Treatment of cartilage disorders
A barrier, cartilage technology, applied in the field of medicine, can solve problems such as ineffectiveness of damaged tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] Example 1: Disease Models and Long-Term Pharmacology in Animals
[0191] The significant in vivo therapeutic effect of FGF-18(170AA) using intra-articular (i.a.) administration was tested on different OA and damaged cartilage disease models. In different species (rats, dogs), the total therapeutically effective dose was demonstrated to be 3-40 μg / animal / week (i.a.). The results obtained from the above animal disease models (OA and cartilage defects) are summarized as follows:
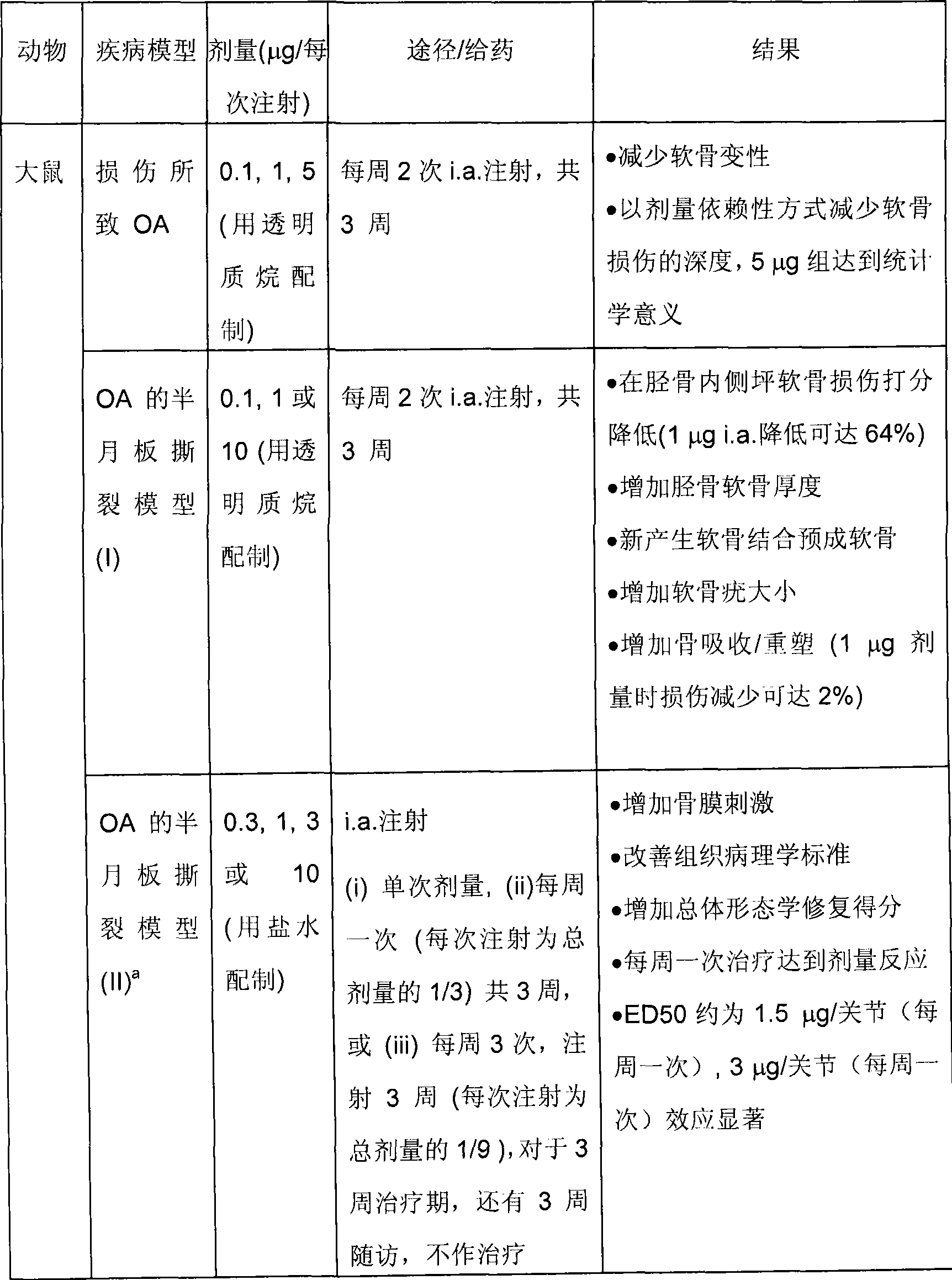
[0192] Table 1: Summary information of pharmacology studies of FGF-18(170AA)
[0193]
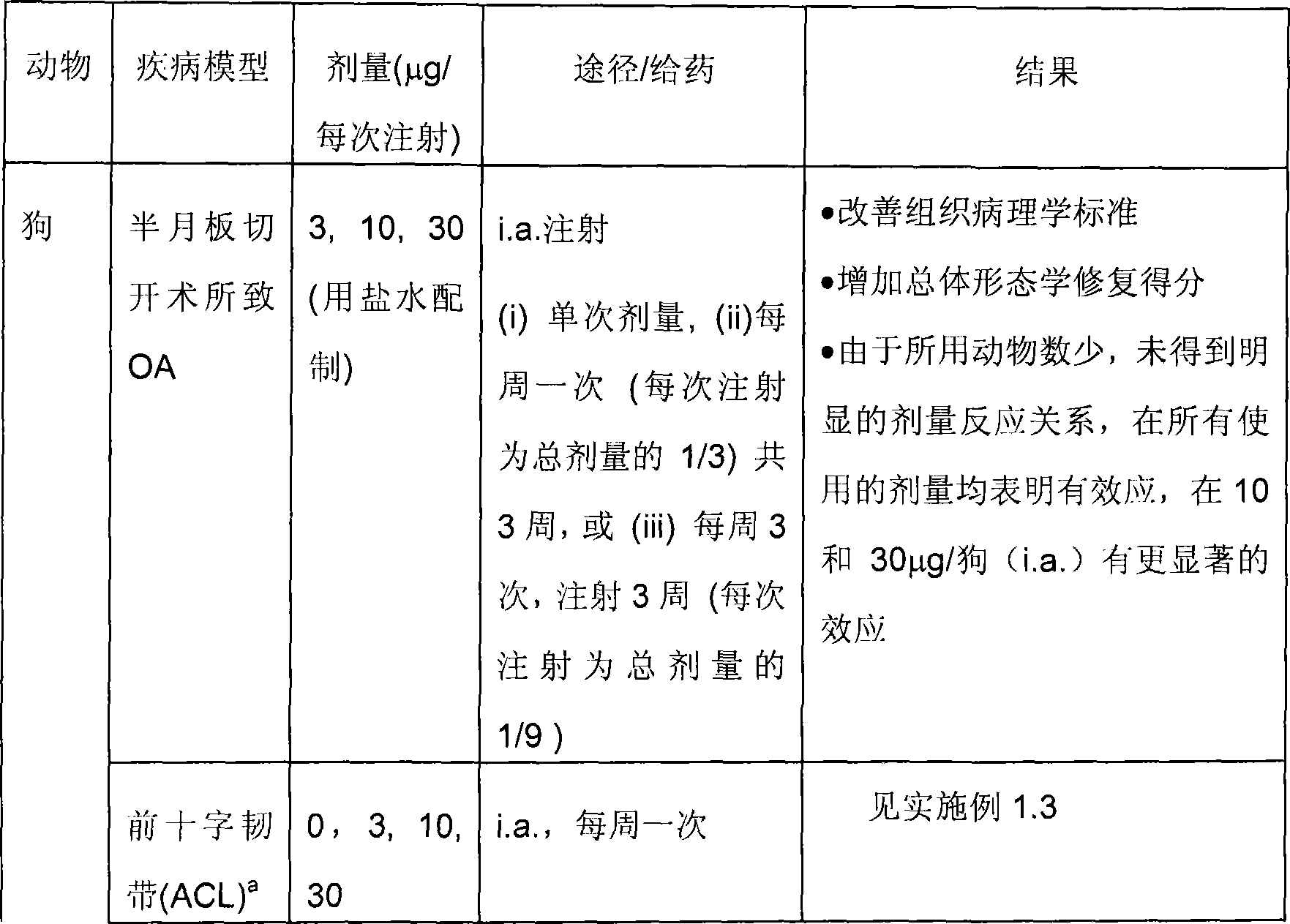
[0194] Table 2: Summary information on pharmacology studies of FGF-18(170AA) (continued)
[0195]
[0196] a Discussed in detail below
Embodiment 11
[0197] Example 1.1: Rat meniscus tear OA model
