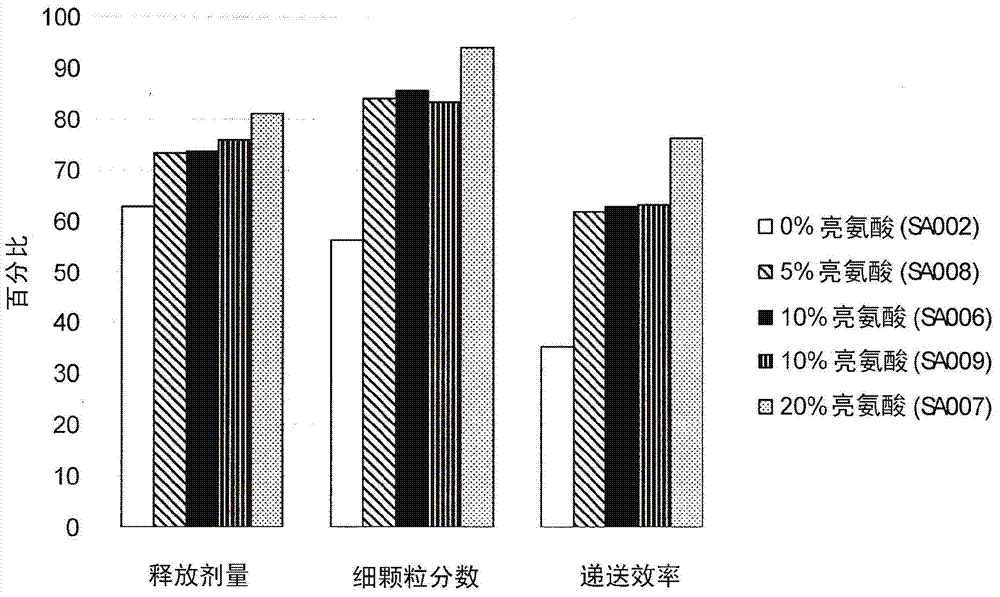
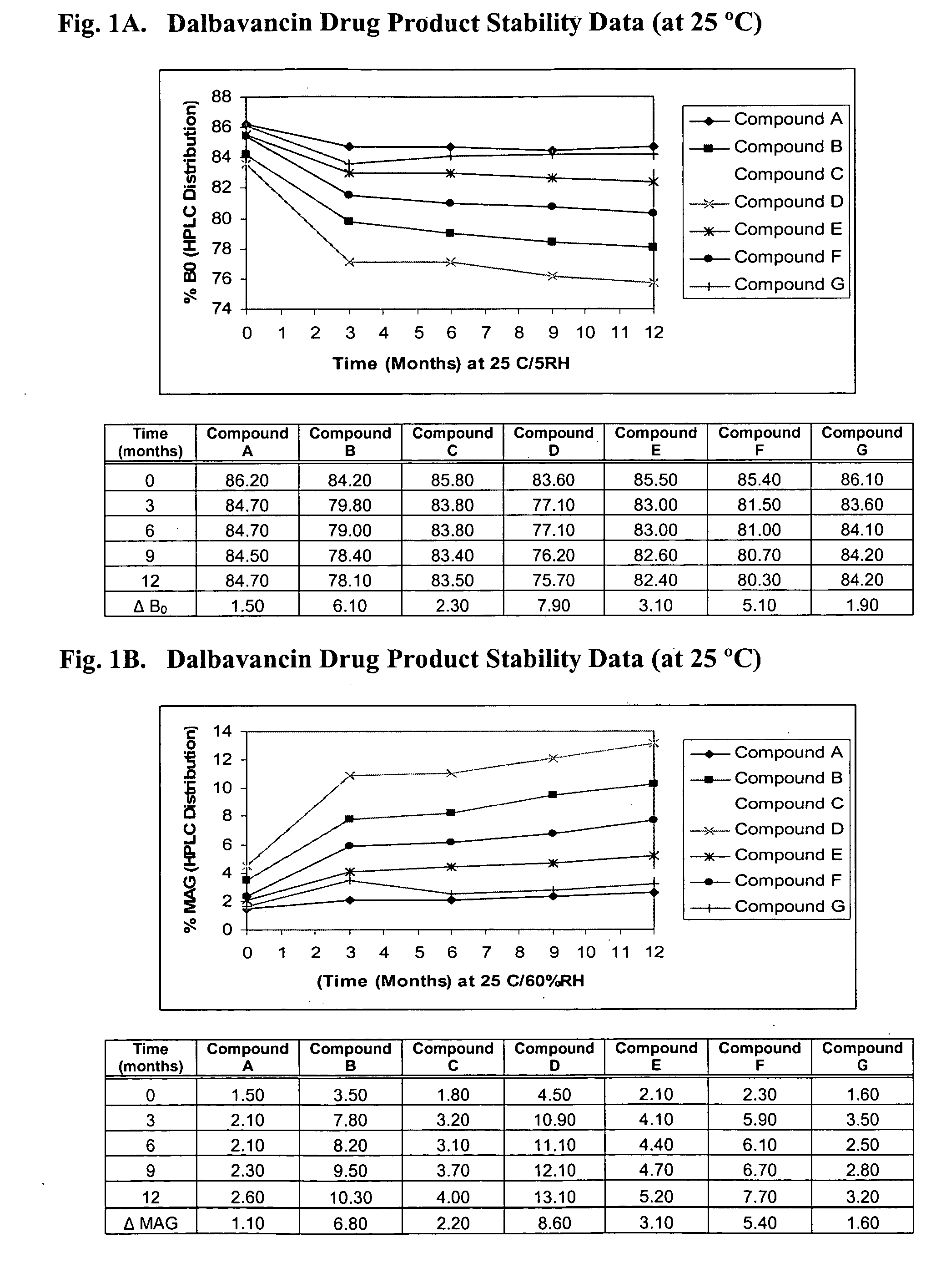
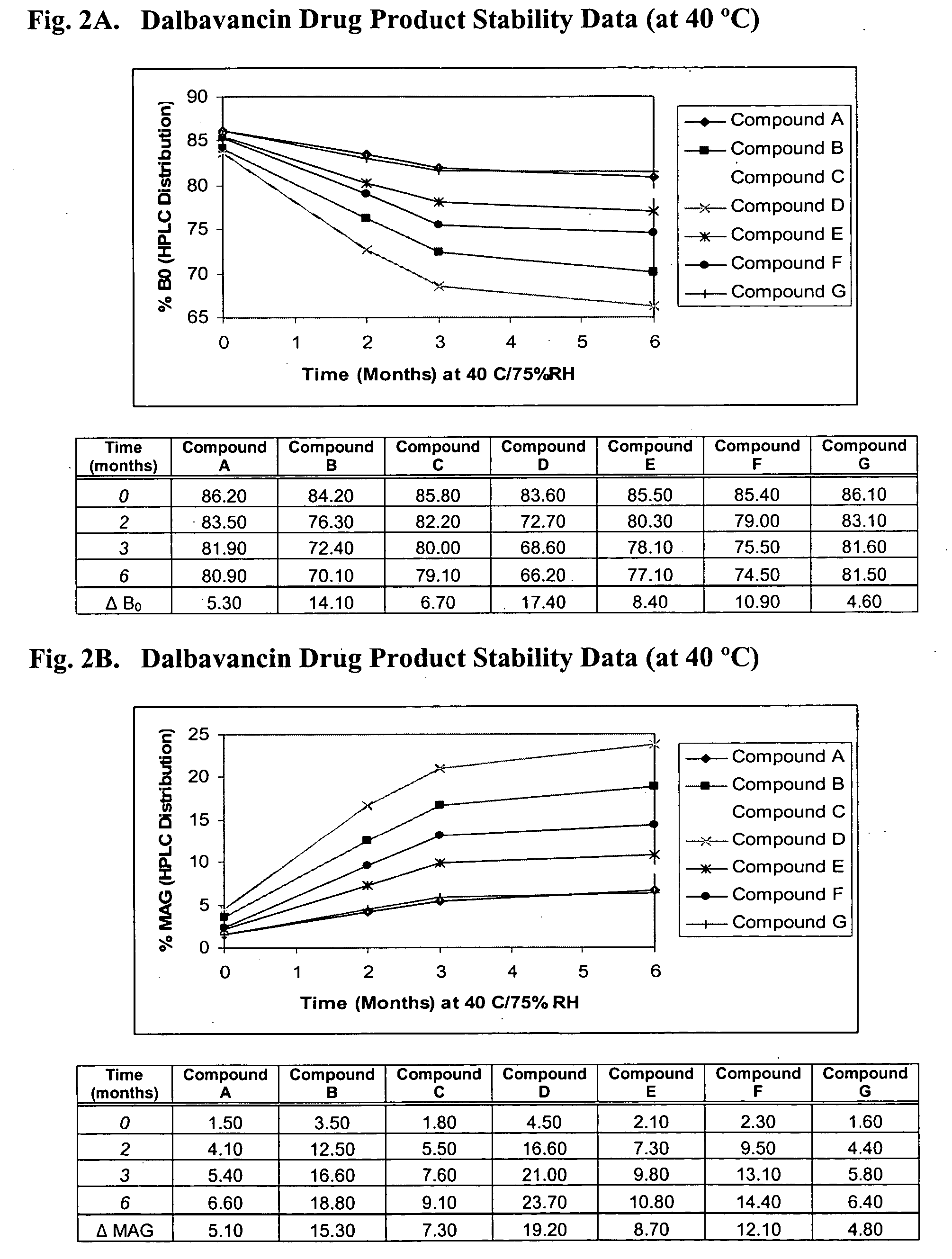
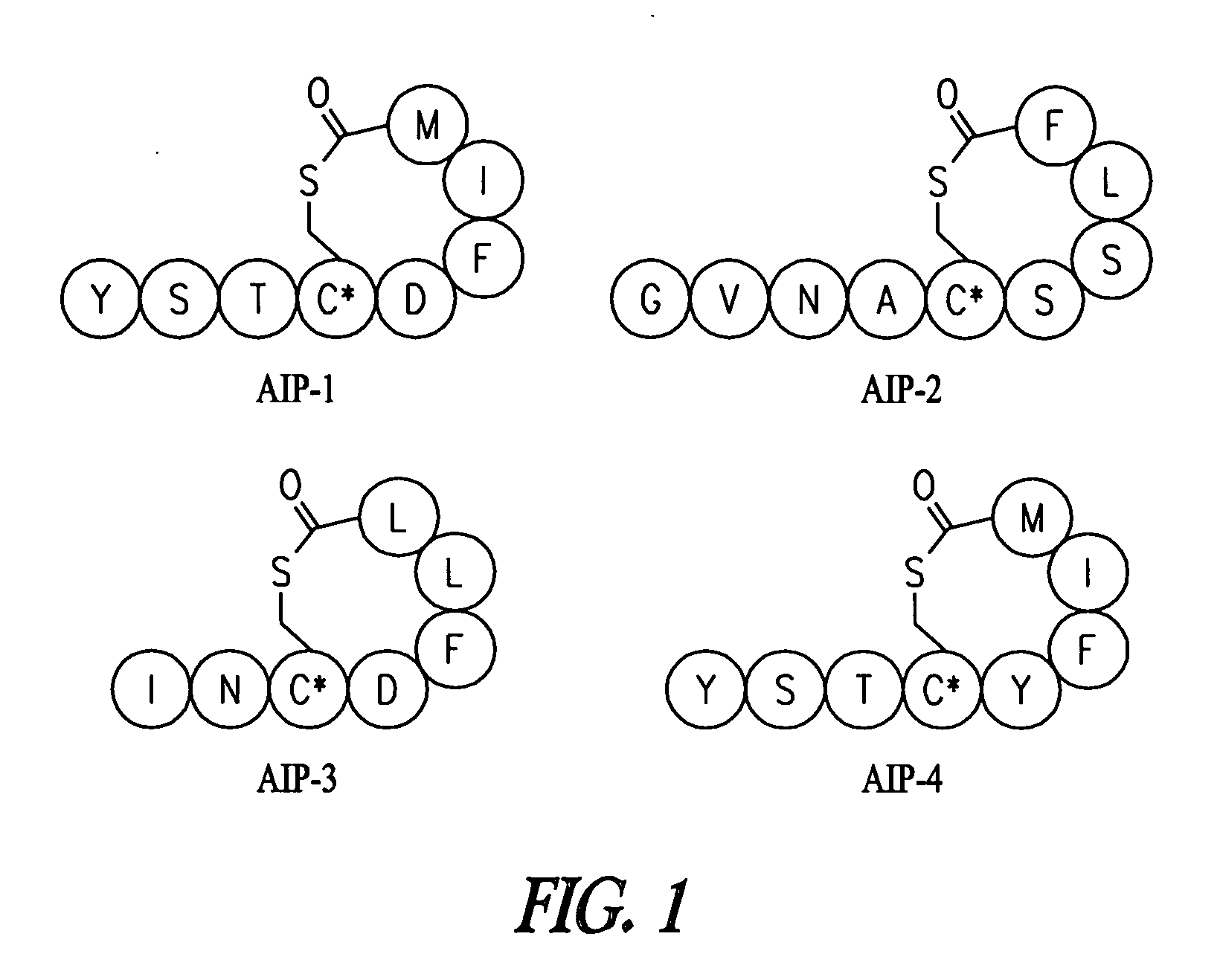
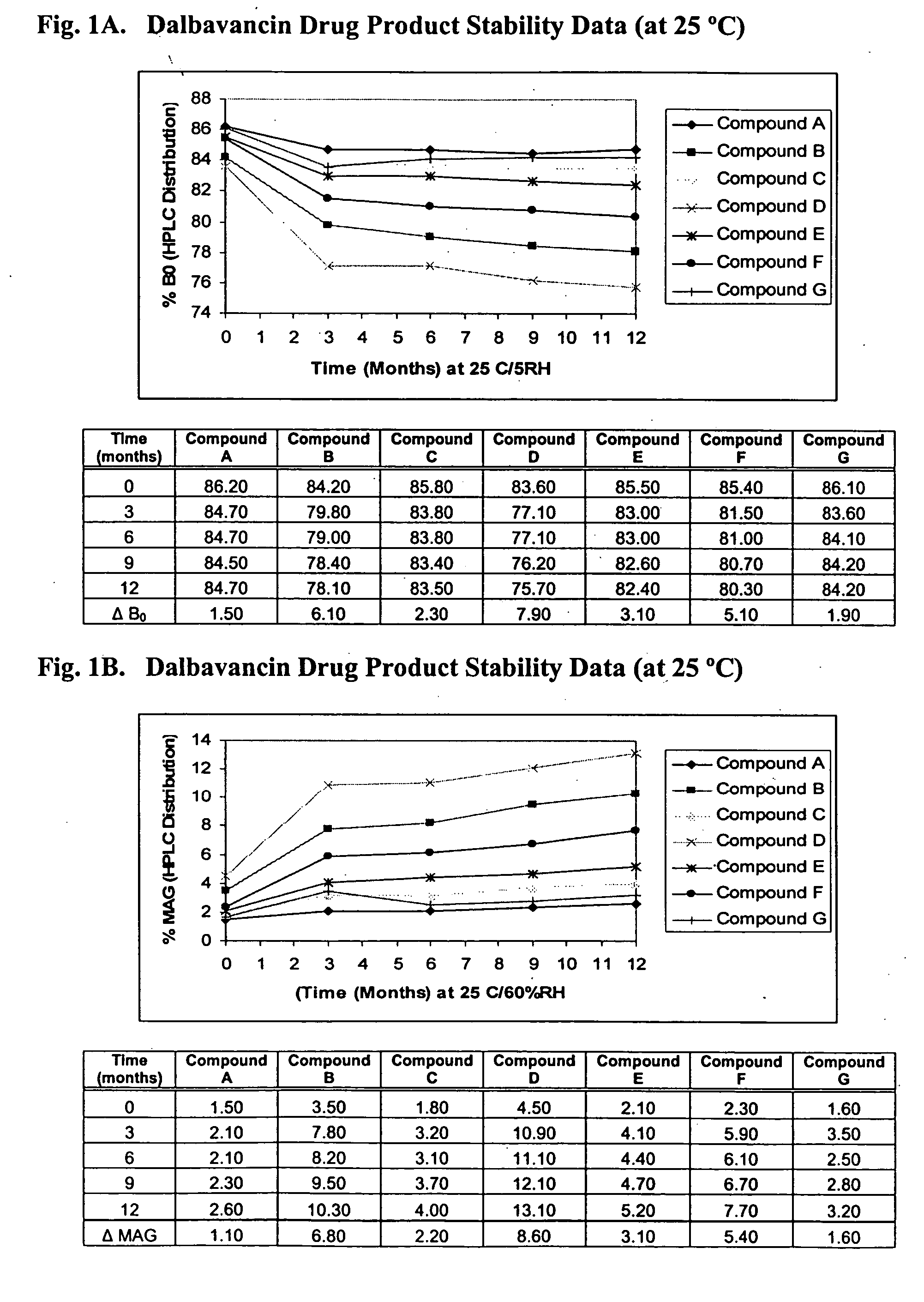
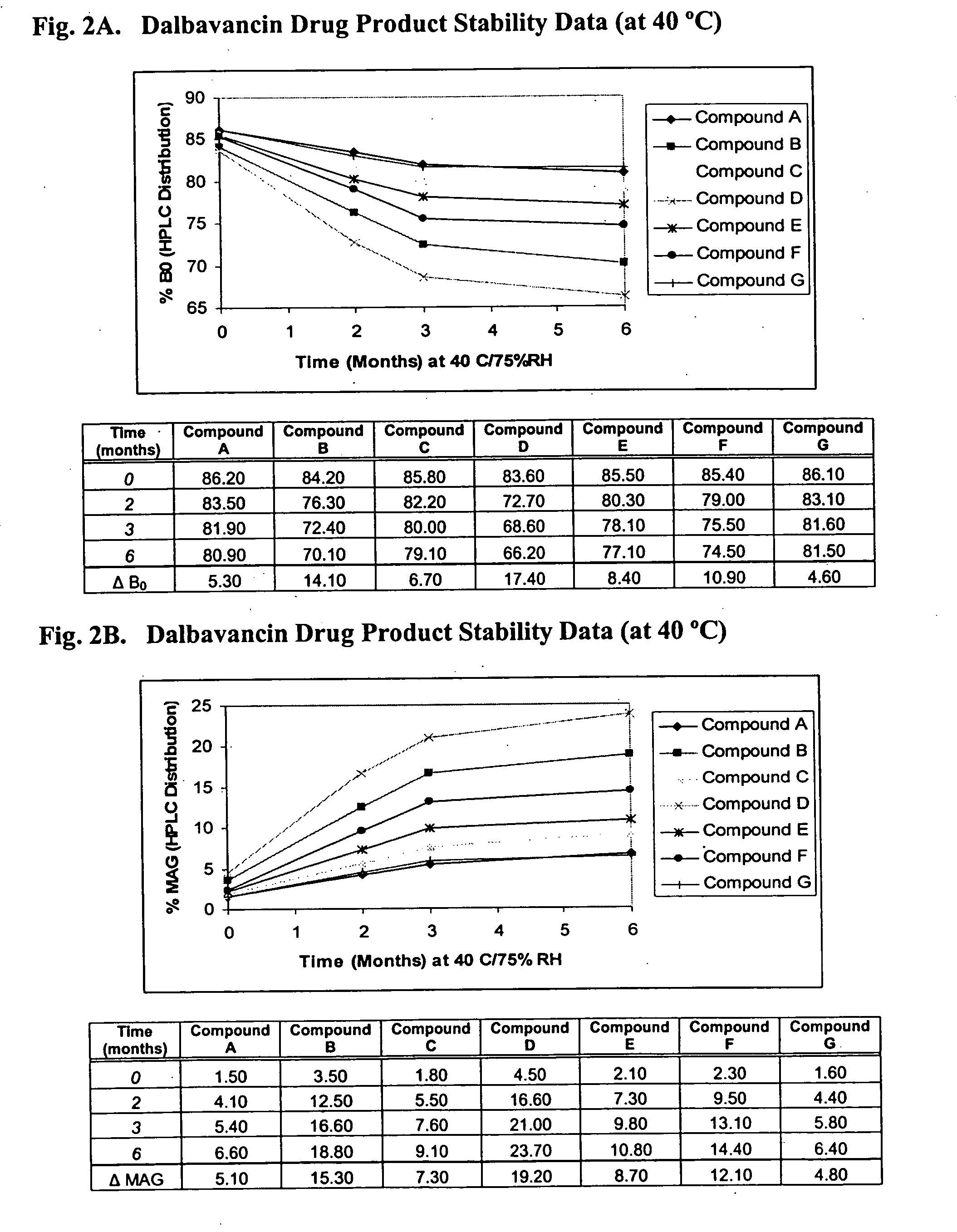
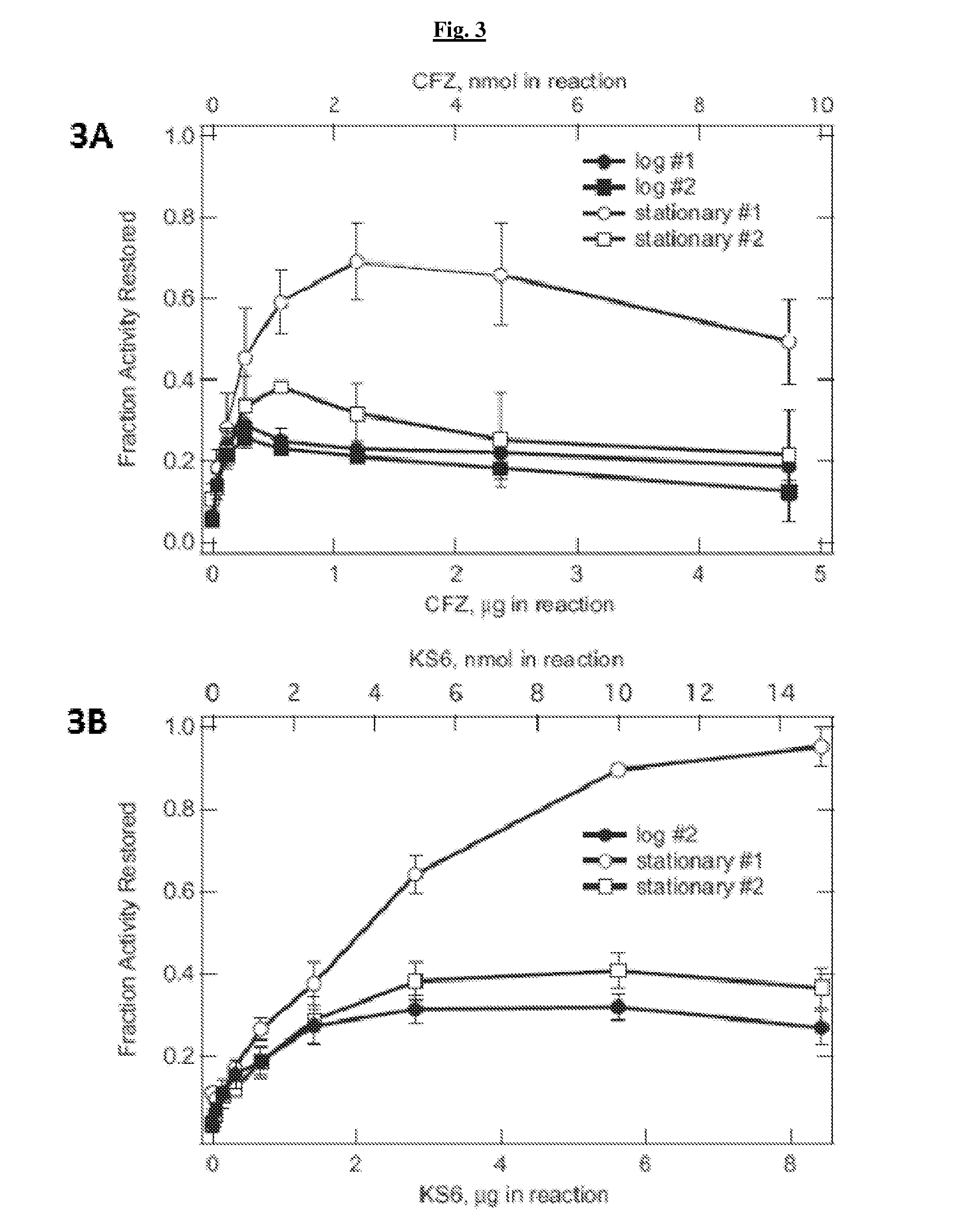
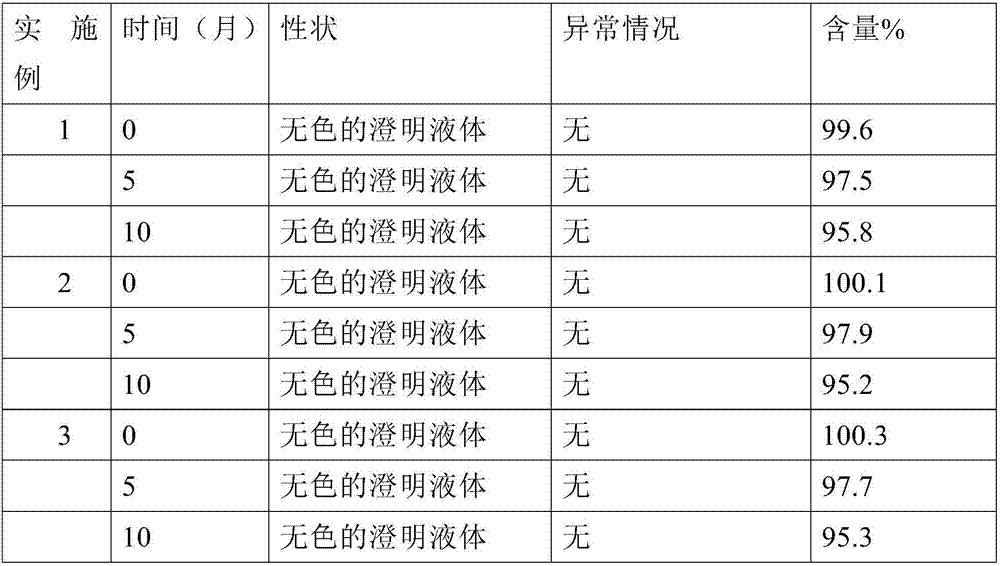
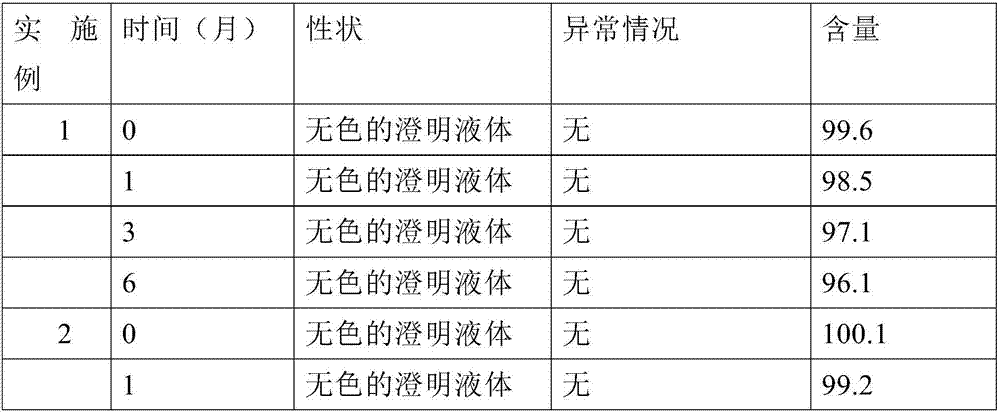
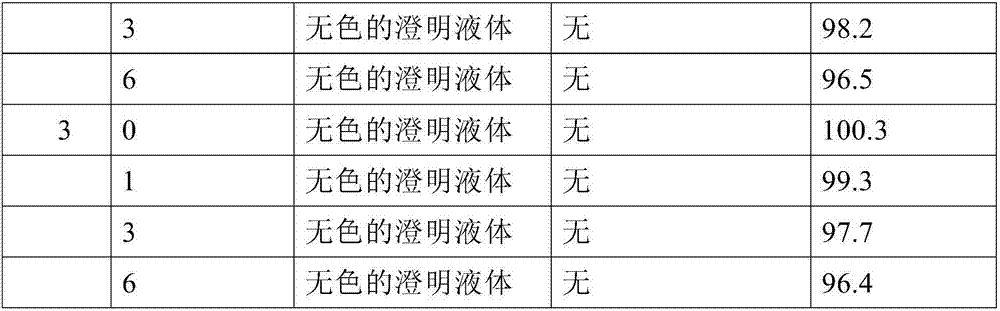
Patents
Literature
59 results about "Gram-positive bacterial infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gram-Positive Bacterial Infections: Infections caused by bacteria that retain the crystal violet stain (positive) when treated by the gram-staining method.
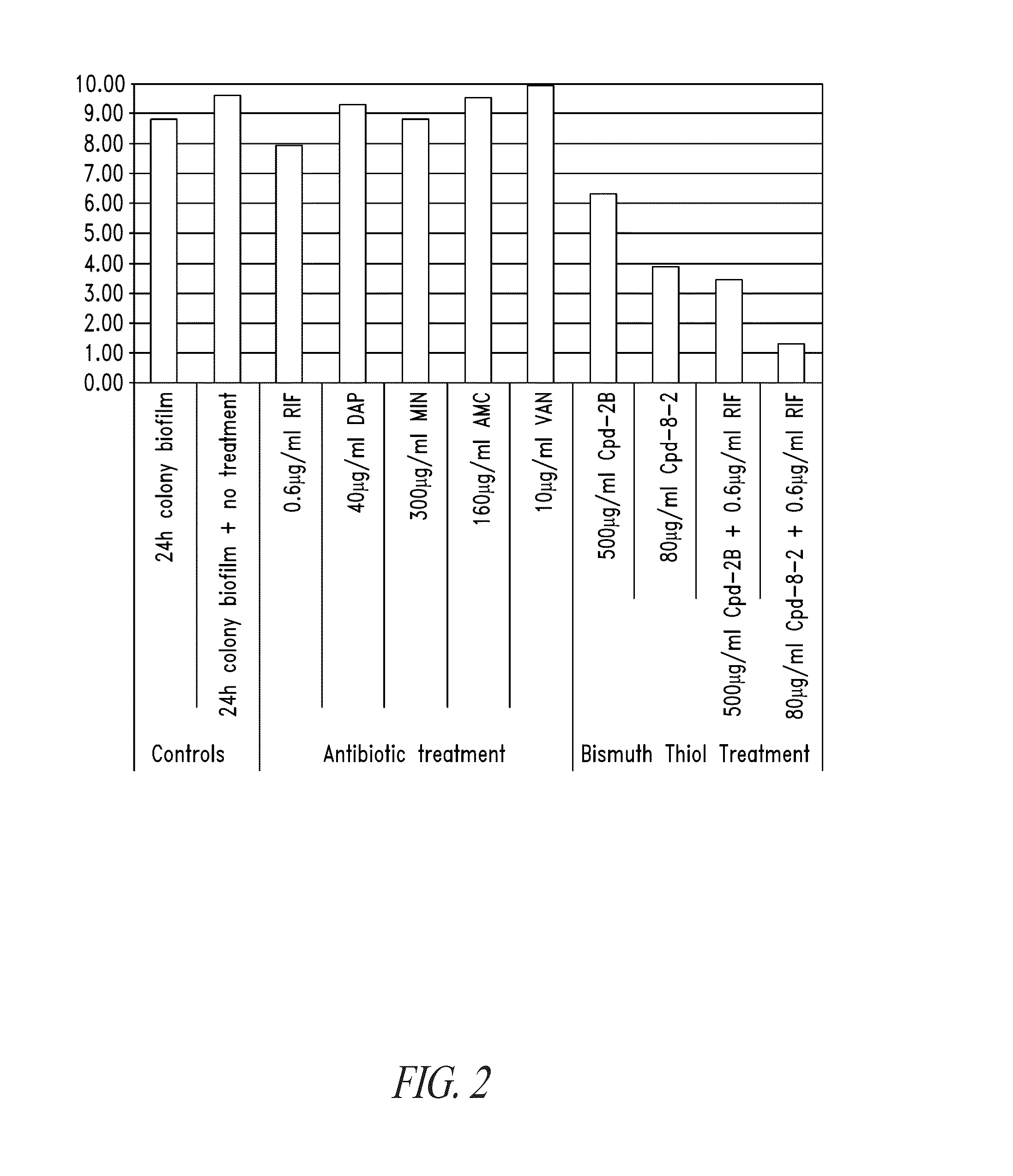
Bismuth-thiols as antiseptics for agricultural, industrial and other uses
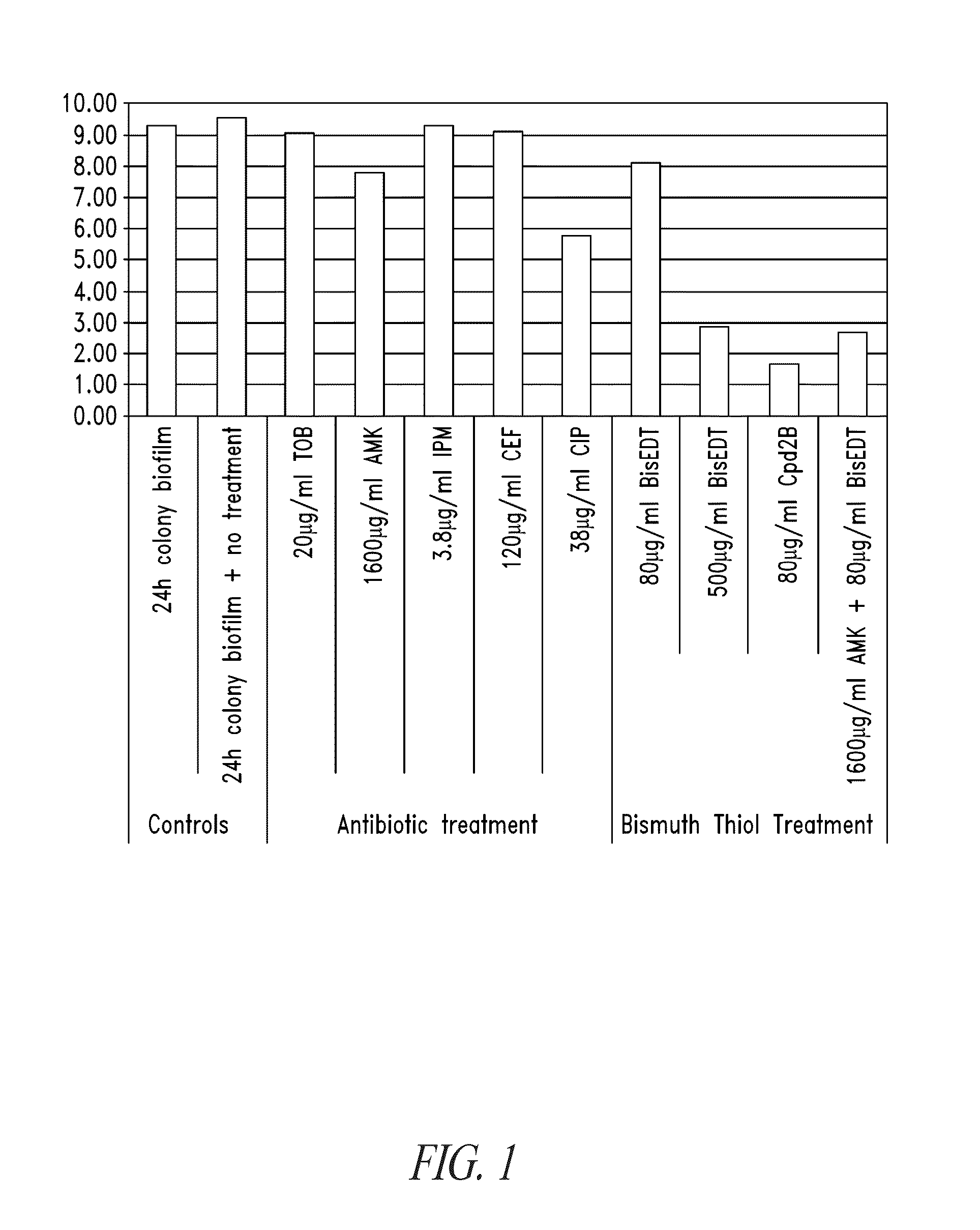
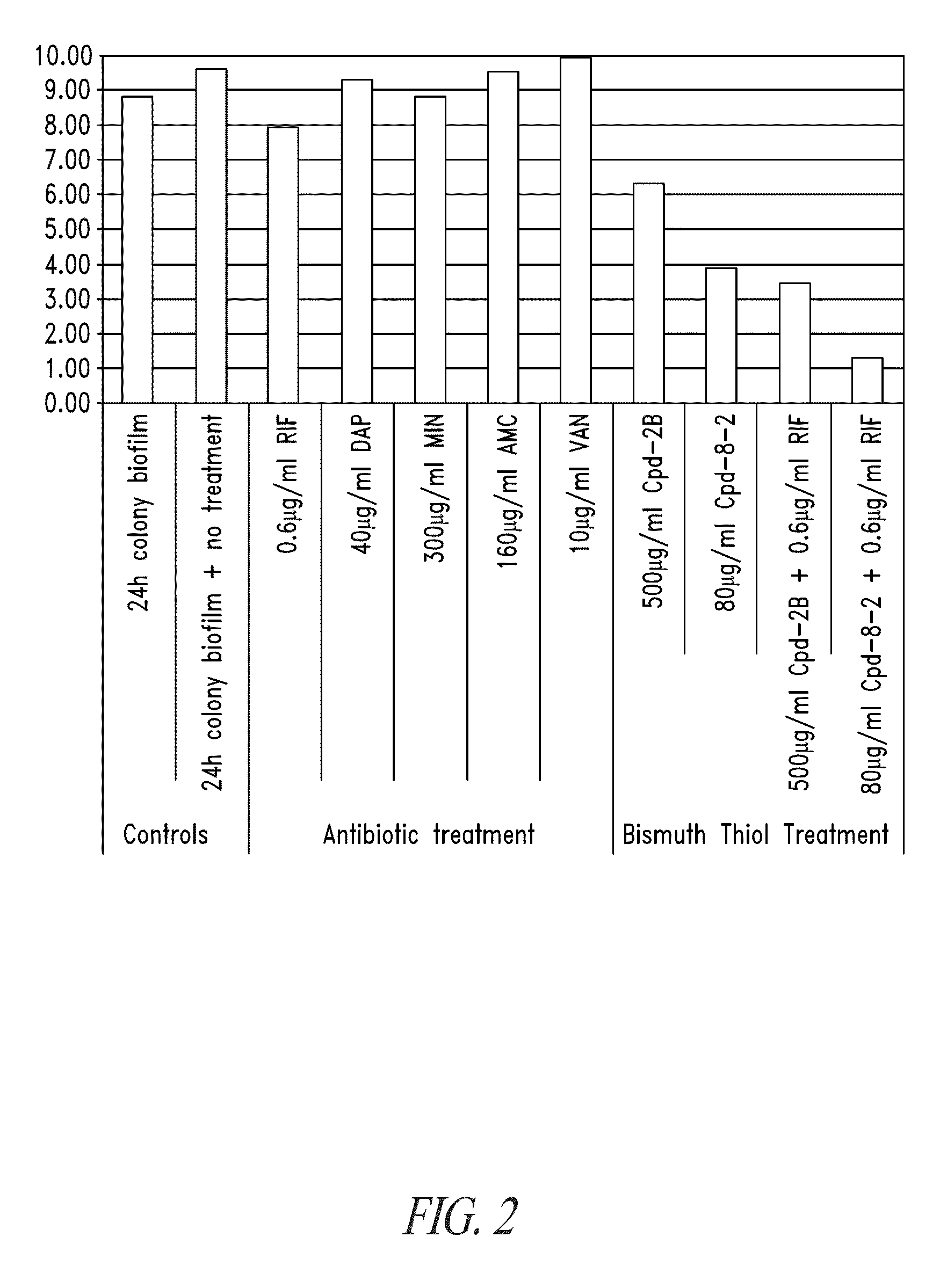
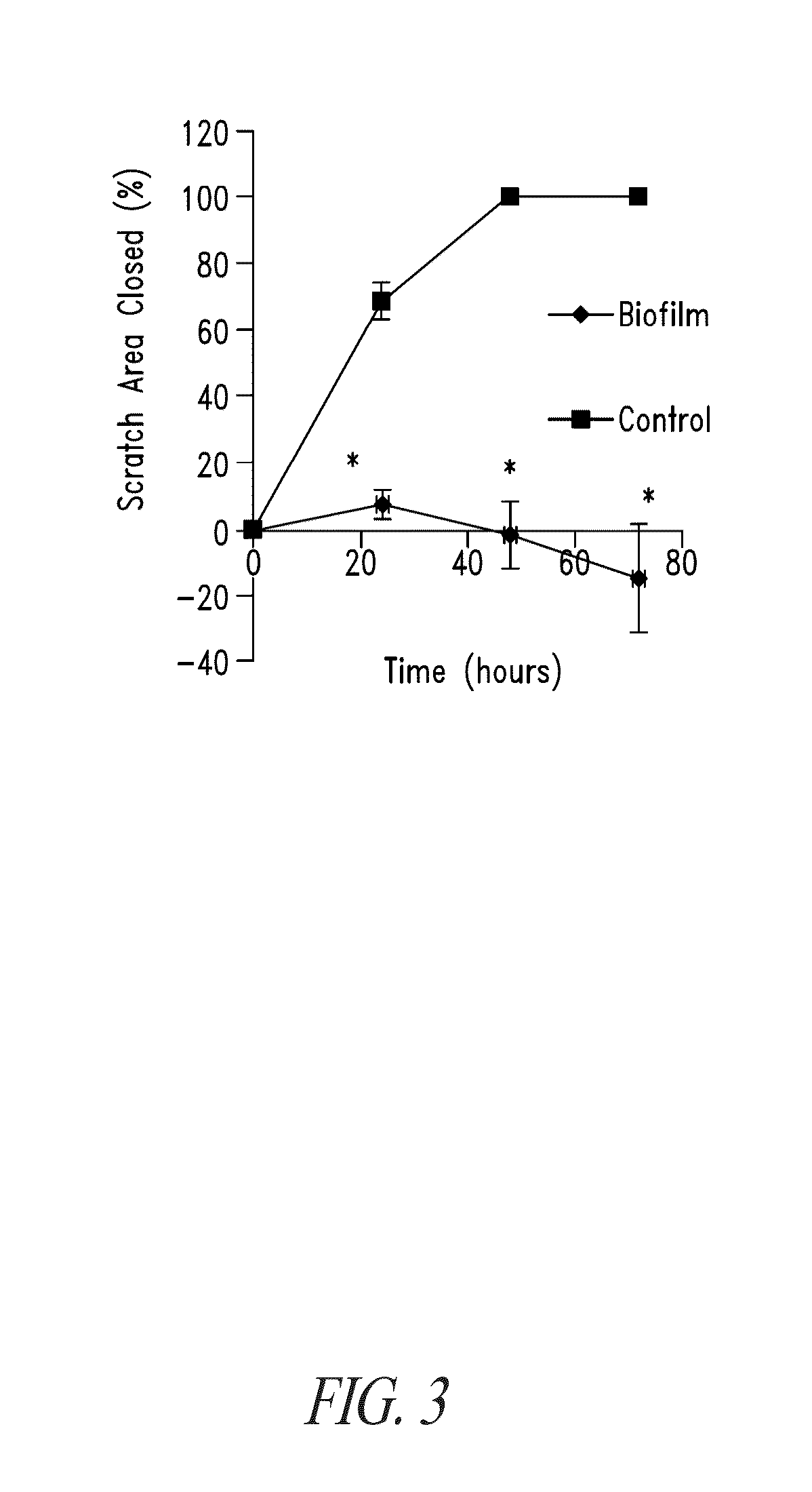
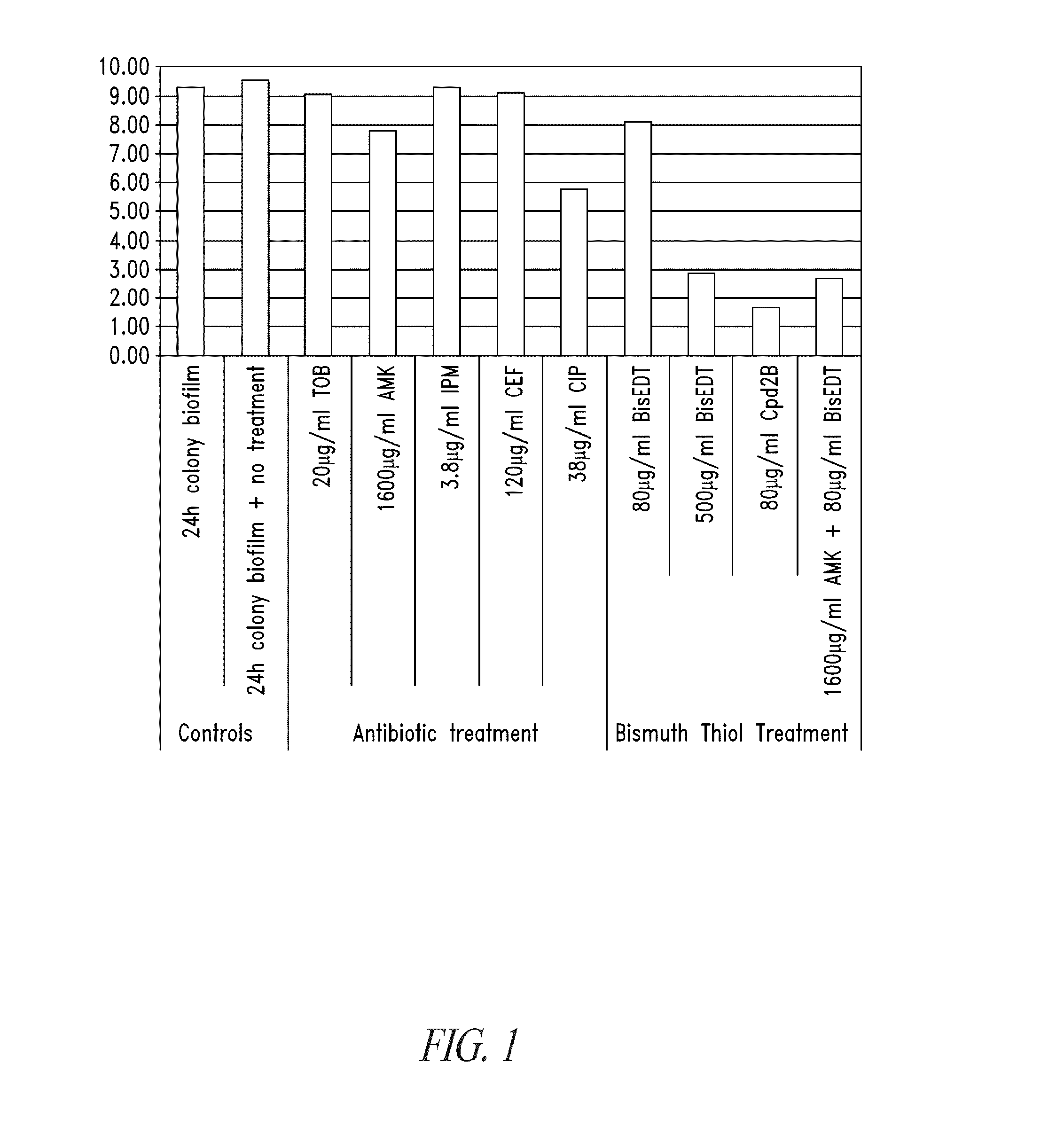
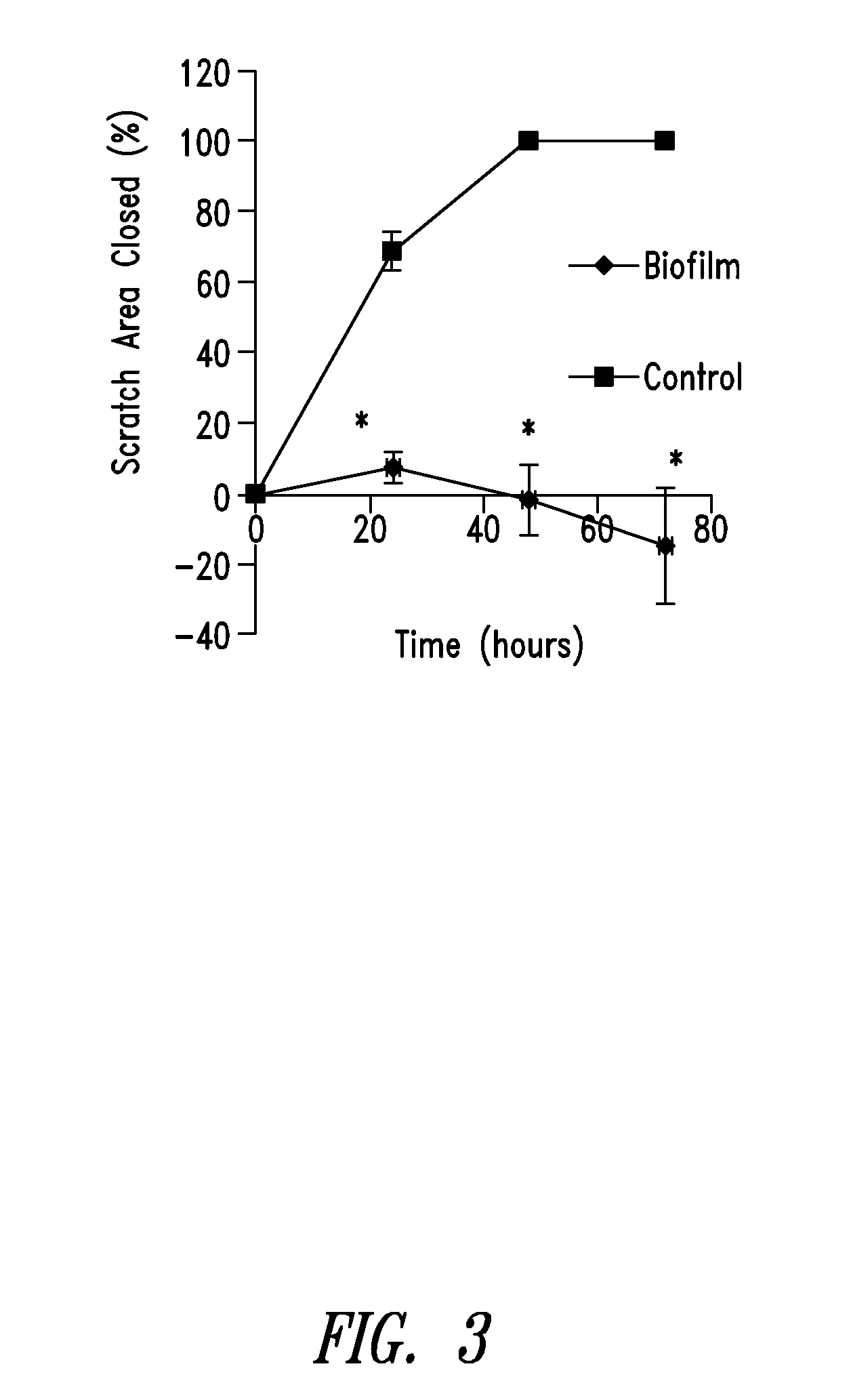
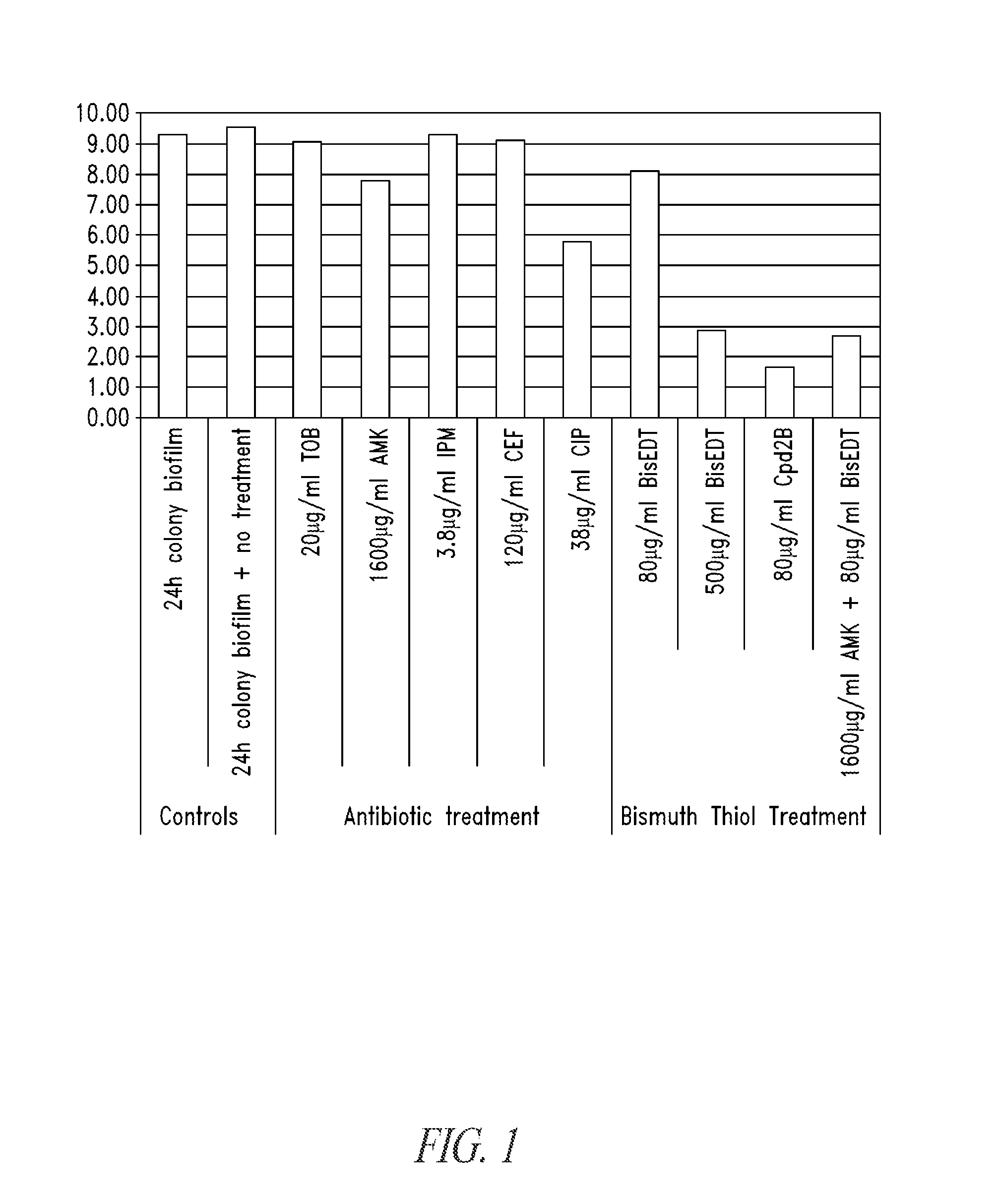
