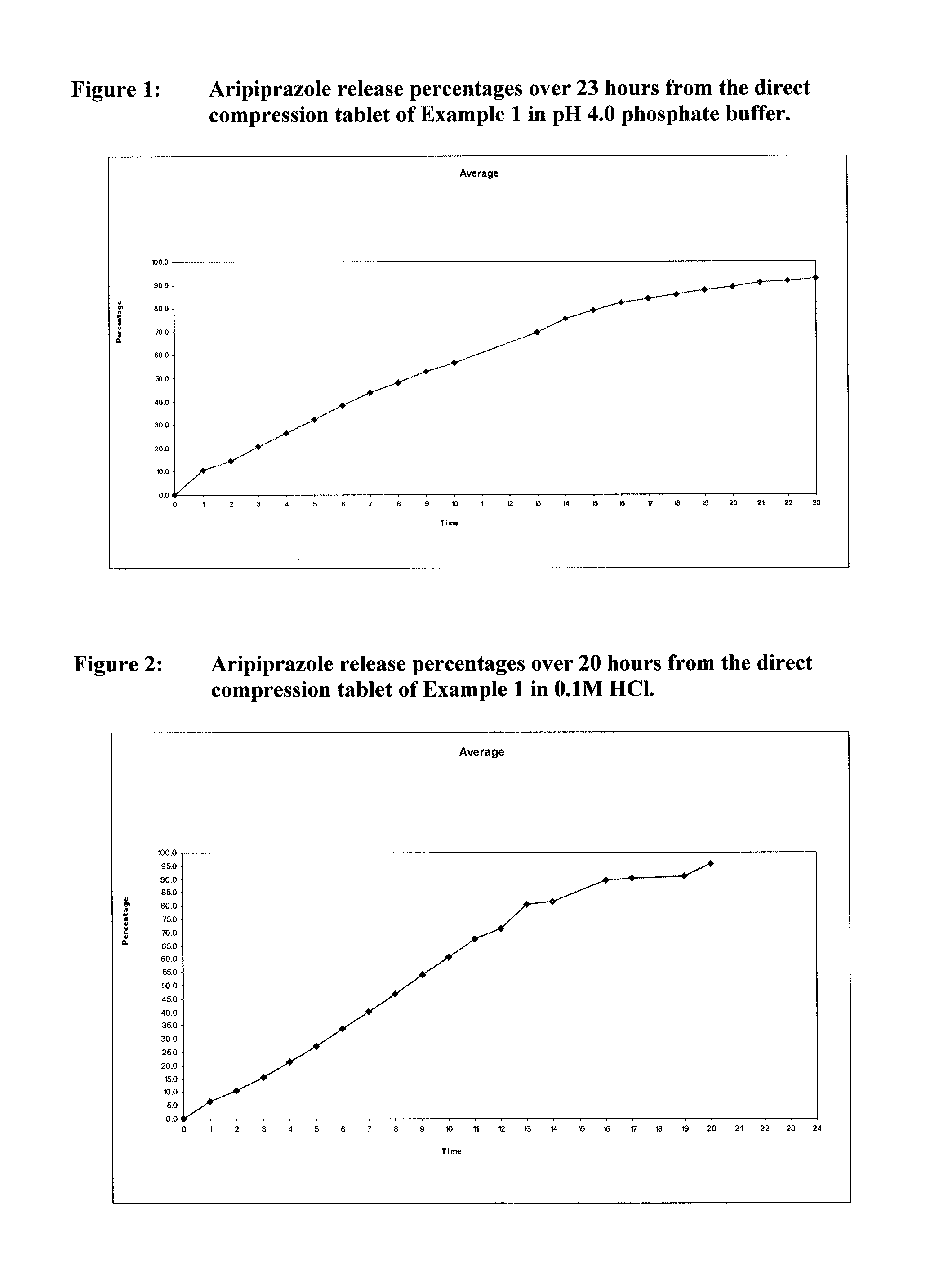
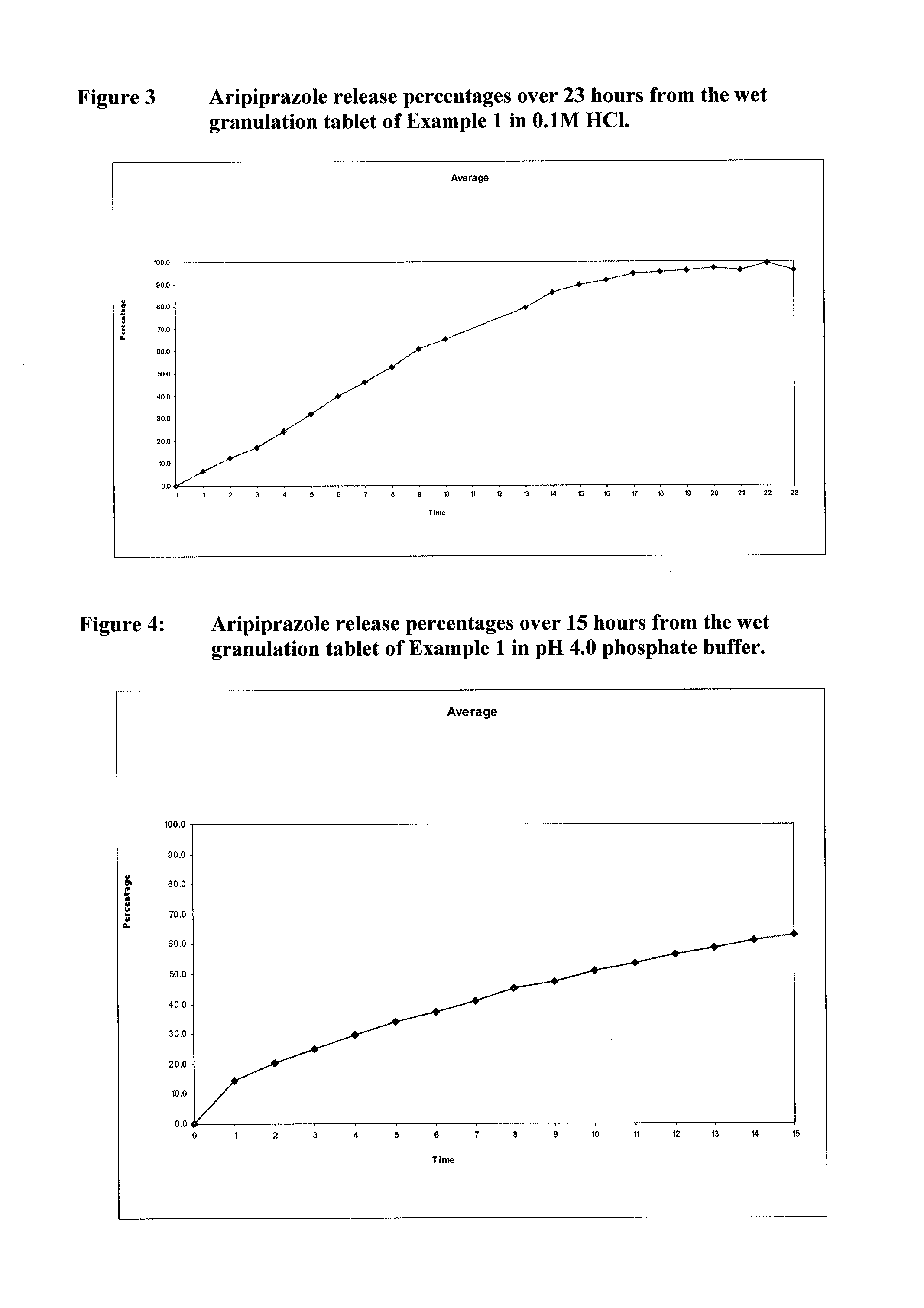
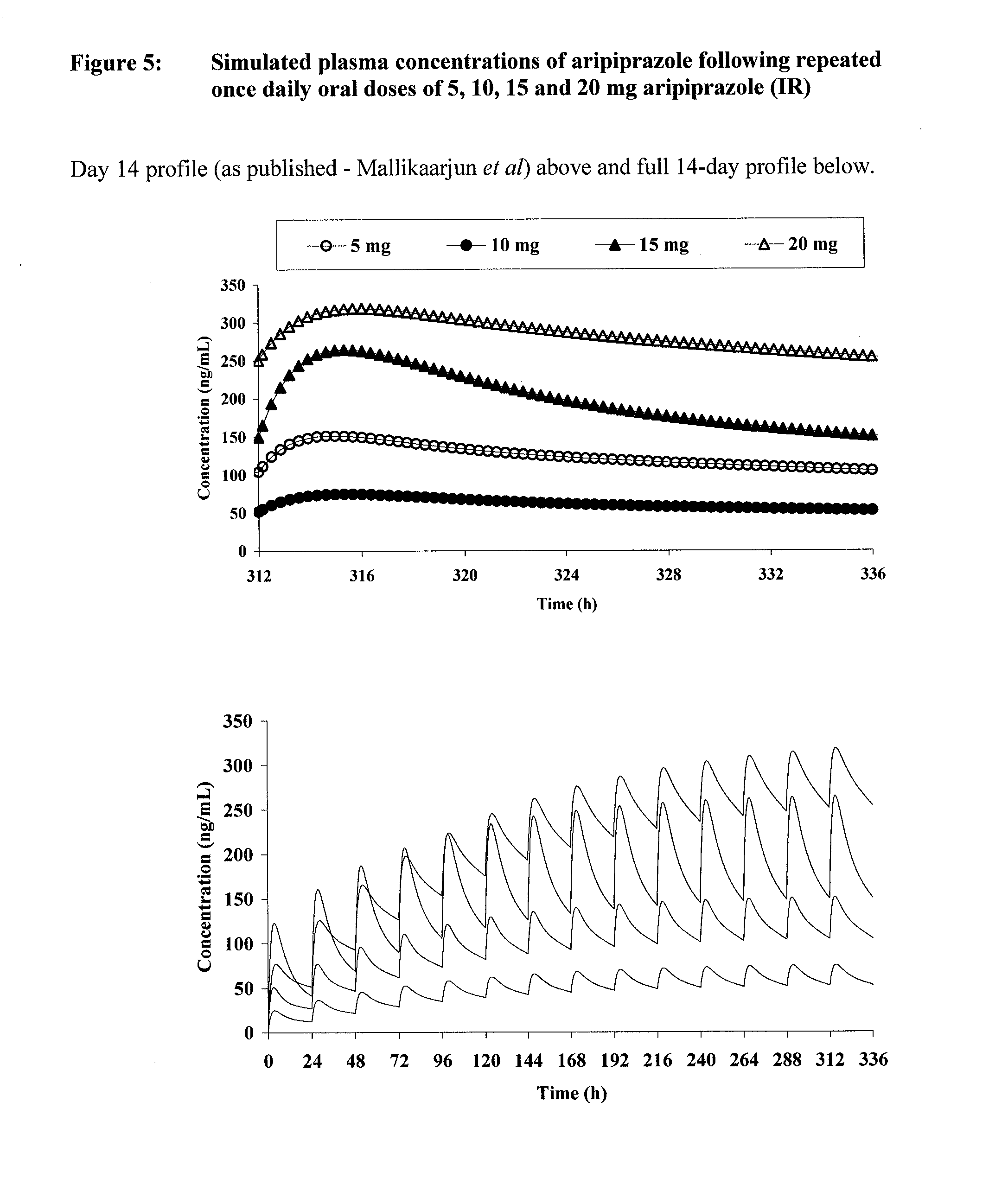
Once-weekly oral administration of aripiprazole
InactiveUS20140044786A1Increasing adverse eventImprove compliancePowder deliveryOrganic active ingredientsPharmacyControlled release
An orally deliverable pharmaceutical composition provides controlled release of aripiprazole. The composition includes a therapeutically effective amount of aripiprazole and at least one pharmaceutically acceptable excipient. The compositions of the invention may exhibit one or more of the release profiles defined in the specification.