[0198] method:
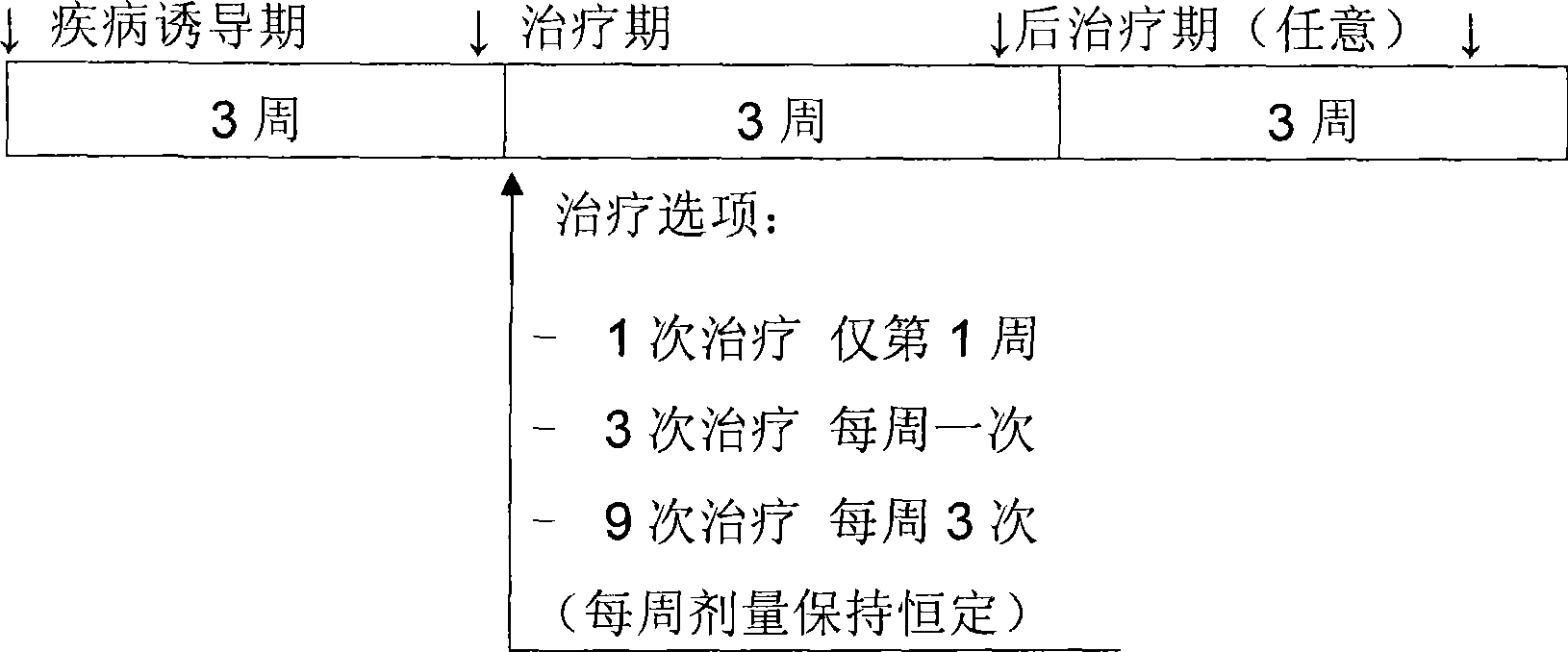
[0199] Male Lewis rats (5-10 / group) were operated to cause meniscus tear in the right knee joint. Doses of 0.3, 1, 3, or 10 μg (in saline) were administered by the i.a. route starting 19-21 days after surgery, and pharmacodynamic effects related to the dosing regimen were determined. These total doses were given as (i) a single dose, (ii) once weekly for 3 weeks (each injection being 1 / 3 of the total dose), or (iii) 3 weekly injections for 3 weeks (each injection being 1 / 3 of the total dose) for 3 weeks 1 / 9 of the total dose). At the end of treatment or 3 weeks after treatment, the right knee was collected for histopathological evaluation of potential effects. Injury assessment was performed in 3 distinct zones: 3 zones per section (1-lateral, 2-medial, 3-medial), taking into account regional differences across the medial tibial plateau. In surgical models of OA, the lateral (z1) and medial (z2) thirds are u...
Embodiment 12
[0223] Example 1.2: Dog Meniscotomy OA Model
[0224] Female beagles (3 / group) underwent unilateral mid-partial meniscotomy on the left knee one month before starting treatment, followed by saline or 3, 10 or 30 μg FGF-18 (170AA) once a week Beneficial effects on established OA were determined with treatment once or thrice weekly (1 / 3 divided doses) for three weeks.
[0225] After three weeks of treatment, the left knee was evaluated for signs of gross (3 / group) and microscopic (3 / group) meniscotomy-induced changes and synthetic responses. All but one dog had normal appetite and activity throughout the study. One dog in group 12 (YLI-8) died before the end of the study (day 17) due to aspiration pneumonia caused by repeated anesthesia for joint injections. All operated dogs in all groups developed typical degenerative changes on the medial tibia characterized by focal localized cartilage degenerative lesions. Femur injuries are rare. All dogs had thickening of the medial j...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com