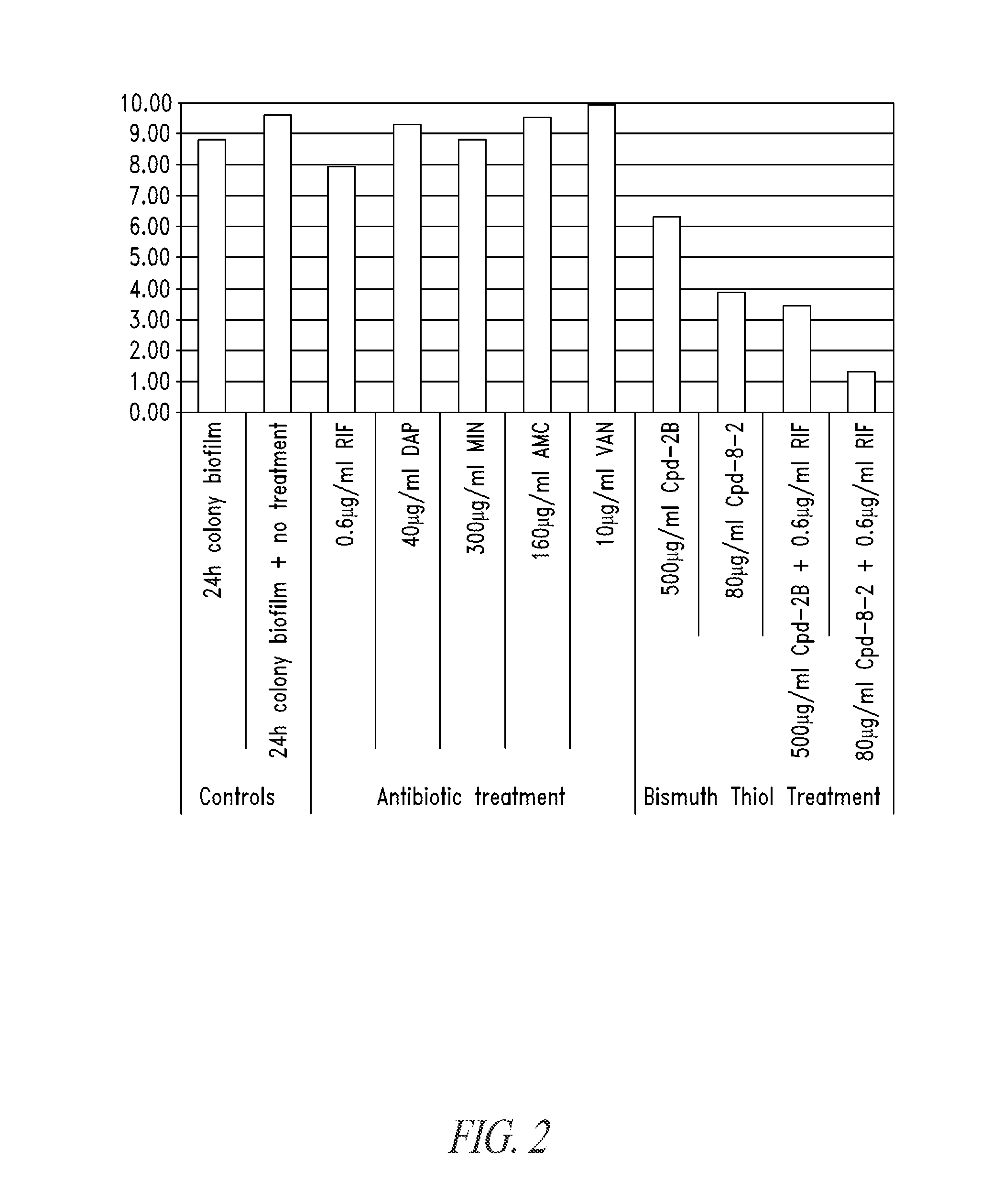
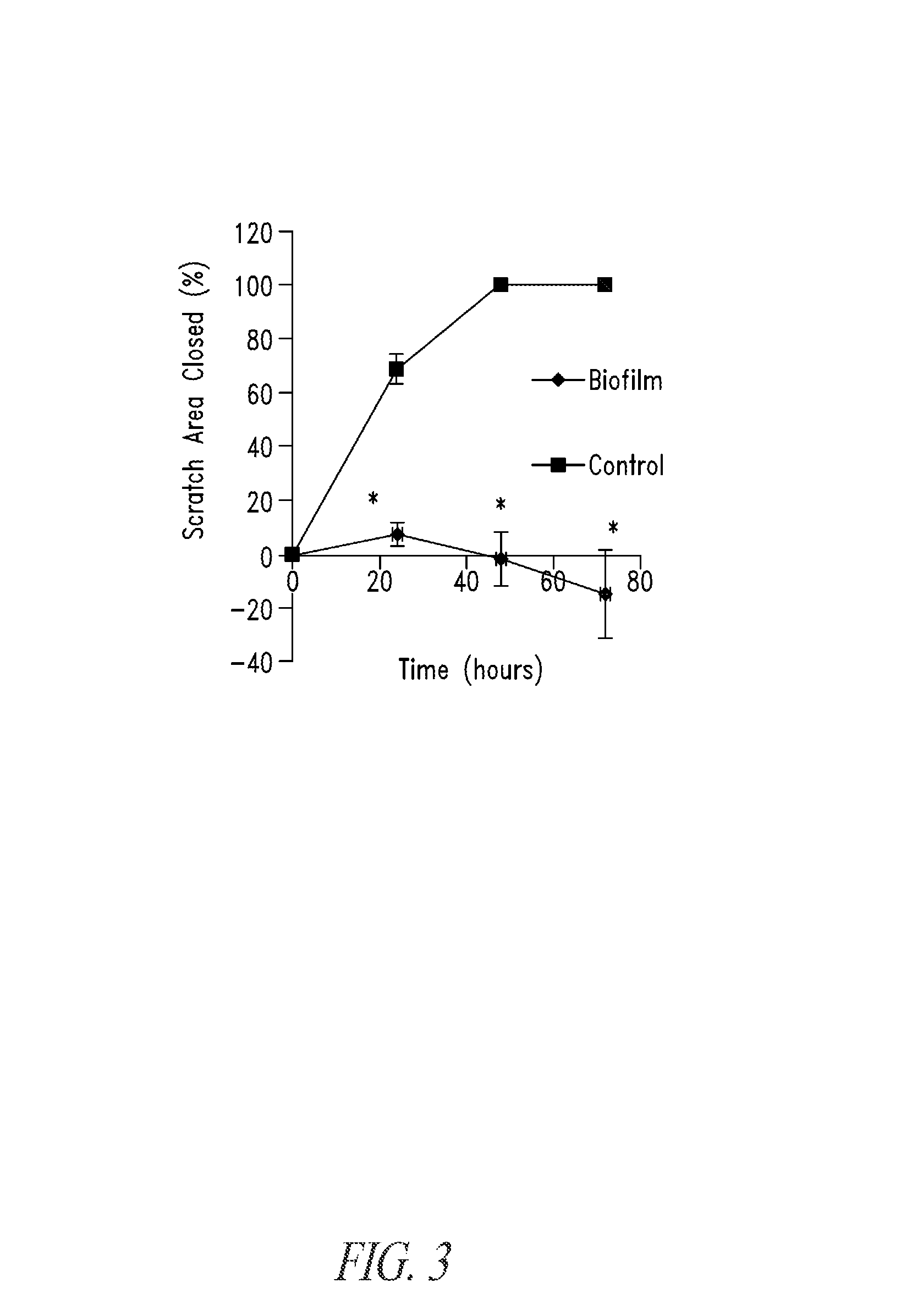
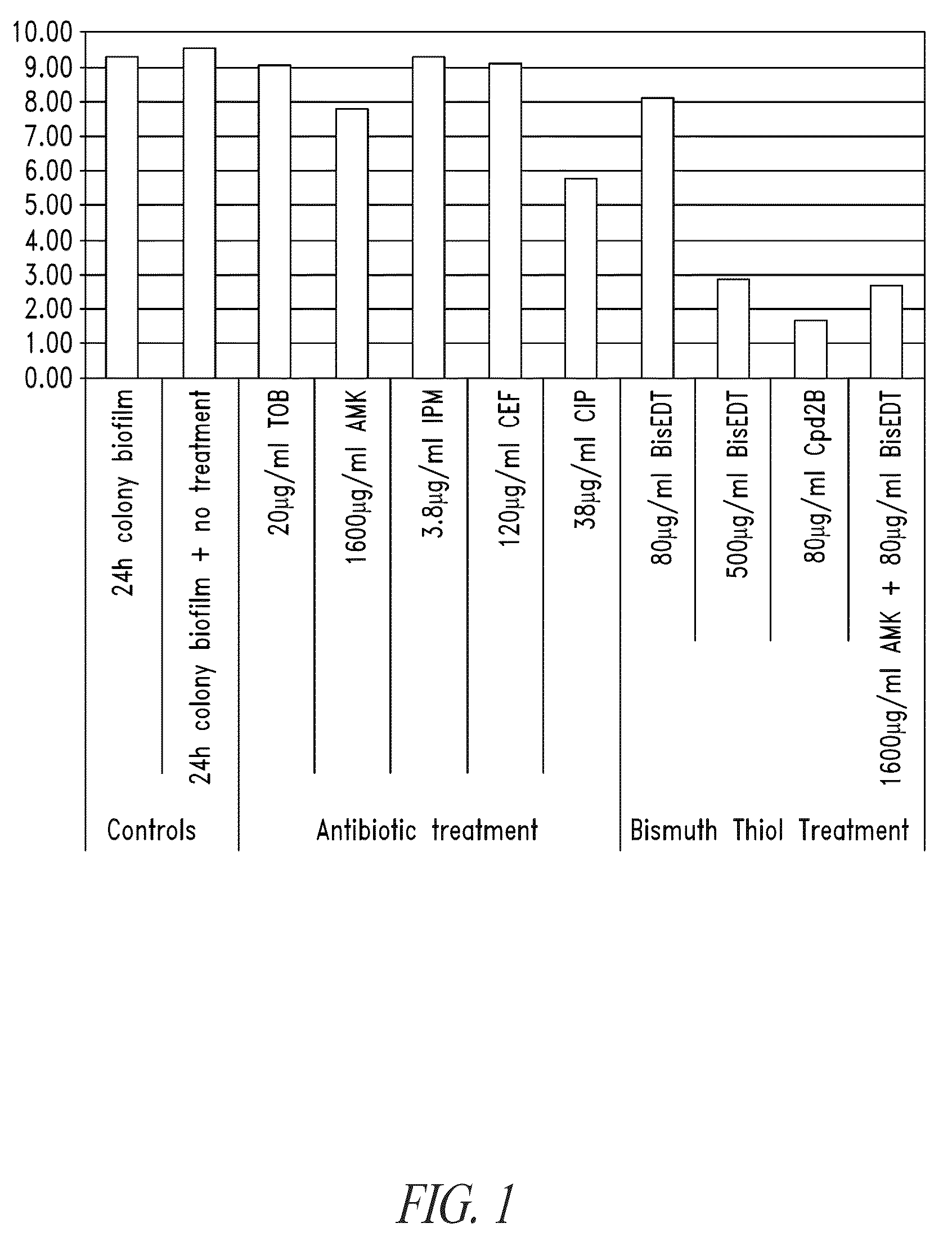
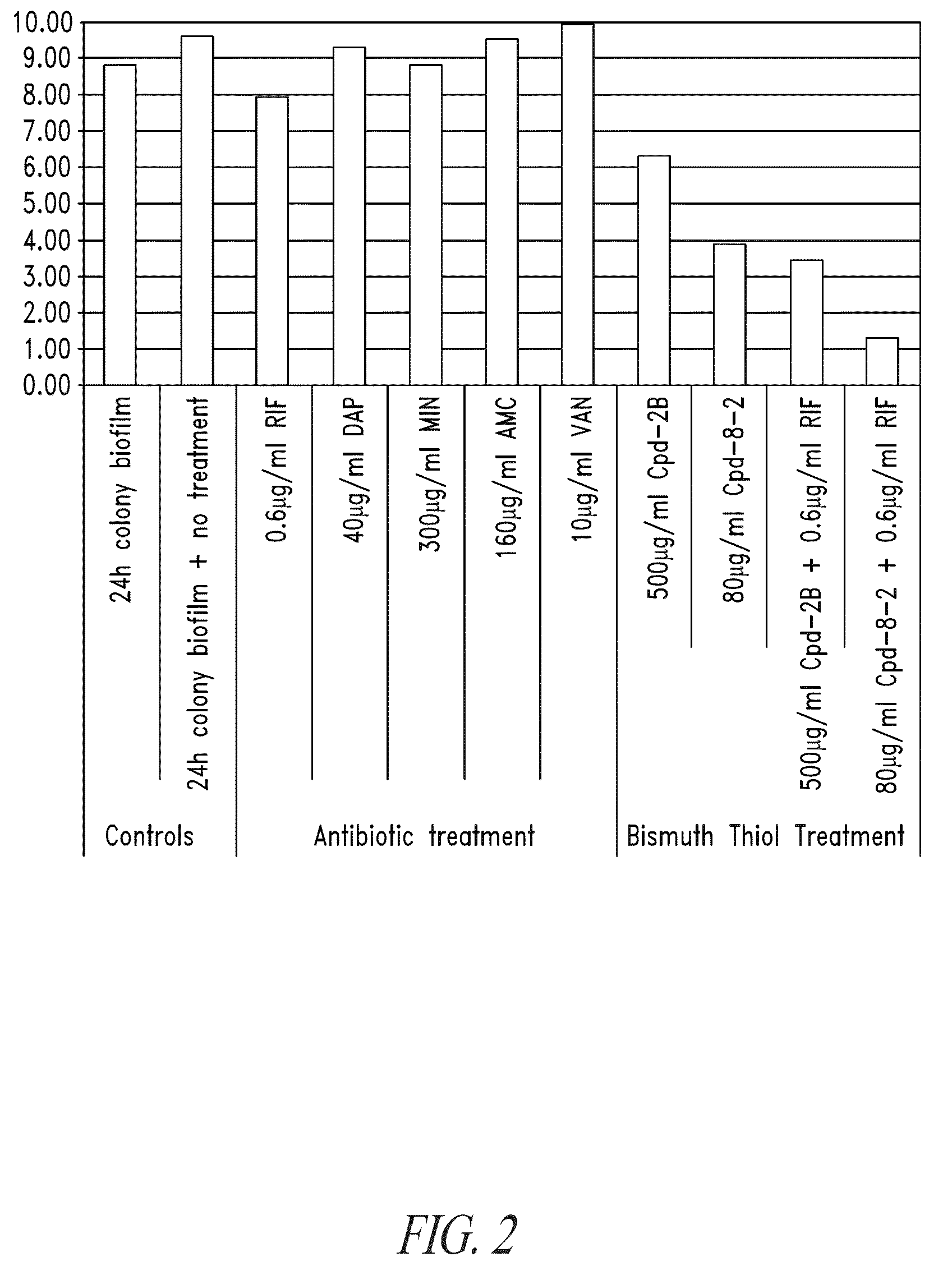
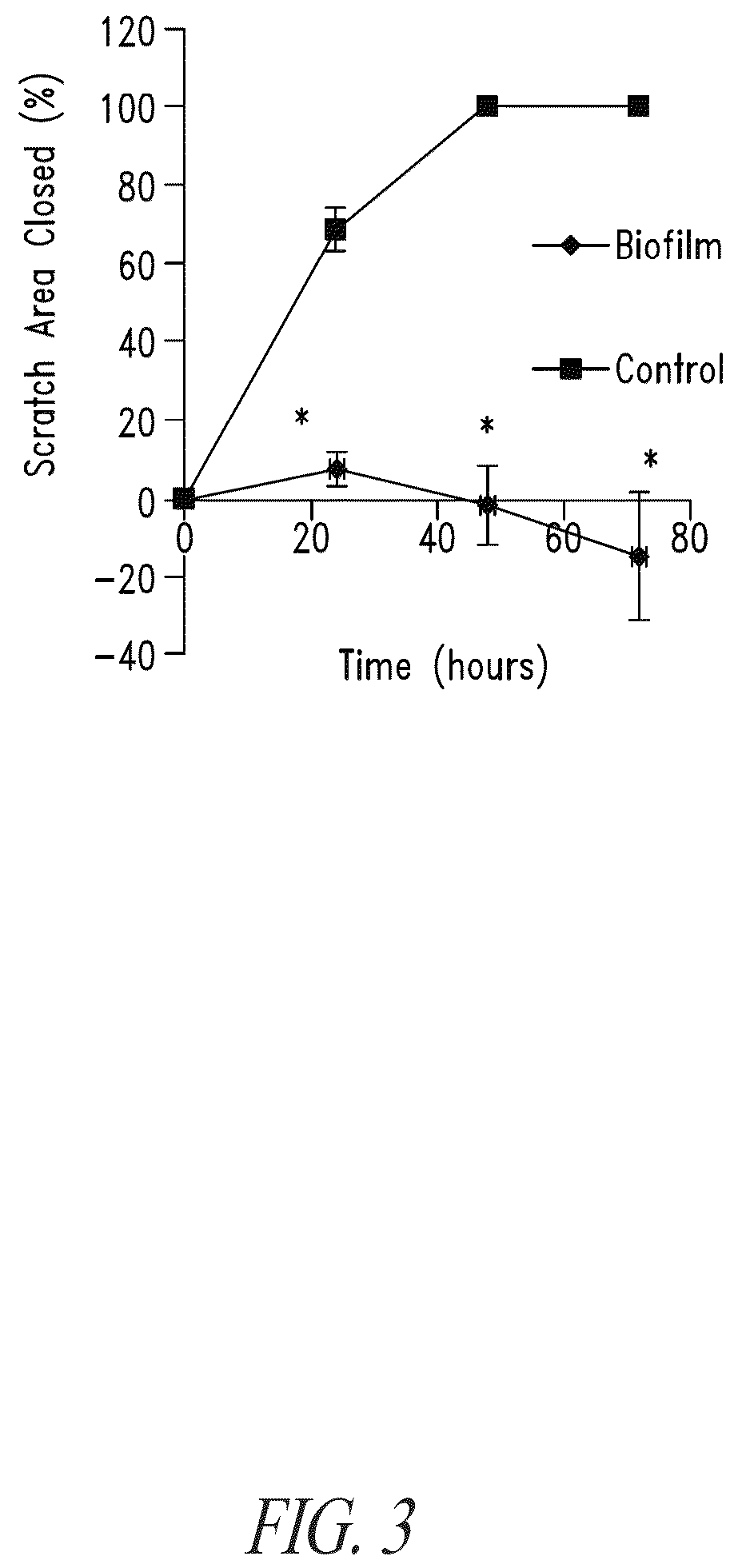
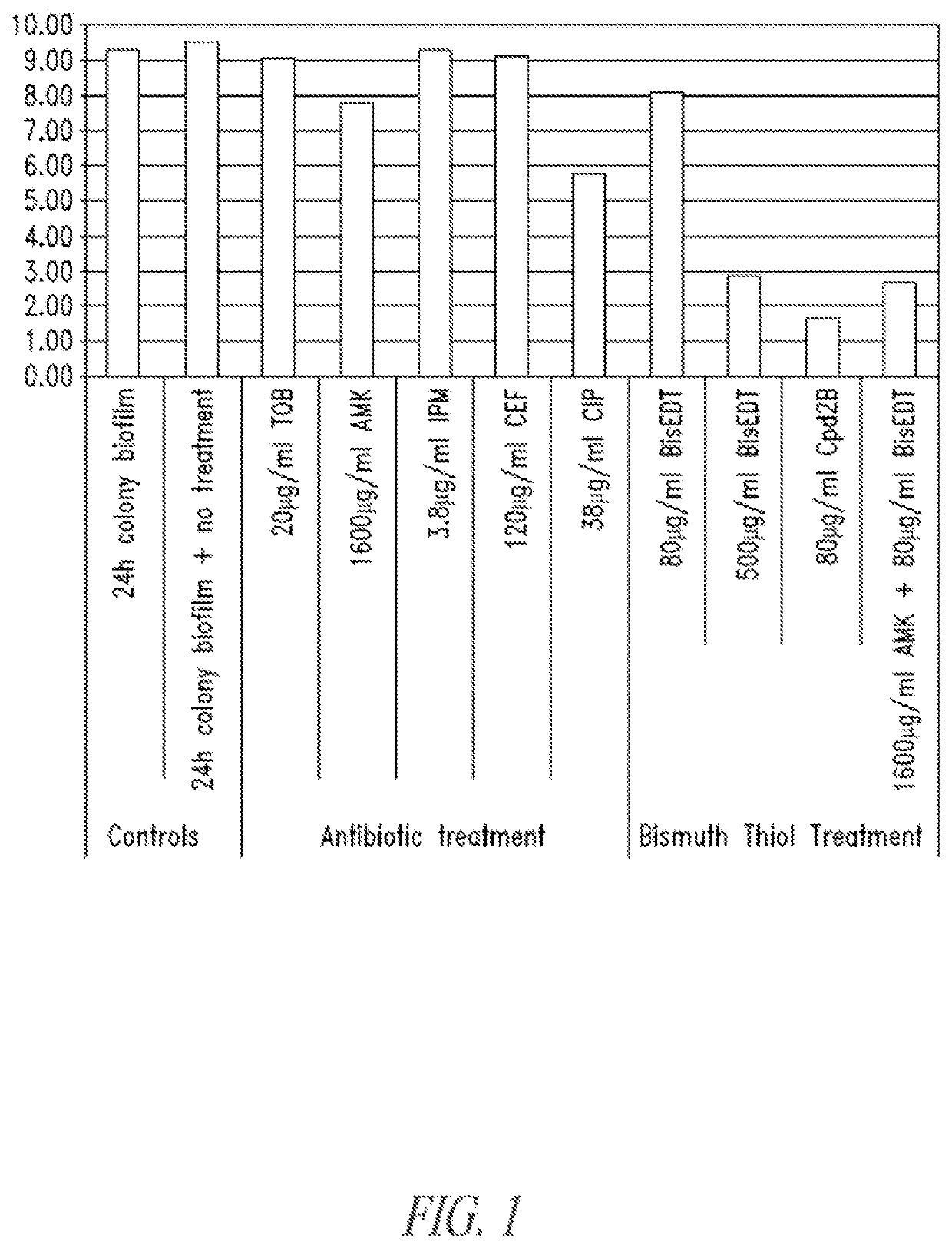
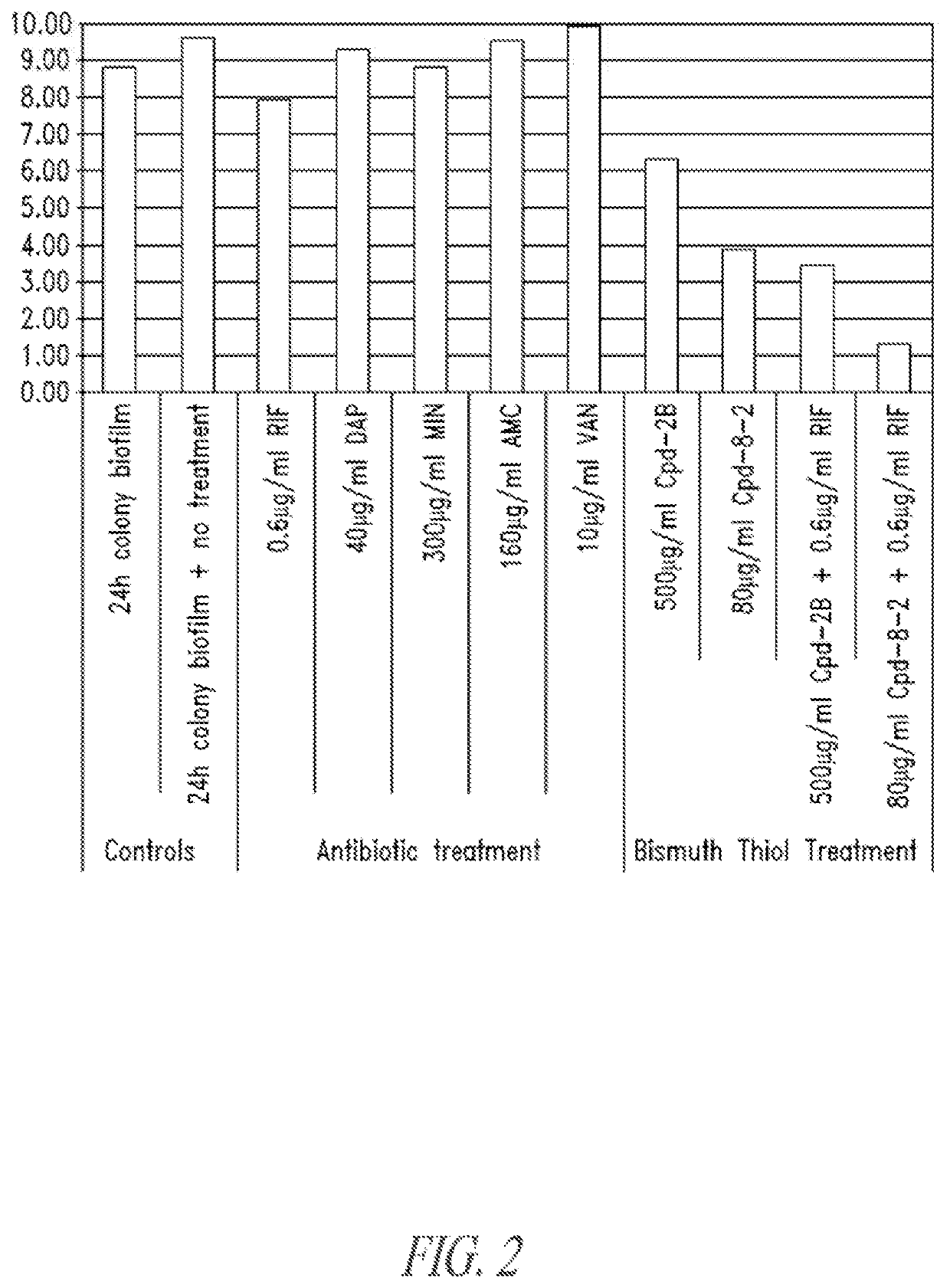
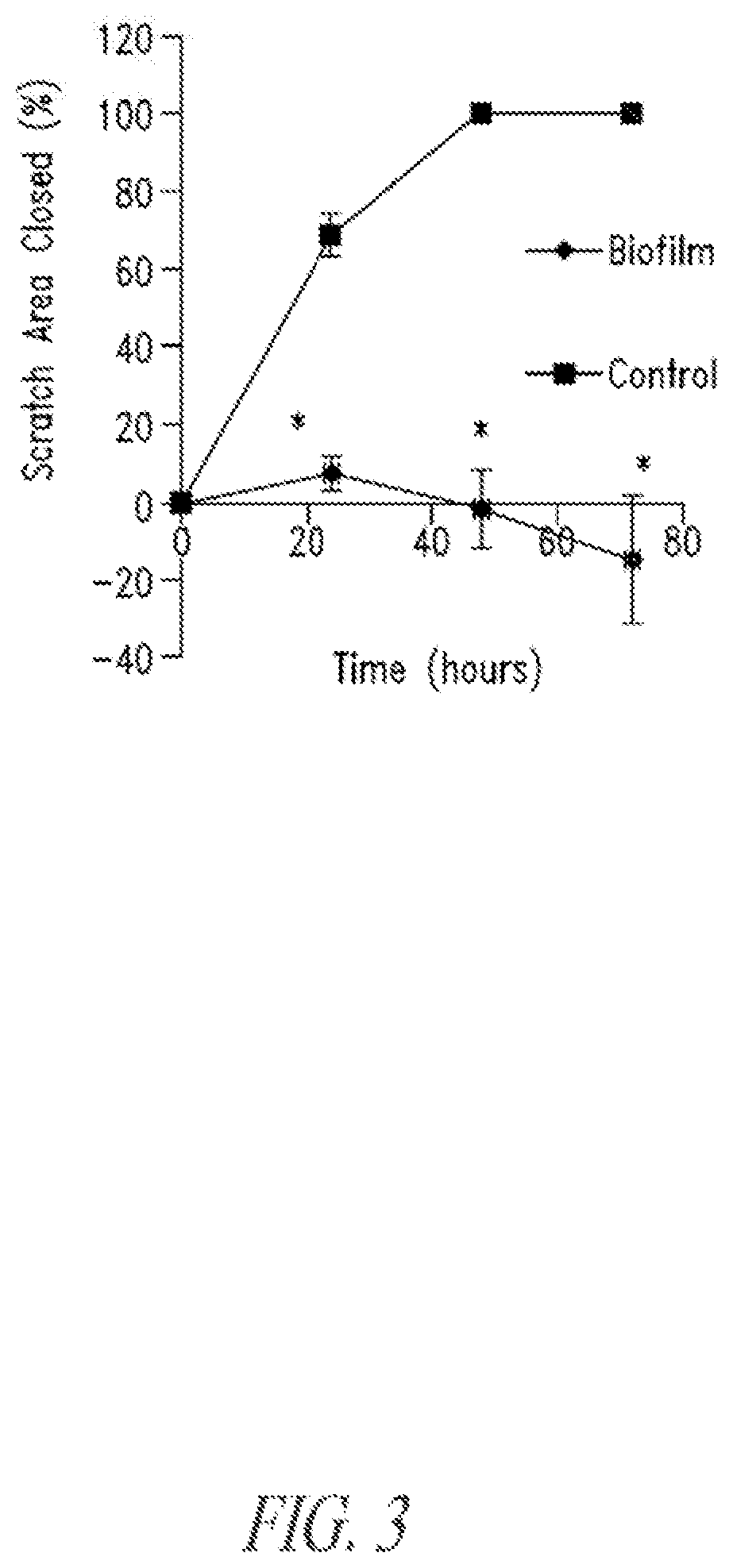
Compositions and methods, including novel homogeneous microparticulate suspensions, are described for treating natural and artificial surfaces that contain bacterial biofilm, including unexpected synergy or enhancing effects between bismuth-thiol (BT) compounds and certain antibiotics, to provide formulations including antiseptic formulations. Previously unpredicted antibacterial properties and anti-biofilm properties of disclosed BT compounds and BT compound-plus-antibiotic combinations are also described, including preferential efficacies of certain such compositions for treating certain gram-positive bacterial infections, and distinct preferential efficacies of certain such compositions for treating certain gram-negative bacterial infections.