Owner:ZYSIS
Dalbavancin compositions for treatment of bacterial infections
InactiveUS7119061B2Reduce adverse side effectsAntibacterial agentsBiocideDalbavancinGram-positive bacterial infections
The invention provides methods and compositions for treatment of bacterial infections. Methods of the invention include administration of dalbavancin formulations for treatment of a bacterial infection, in particular a Gram-positive bacterial infection of skin and soft tissue. Dosing regimes include once weekly administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection.
Owner:APTALIS PHARMA
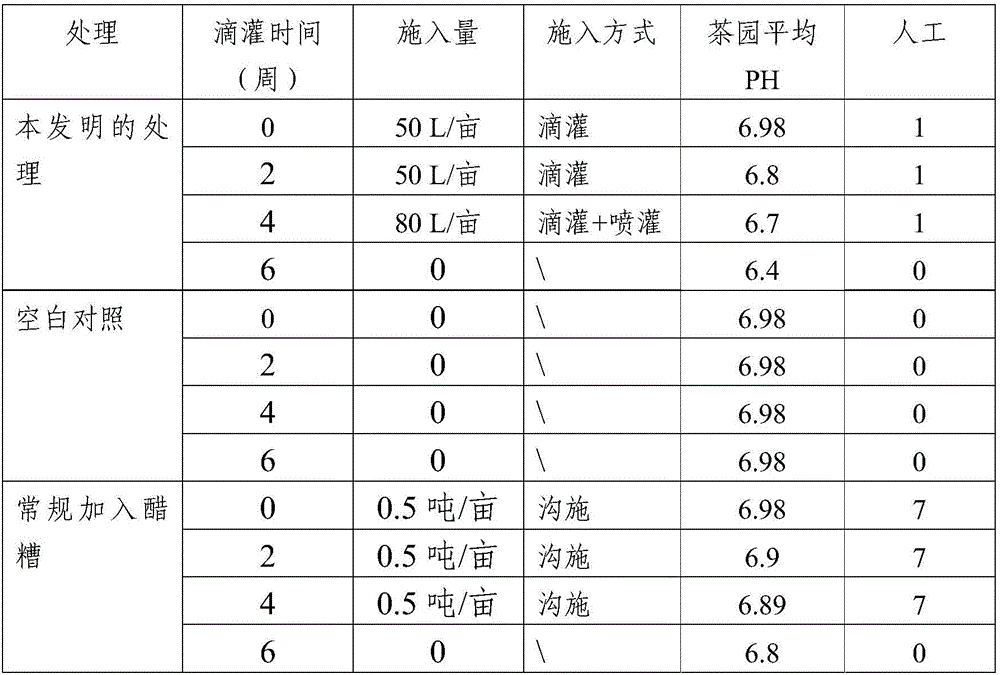
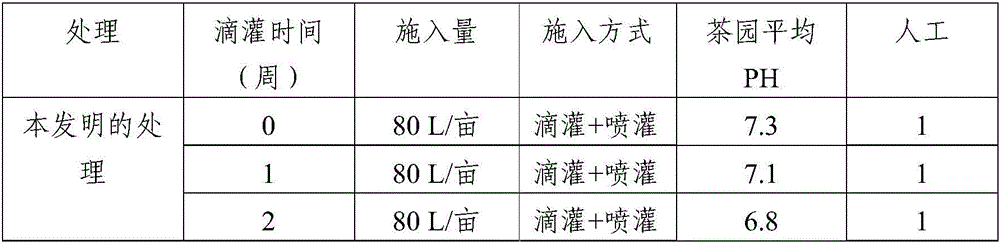
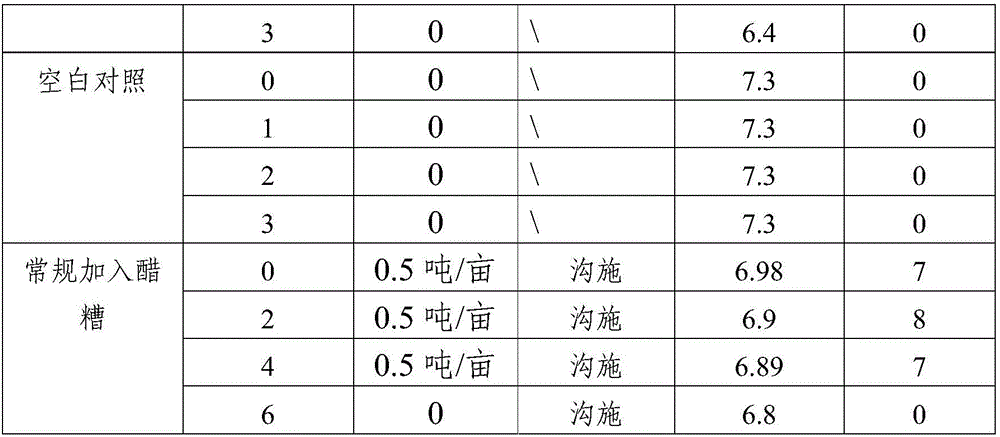
Method for amending alkalescent tea garden by vinegar residue digested effluent
InactiveCN105814999AIncrease profitAdjust the dosageBio-organic fraction processingOther chemical processesDrip irrigationOnce weekly
The invention discloses a method for amending an alkalescent tea garden by vinegar residue digested effluent. The method comprises the step: carrying out drip irrigation on tea garden soil according to certain frequency by using the vinegar residue digested effluent until the pH value of the tea garden soil is smaller than 6.5, wherein the frequency is arranged as follows: drip irrigation is carried out once biweekly when the