Owner:MICROBION
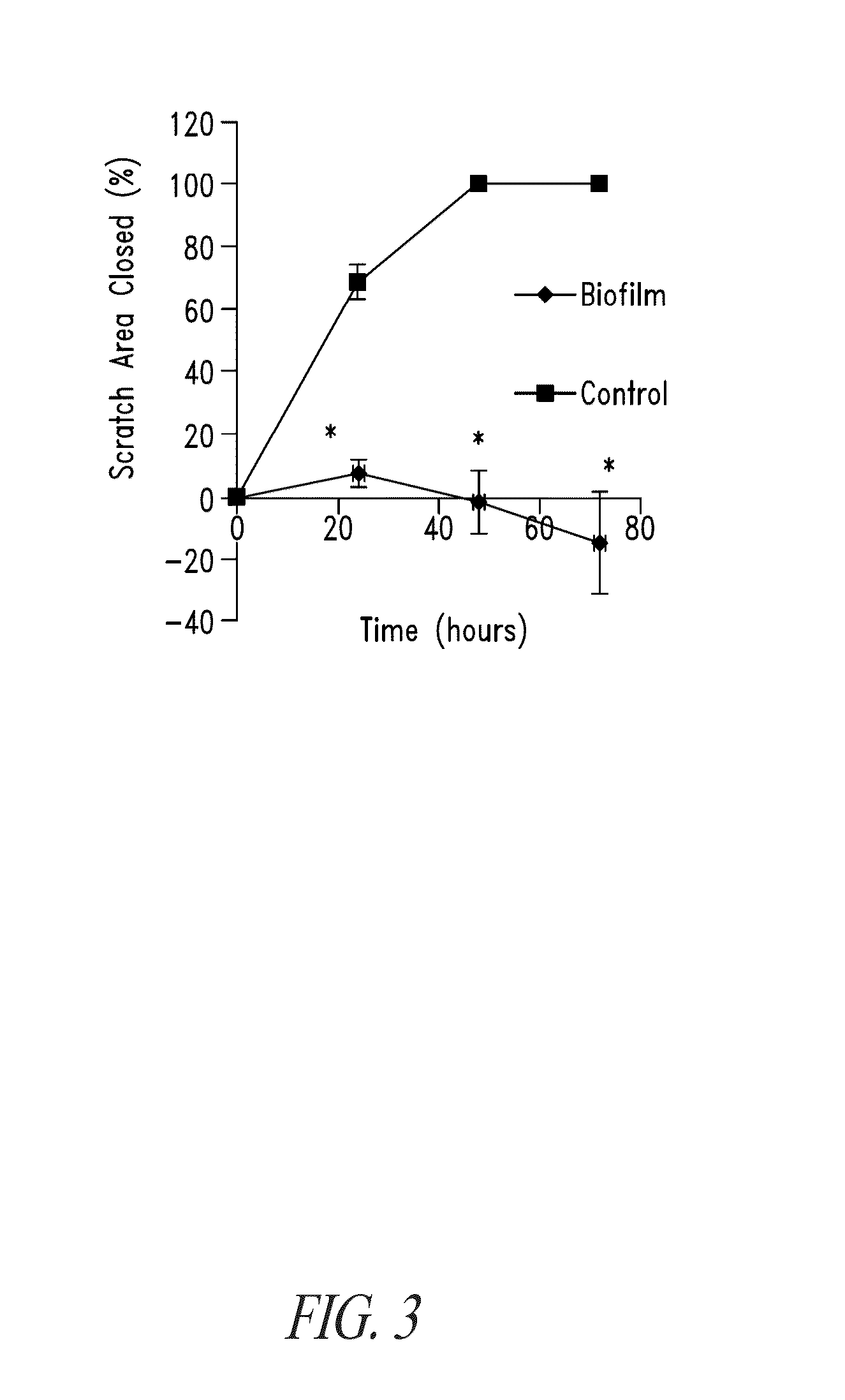
Bismuth-thiols as antiseptics for biomedical uses, including treatment of bacterial biofilms and other uses
ActiveUS20130058983A1Avoid infectionLow costAntibacterial agentsPowder deliveryNatural surfaceGram-positive bacterial infections
Compositions and methods, including novel homogeneous microparticulate suspensions, are described for treating natural surfaces that contain bacterial biofilm, including unexpected synergy or enhancing effects between bismuth-thiol (BT) compounds and certain antibiotics, to provide formulations including antiseptic formulations. Previously unpredicted antibacterial properties and anti-biofilm properties of disclosed BT compounds and BT compound-plus-antibiotic combinations are also described, including preferential efficacies of certain such compositions for treating certain gram-positive bacterial infections, and distinct preferential efficacies of certain such compositions for treating certain gram-negative bacterial infections.
Owner:MICROBION
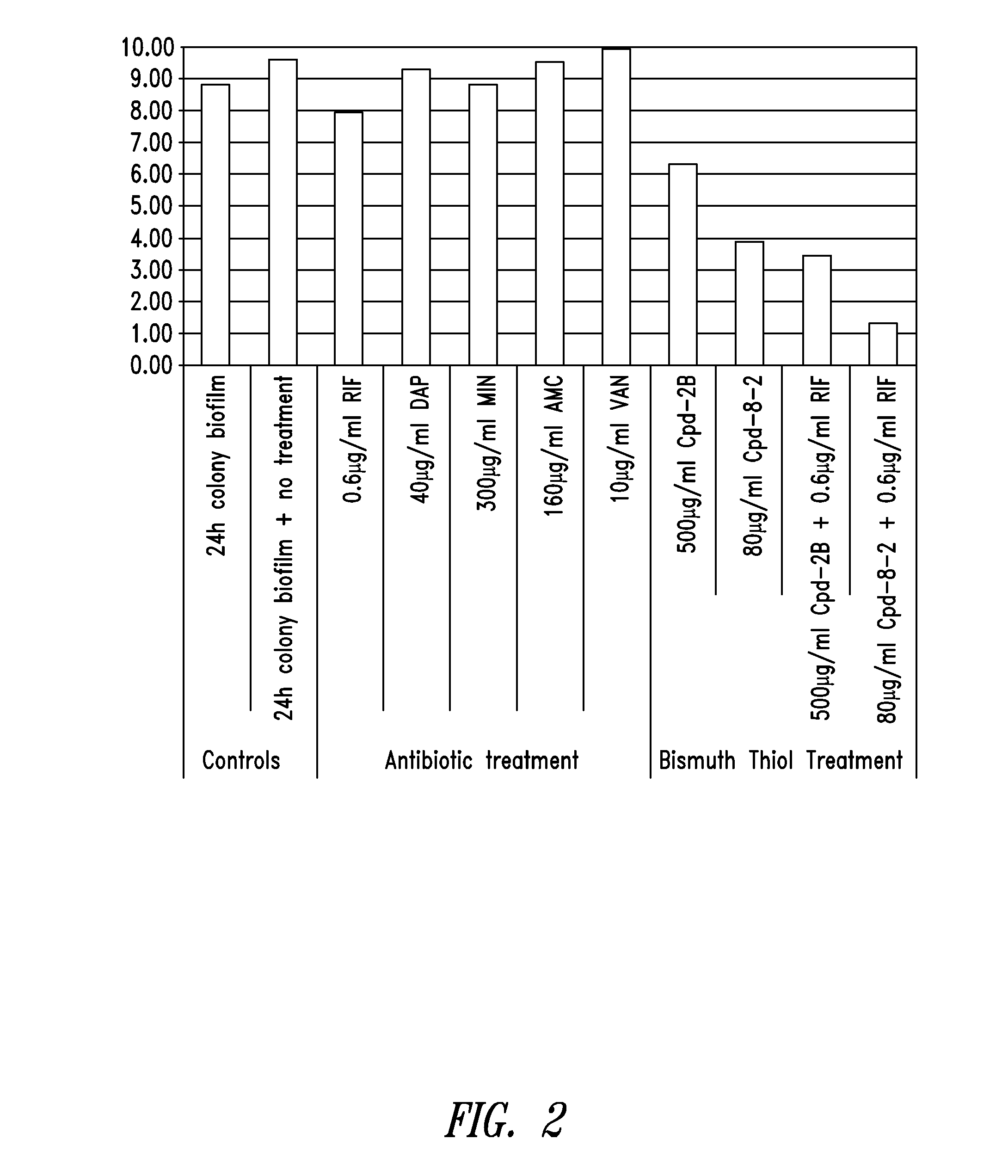
Bismuth-thiols as antiseptics for epithelial tissues, acute and chronic wounds, bacterial biofilms and other indications
ActiveUS8389021B2Reduce in quantityLow costAntibacterial agentsPowder deliveryBacteroidesInjury mouth
Compositions and methods, including novel homogeneous microparticulate suspensions, are described for treating acute wounds, chronic wounds and / or a wound or epithelial tissue surface that contains bacterial biofilm, including unexpected synergy between bismuth-thiol (BT) compounds and certain antibiotics, to provide topical formulations including antiseptic formulations, for management and promotion of wound healing and in particular infected wounds. Previously unpredicted antibacterial properties and anti-biofilm properties of disclosed BT compounds and BT compound-plus-antibiotic combinations are also described, including preferential efficacies of certain such compositions for treating gram-positive bacterial infections, and distinct preferential efficacies of certain such compositions for treating gram-negative bacterial infections.
Owner:MICROBION
Bismuth-thiols as antiseptics for agricultural, industrial and other uses
ActiveUS20130224258A1Low costBiocideSurgical adhesivesGram-positive bacterial infectionsAntibiotic Y
Compositions and methods, including novel homogeneous microparticulate suspensions, are described for treating natural and artificial surfaces that contain bacterial biofilm, including unexpected synergy or enhancing effects between bismuth-thiol (BT) compounds and certain antibiotics, to provide formulations including antiseptic formulations. Previously unpredicted antibacterial properties and anti-biofilm properties of disclosed BT compounds and BT compound-plus-antibiotic combinations are also described, including preferential efficacies of certain such compositions for treating certain gram-positive bacterial infections, and distinct preferential efficacies of certain such compositions for treating certain gram-negative bacterial infections.