pH value of the tea garden soil is not smaller than 6.5 and is not greater than 7; drip irrigation is carried out once weekly when the pH value of the tea garden soil is greater than 7 and is not higher than 7.5. The method for amending the alkalescent tea garden by the vinegar residue digested effluent further comprises the step of carrying out sprinkling irrigation on tea trees once every two days by using water-diluted vinegar residue digested effluent when the growth vigor of the tea trees is poor until the pH value of the tea garden soil is smaller than 6.5. According to the method, a drip irrigation mode is adopted and is combined with sprinkling irrigation if necessary, so that the aim of amending the tea garden is achieved while the utilization ratio of the vinegar residue digested effluent is increased; the flexibility is improved, so that the volume of the vinegar residue digested effluent applied is convenient to adjust according to the pH value of the tea garden soil; automation is achieved, the labor is saved, and the cost is reduced.
Owner:SHANDONG INST OF POMOLOGY
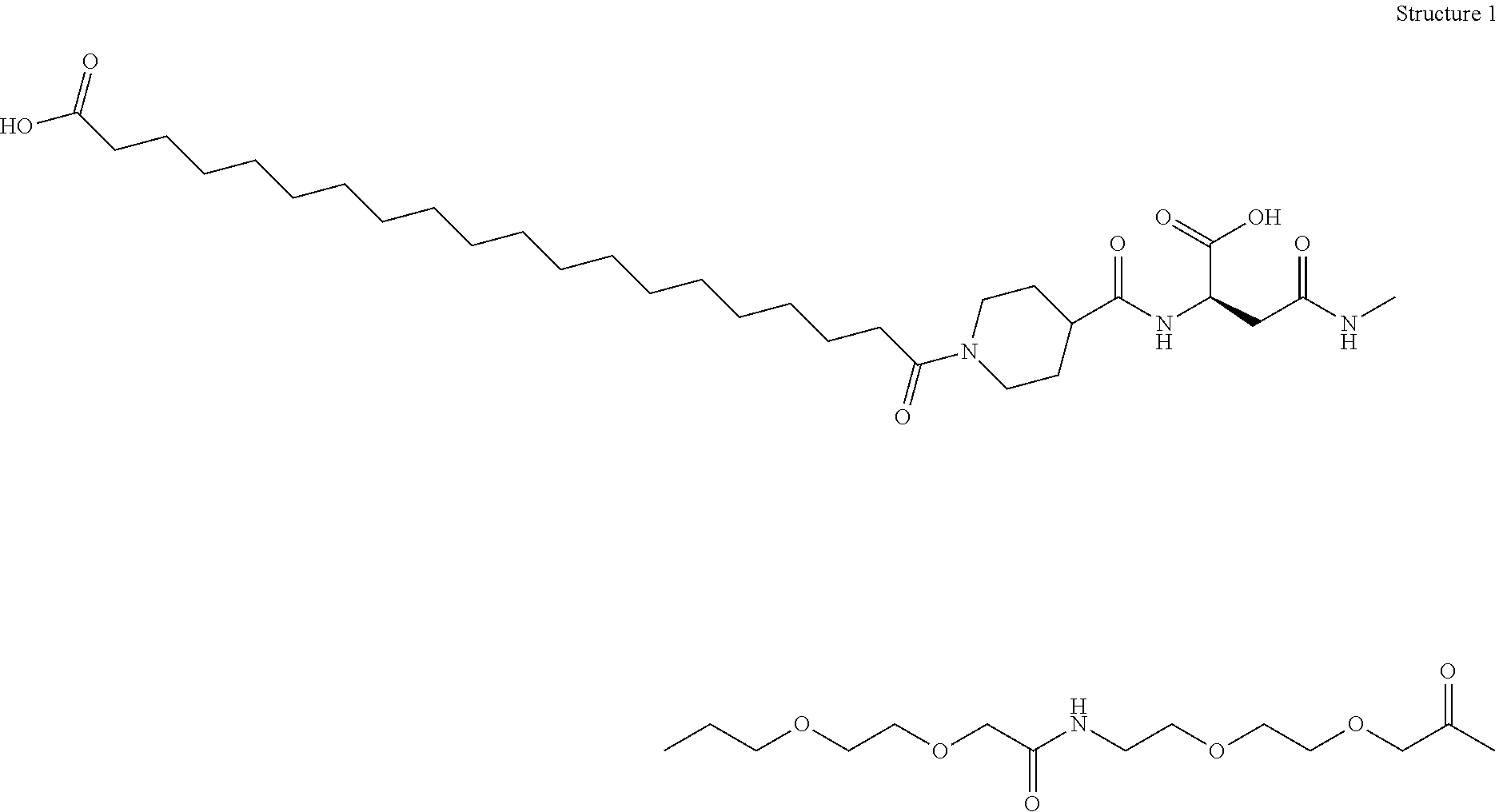
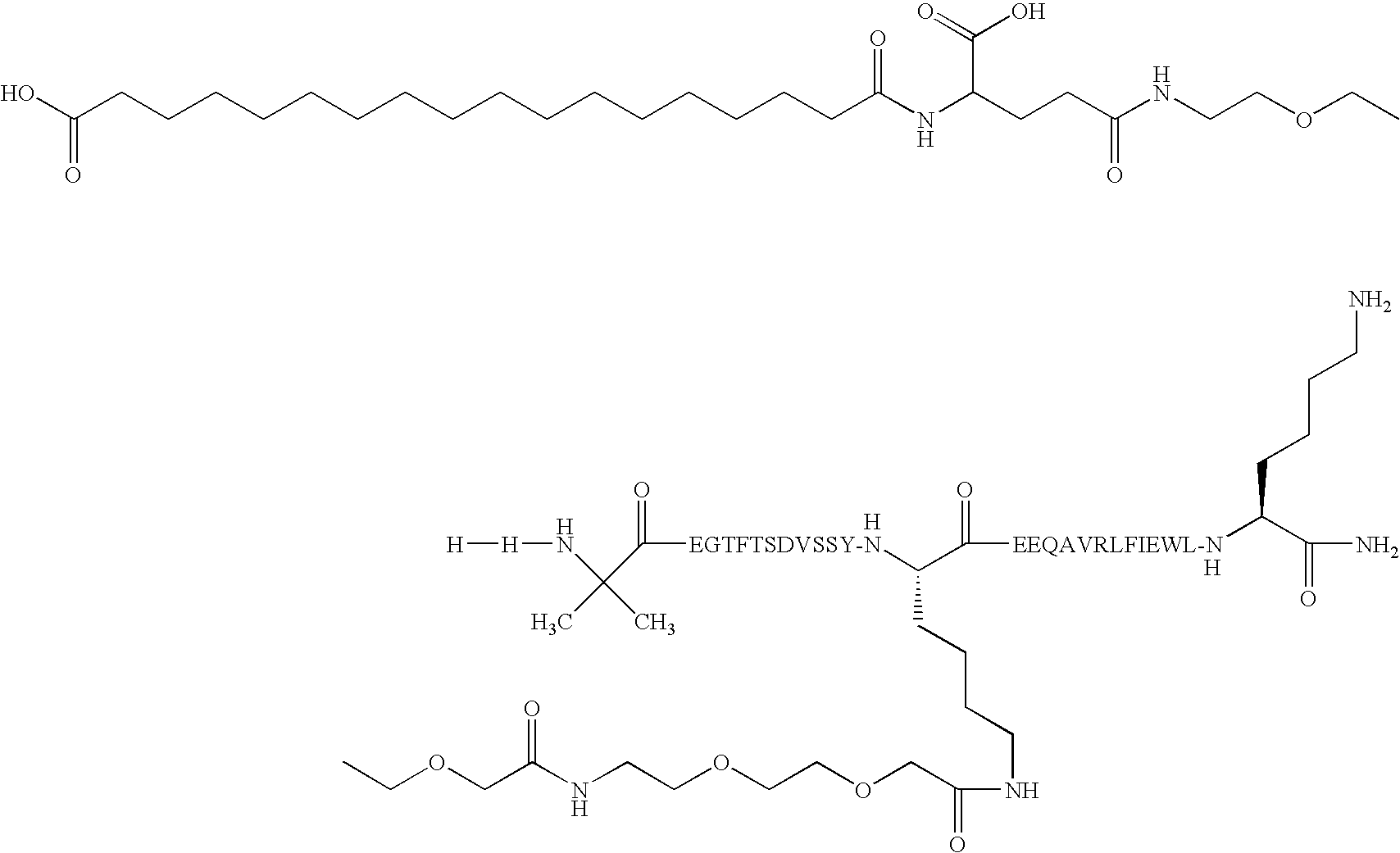
Engineered polypeptides having enhanced duration of action and reduced immunogenicity