Owner:MICROBION
Antibacterial combination therapy for the treatment of gram positive bacterial infections
InactiveUS20110257078A1Reduce and eliminate contaminationAvoid developmentAntibacterial agentsBiocideBacteroidesMedicine
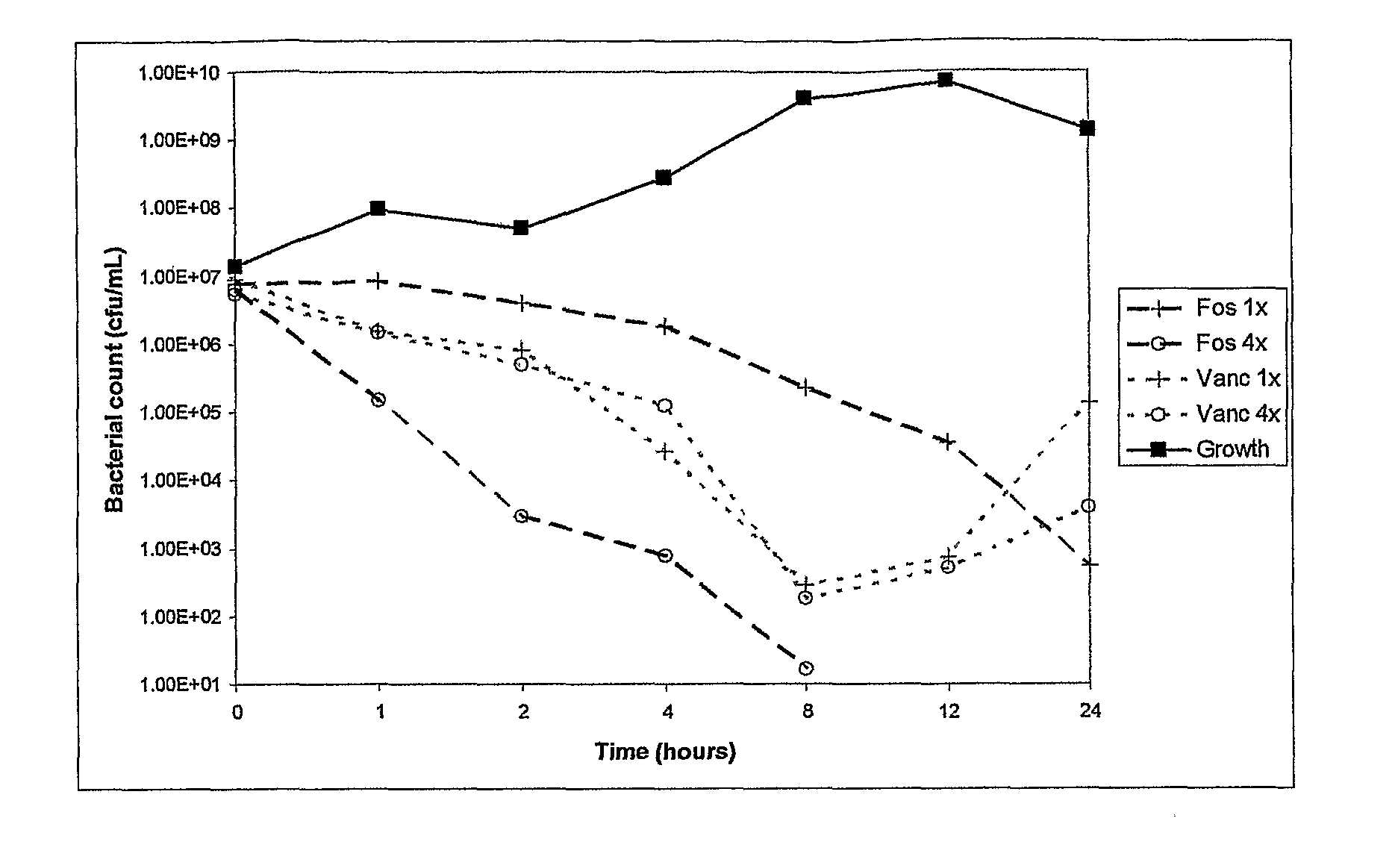
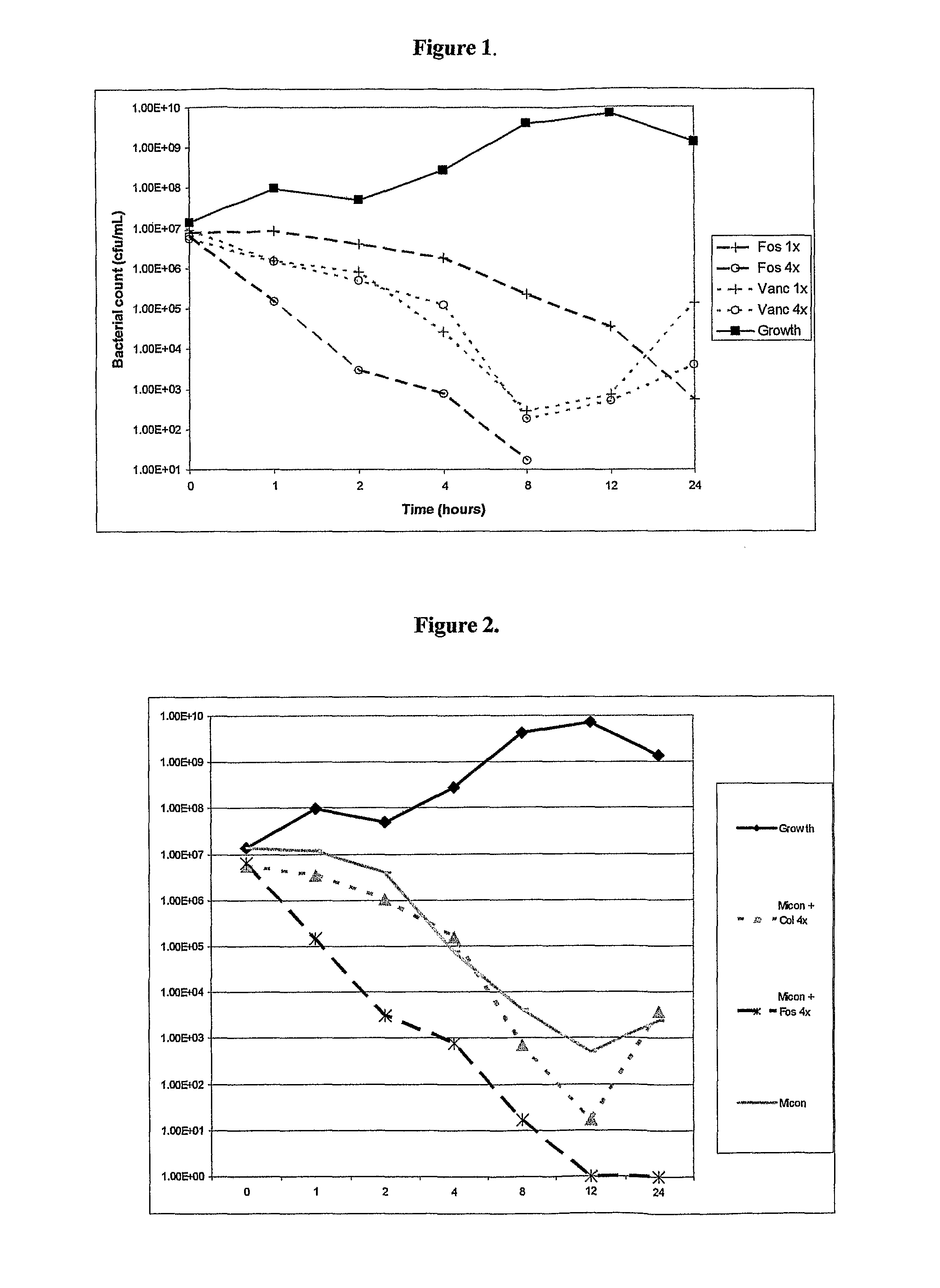
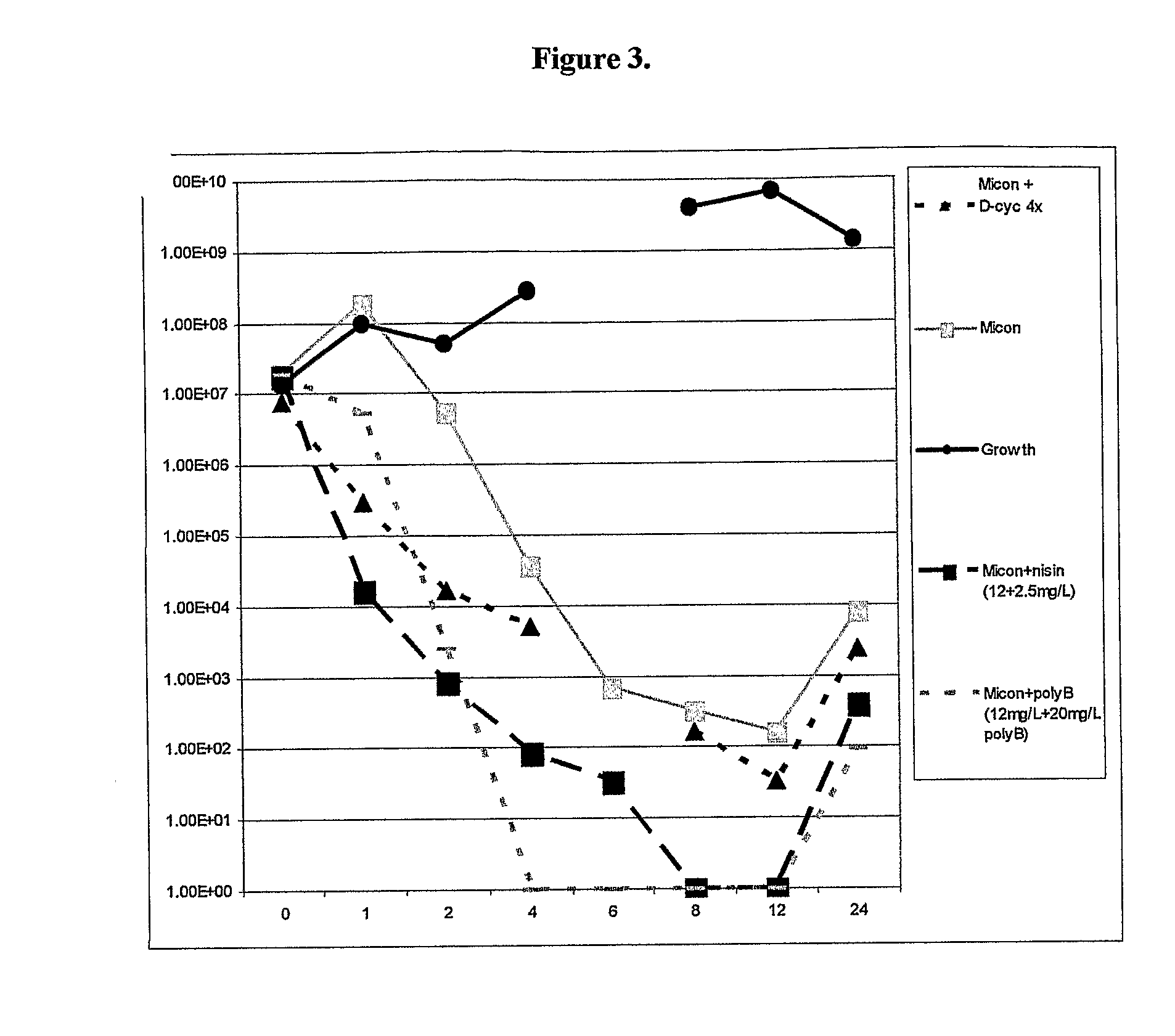
There is described a composition comprising a therapeutically active imidazole, and derivatives thereof, and an agent active on a bacterial cell surface selected from the group consisting of one or more of colistin, nisin, D-cycloserine, fosfomycin, fosfomycin trometamol, fosfomycin disodium and polymixin B, and derivatives thereof.
Owner:E THERAPEUTICS LTD
Compositions and methods for treating bacterial infections with protein-dalbavancin complexes
InactiveUS20050004011A1Improve antibacterial propertiesAntibacterial agentsBiocideDalbavancinMedicine
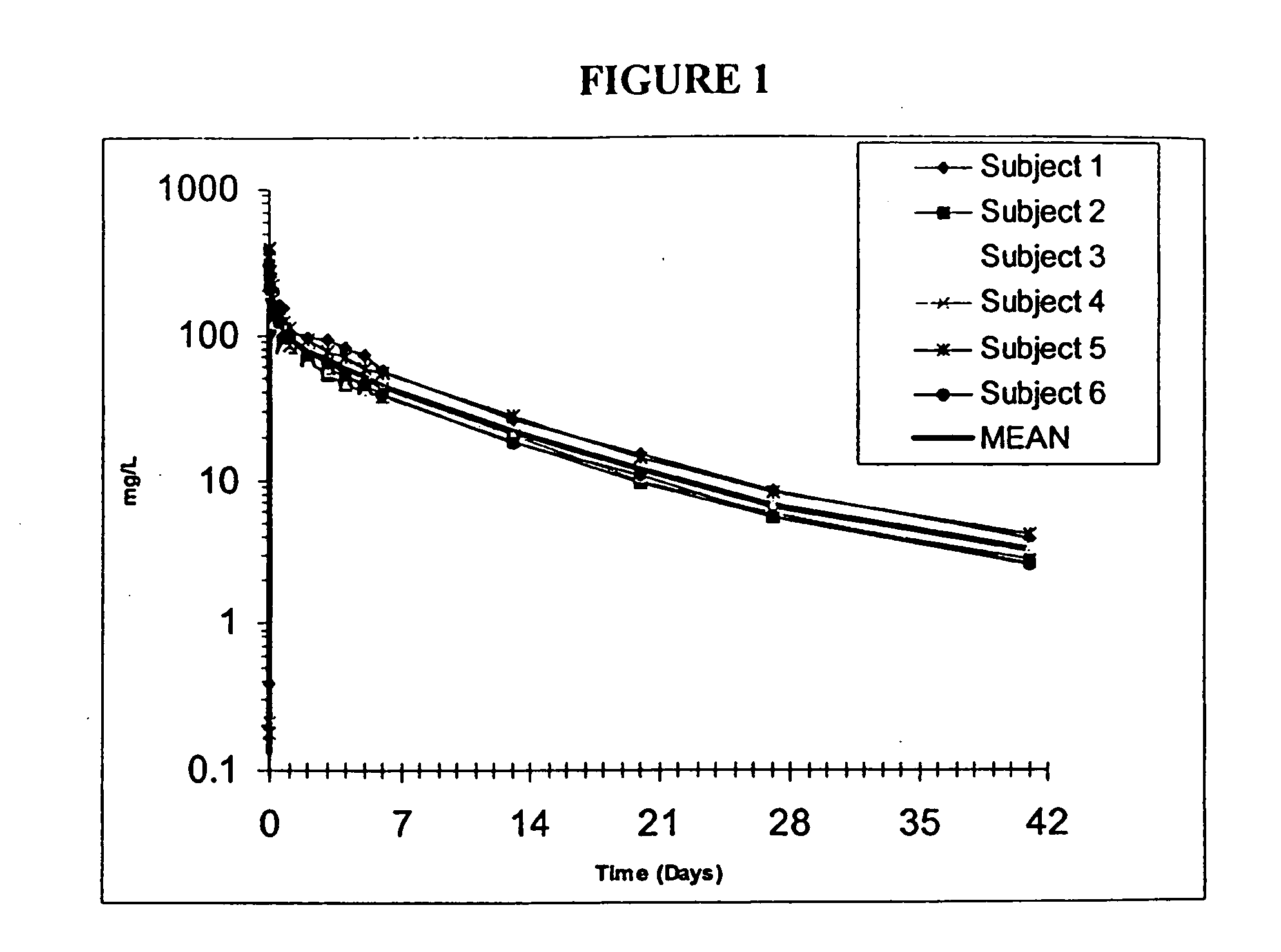
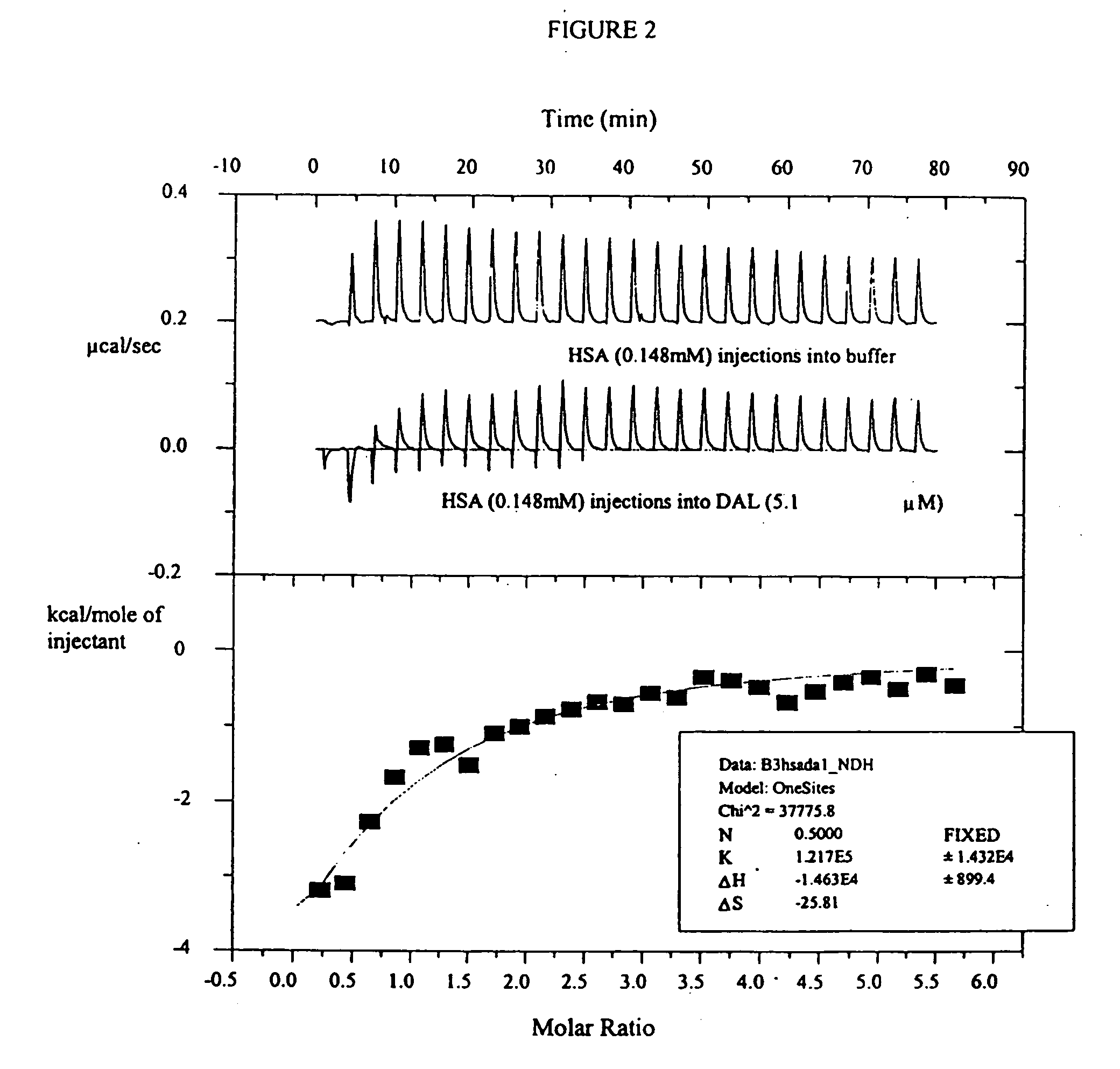
The invention provides methods and compositions for treatment of bacterial infections. Methods of the invention include administration of dalbavancin for treatment of a bacterial infection, in particular a Gram-positive bacterial infection of skin and soft tissue, under conditions where a protein-dalbavancin complex forms, or administering a protein-dalbavancin complex. Dosing regimes include once weekly administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection.
Owner:VICURON PHARM INC
Bioinformatic method for identifying surface-anchored proteins from gram-positive bacteria and proteins obtained thereby
ActiveUS7615616B2Antibacterial agentsPeptide/protein ingredientsSAA proteinGram-positive bacterial infections
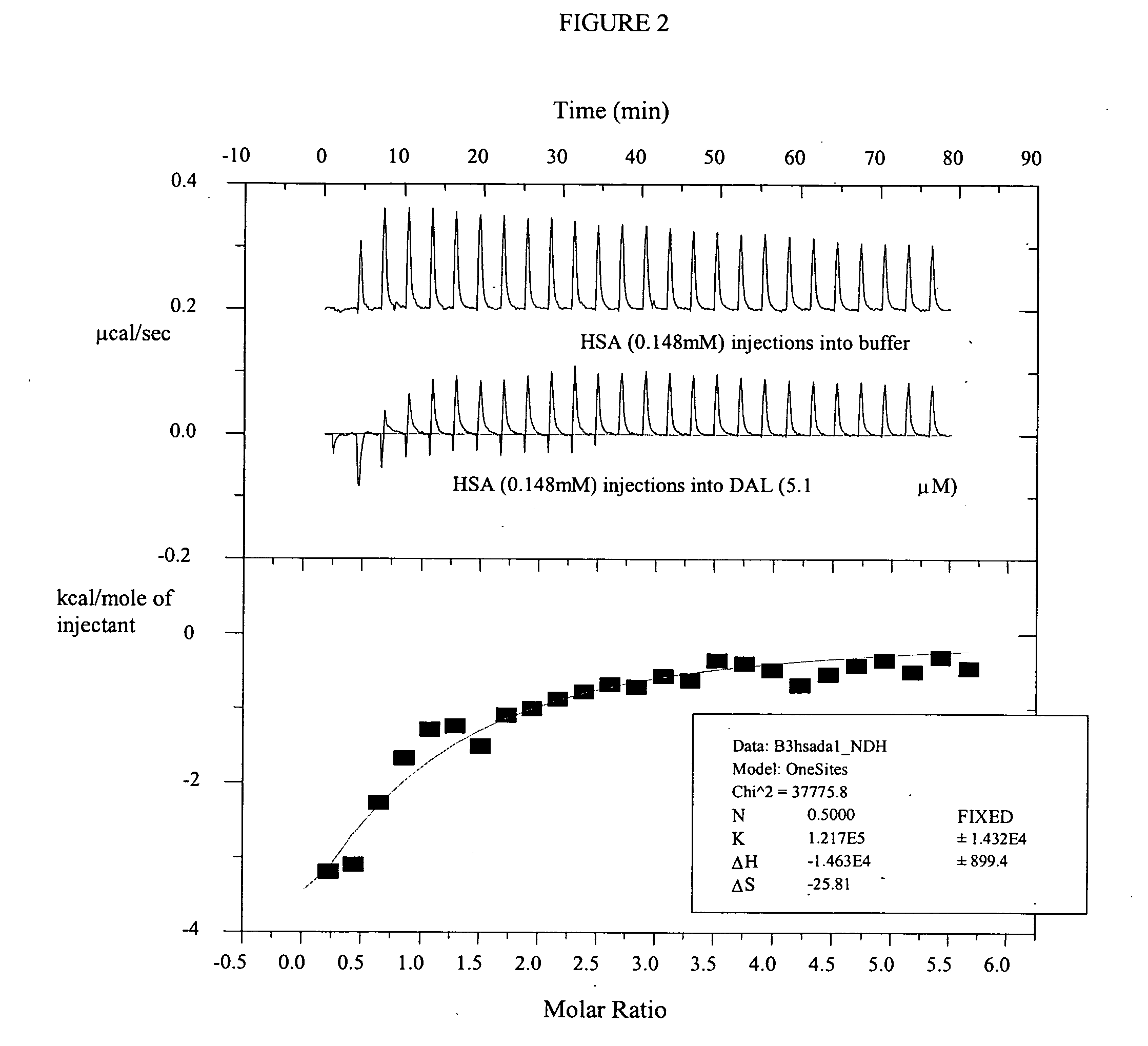
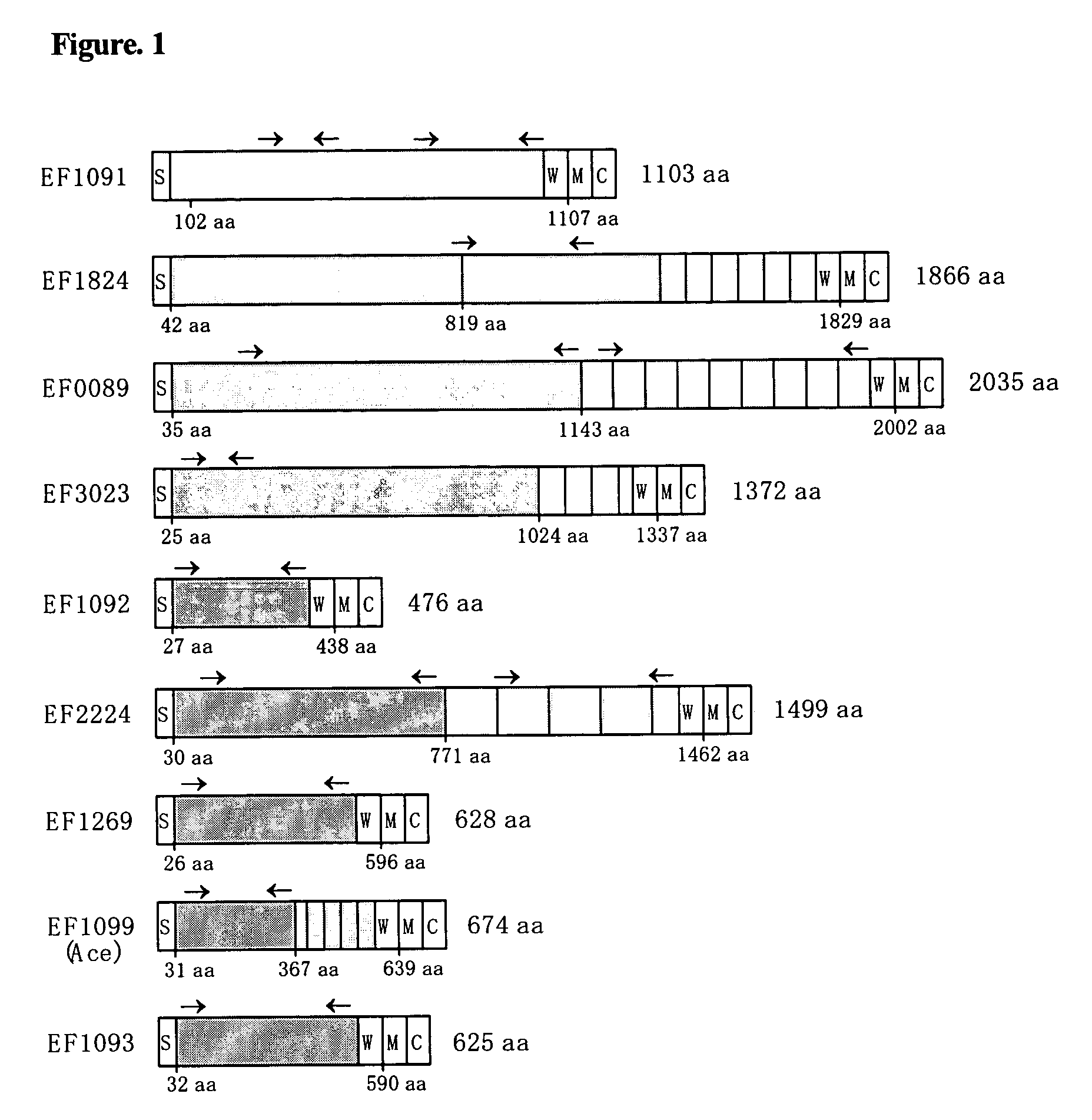
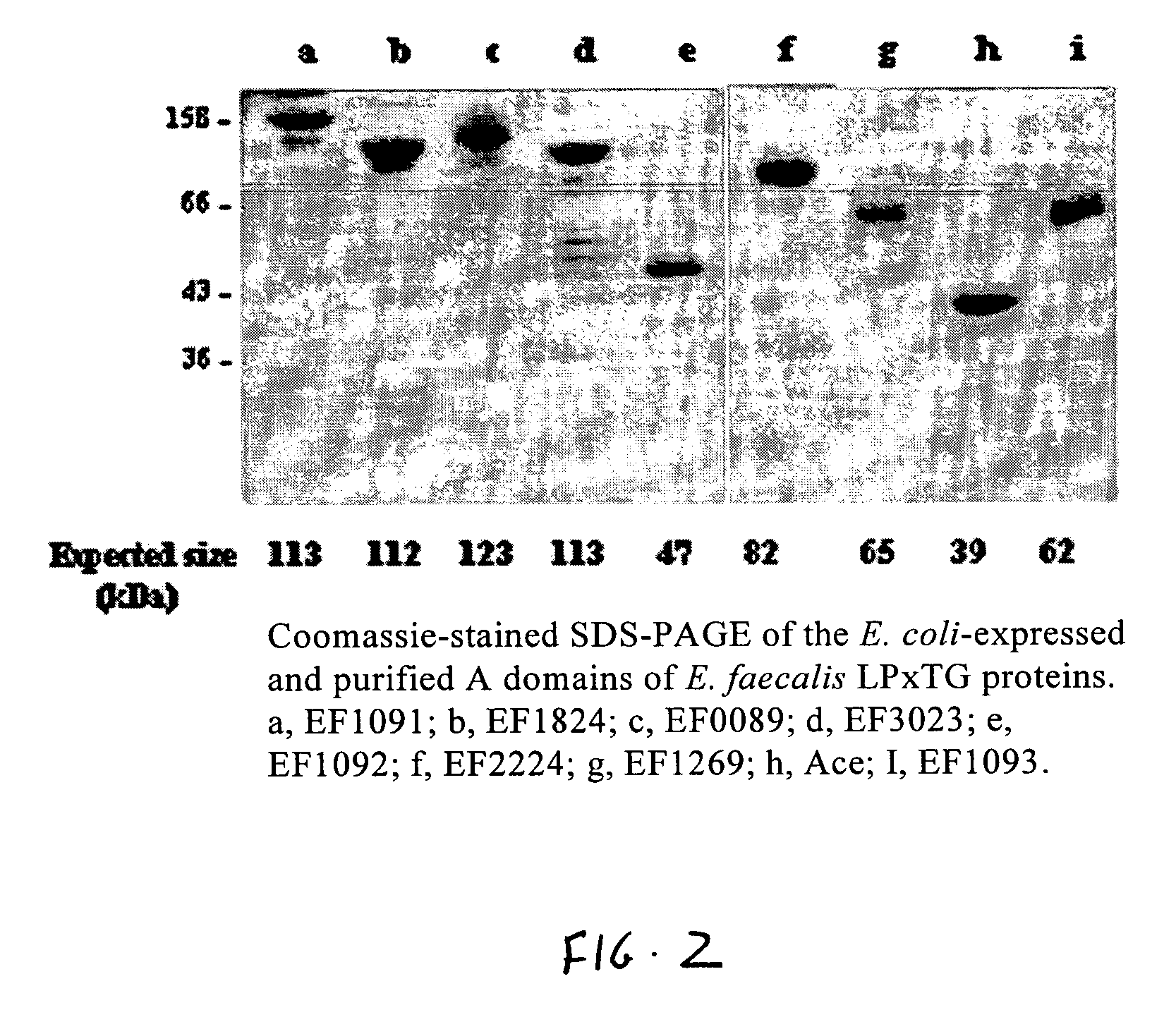
A bioinformatic method is provided for identifying and isolating proteins with MSCRAMM®—like characteristics from Gram positive bacteria, such as Enterococcus, Staphylococcus, Streptococcus and Bacillus bacteria, which can then be utilized in methods to prevent and treat infections caused by Gram-positive bacteria. The method involves identifying from sequence information those proteins with a putative C-terminal LPXTG (SEQ ID NO:1) cell wall sorting signal and other structural similarities to MSCRAMM® proteins having the LPXTG-anchored cell wall proteins. The MSCRAMM® proteins and immunogenic regions therein that are identified and isolated using the present invention may be used to generate antibodies useful in the diagnosis, treatment or prevention of Gram positive bacterial infections.
Owner:TEXAS A&M UNIVERSITY +2
Bismuth-thiols as antiseptics for biomedical uses, including treatment of bacterial biofilms and other uses
Owner:MICROBION
Dry powder vancomycin compositions and associated methods
InactiveCN103717231AAntibacterial agentsPowder deliveryGram-positive bacterial infectionsIndoschulzia
Dry powder vancomycin compositions and methods for administering and preparing such compositions. A composition of the present disclosure may be administered to a subject via pulmonary administration in an amount effective to treat and / or prevent a bacterial infection in the subject. Administration of an effective amount of a composition of the present disclosure may be particularly useful in treating a gram-positive bacterial infection in a subject suffering from pneumonia, cystic fibrosis, bronchiectasis, or other chronic lung disease with a bacterial infection of the subject's airway and / or lung.