Compounds are provided having inter alia good duration of action, high potency and / or convenient dosing regimens including once weekly administration. The compounds are engineered polypeptides which incorporate an albumin binding domain in combination with one or more biologically active polypeptides. Also provided are pharmaceutical compositions and methods of treatment for diseases and disorders including lipodystrophy, dyslipidemia, hyperlipidemia, overweight, obesity, hypothalamic amenorrhea, Alzheimer's disease, leptin deficiency, fatty liver disease or diabetes (including type I and type II). Additional diseases and disorders which can be treated by the compounds and methods described herein include nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver disease (NAFLD), metabolic syndrome X and Huntington's Disease.
Owner:安瑞特制药公司
Crater detection method
InactiveCN107796817AIncrease monitoring frequencyFew experimental toolsSemiconductor/solid-state device testing/measurementMaterial analysis by optical meansAlcoholBonding process
The crater detection method of the present invention is used for detecting craters of copper wire products during product variety, packaging model, production equipment replacement and parameter adjustment, and the monitoring frequency is very high. The beneficial effects of the present invention are: ① reproducibility, the use of mixed acid solution can be reused, no need to prepare each time, just replace it once a week; ② simple and convenient, less experimental tools, such as probes or knives, microscopes, beakers , pure water and alcohol are common in the construction process, and the bonding process employees can operate according to the process when necessary.
Owner:江阴苏阳电子股份有限公司
Low dose therapy for treating viral infections
InactiveUS20070208047A1Patient compliance is goodBiocideAntiviralsReverse transcriptasePatient compliance
A method of treating viral infections, particularly Hepatitis B (HBV) and Human Immunodeficiency Virus (HIV), by administering a low dose of Elvucitabine to a patient suffering viral infection is provided herein. The Elvucitabine dosages provided herein for effective anti-viral therapy are approximately 10-fold less than the effective dosages of currently marketed reverse transcriptase inhibitors. The Elvucitabine dosage may be given BID, daily, once every 48 hours, or once weekly. Also provided herein are packaged pharmaceutical formulations comprising Elvucitabine and instructions for treating a viral infection by administering a low BID, daily, once / 48 hour, or weekly dosage of Elvucitabine. The low dose Elvucitabine formulations provided herein have the additional benefit of improving patient compliance with anti-viral therapy.
Owner:ACHILLION PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com