Owner:SAVARA
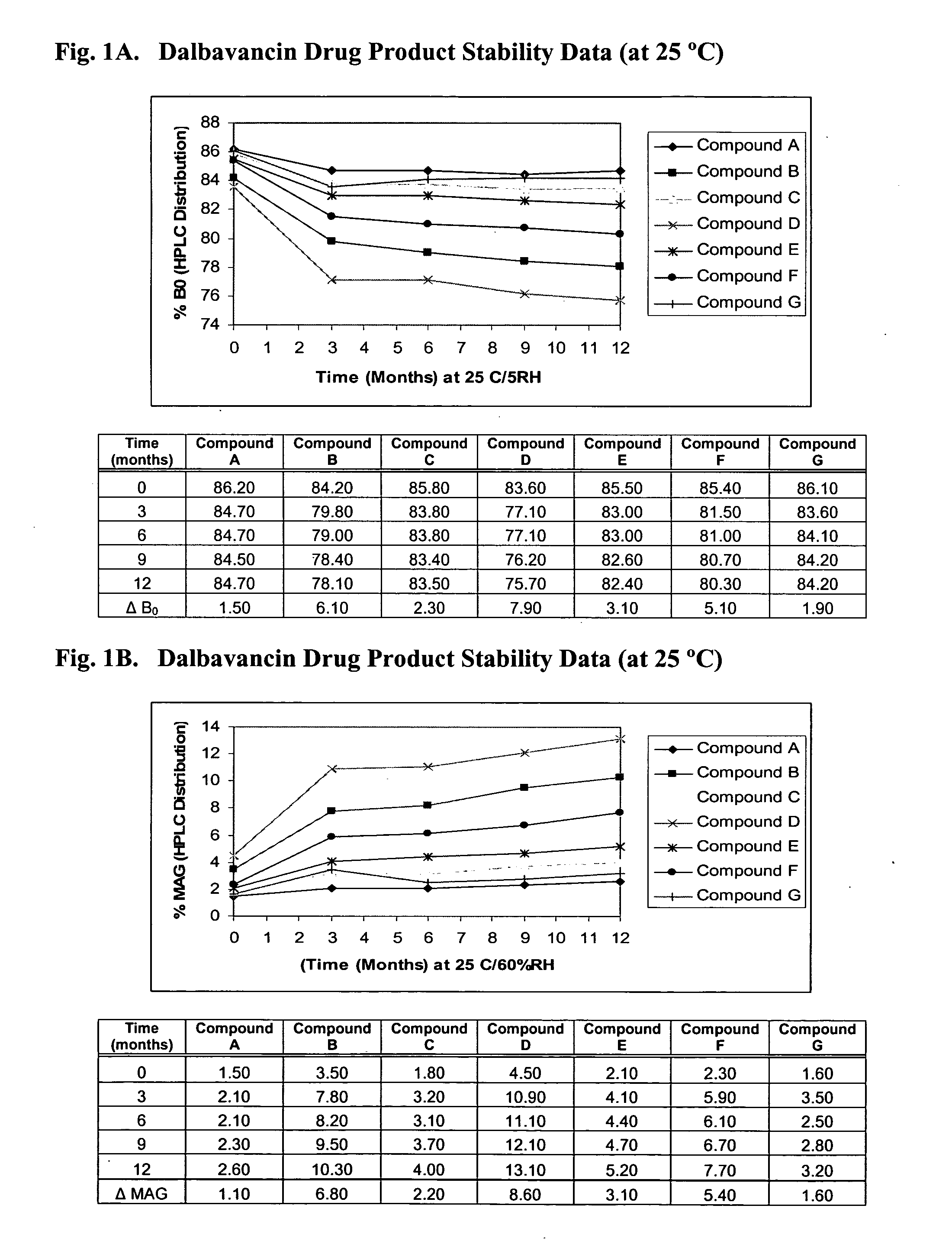
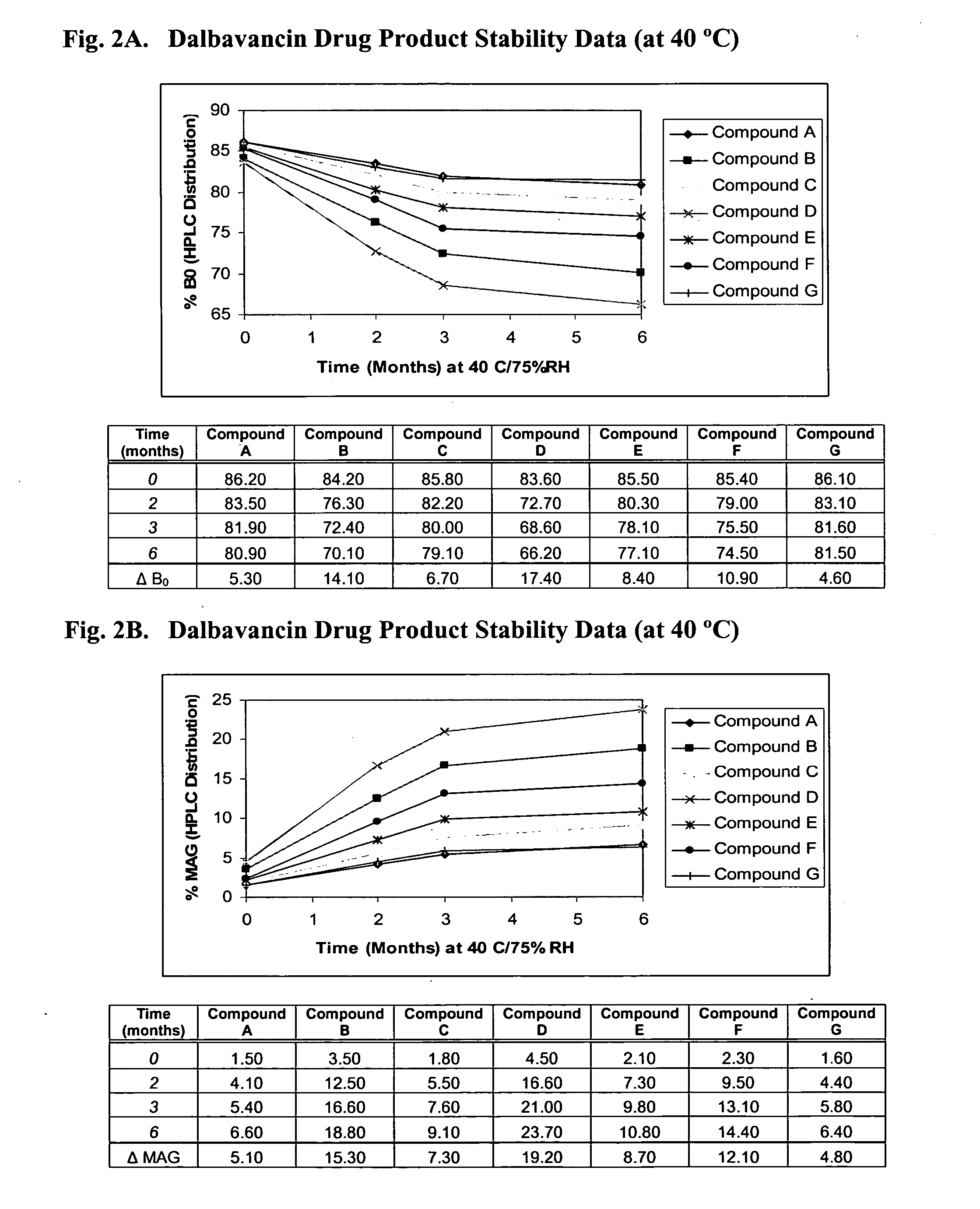
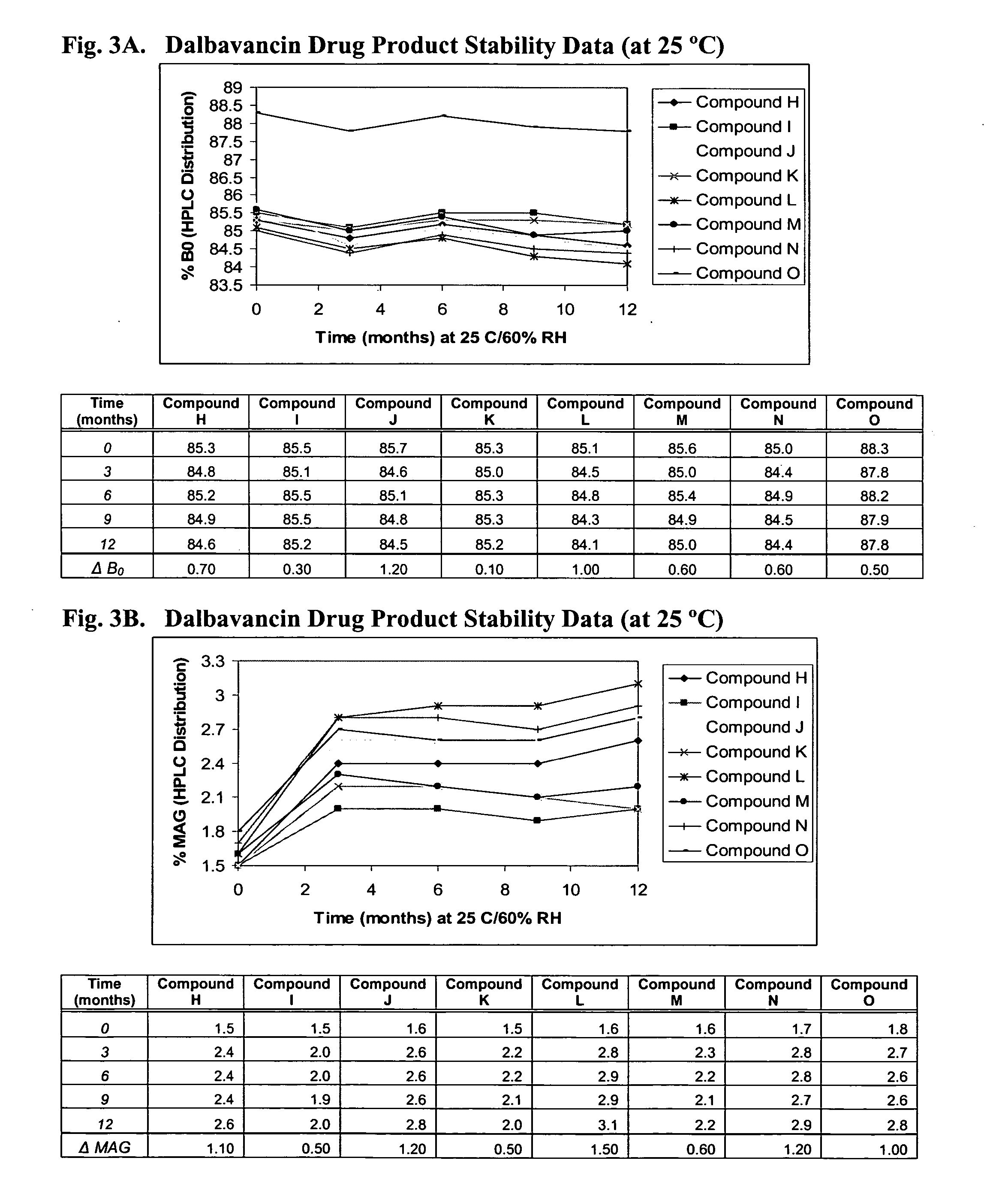
Dalbavancin compositions for treatment of bacterial infections
InactiveUS20050277581A1Reduce adverse side effectsReduce the amount requiredBiocideSaccharide peptide ingredientsRegimenPediatric patient
The invention provides methods and compositions for treatment of bacterial infections in adult and pediatric patients. Methods of the invention include administration of dalbavancin formulations for treatment of a bacterial infection, in particular a Gram-positive bacterial infection of skin and skin structure. Dosing regimens include multiple dose administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection.
Owner:VICURON PHARM INC
Methods of administering dalbavancin for treatment of skin and soft tissue infections
InactiveUS20050032721A1Reduce adverse side effectsAntibacterial agentsBiocideDalbavancinSoft tissue infection
The invention provides methods and compositions for treatment of bacterial infections. Methods of the invention include administration of dalbavancin for treatment of a bacterial infection, in particular an uncomplicated Gram-positive bacterial infection of skin and soft tissue. Dosing regimes include once weekly administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection.
Owner:CAVALERI MARCO +6
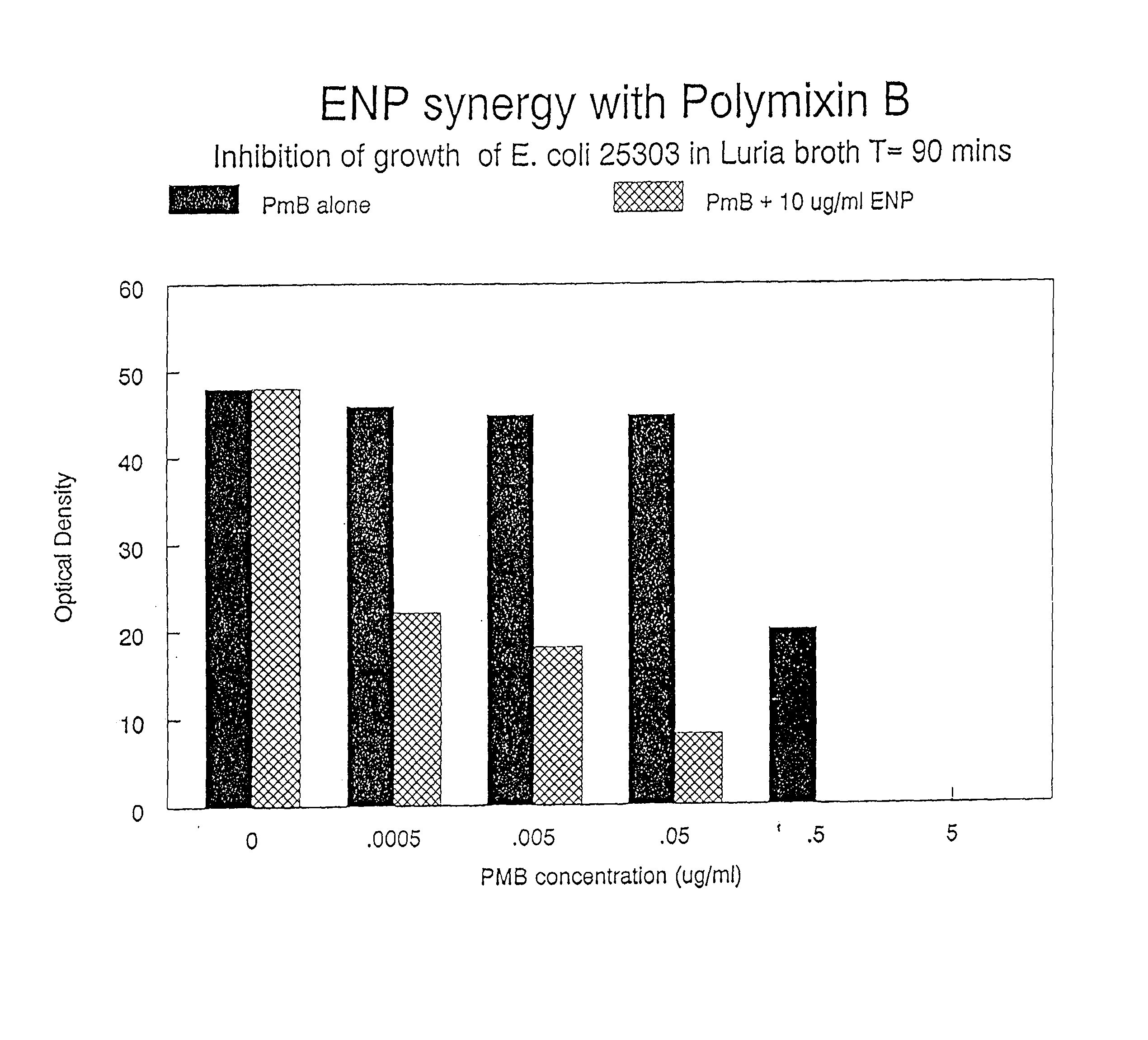
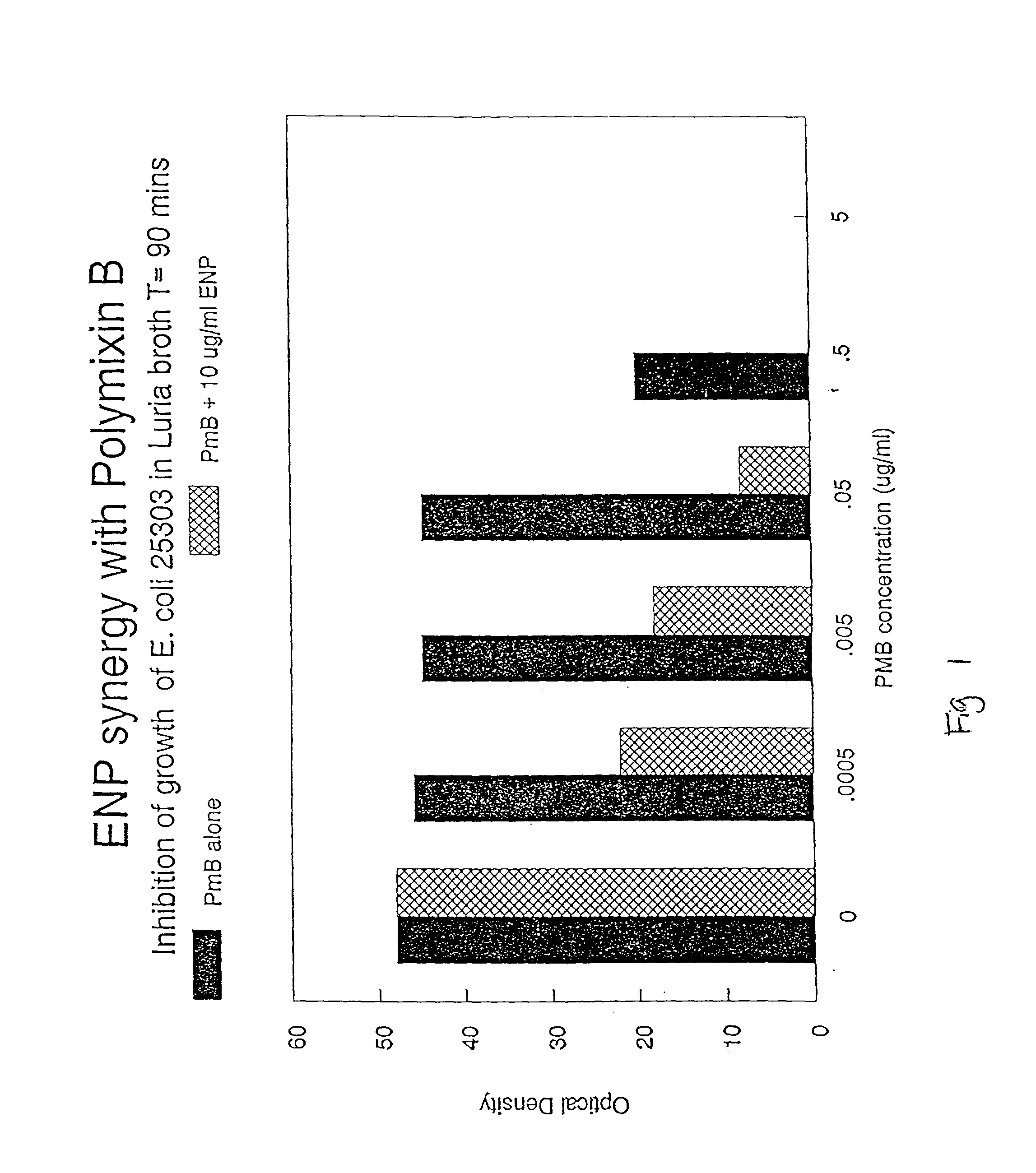
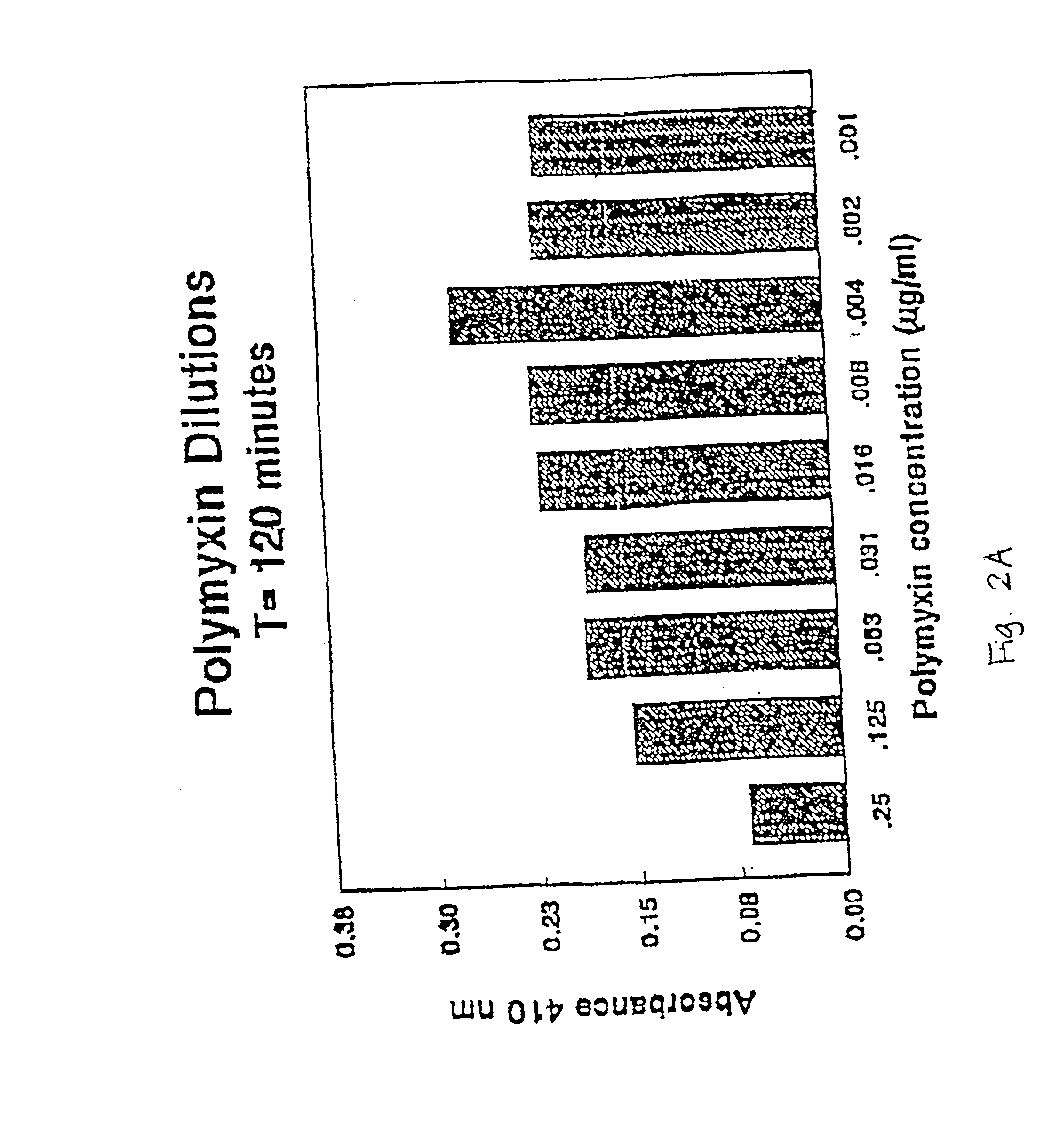
Composition comprising endotoxin neutralizing protein and derivatives and uses thereof
InactiveUS20020107174A1Prevent and inhibit growth of bacteriaAntibacterial agentsBiocidePersonal careGram-positive bacterial infections
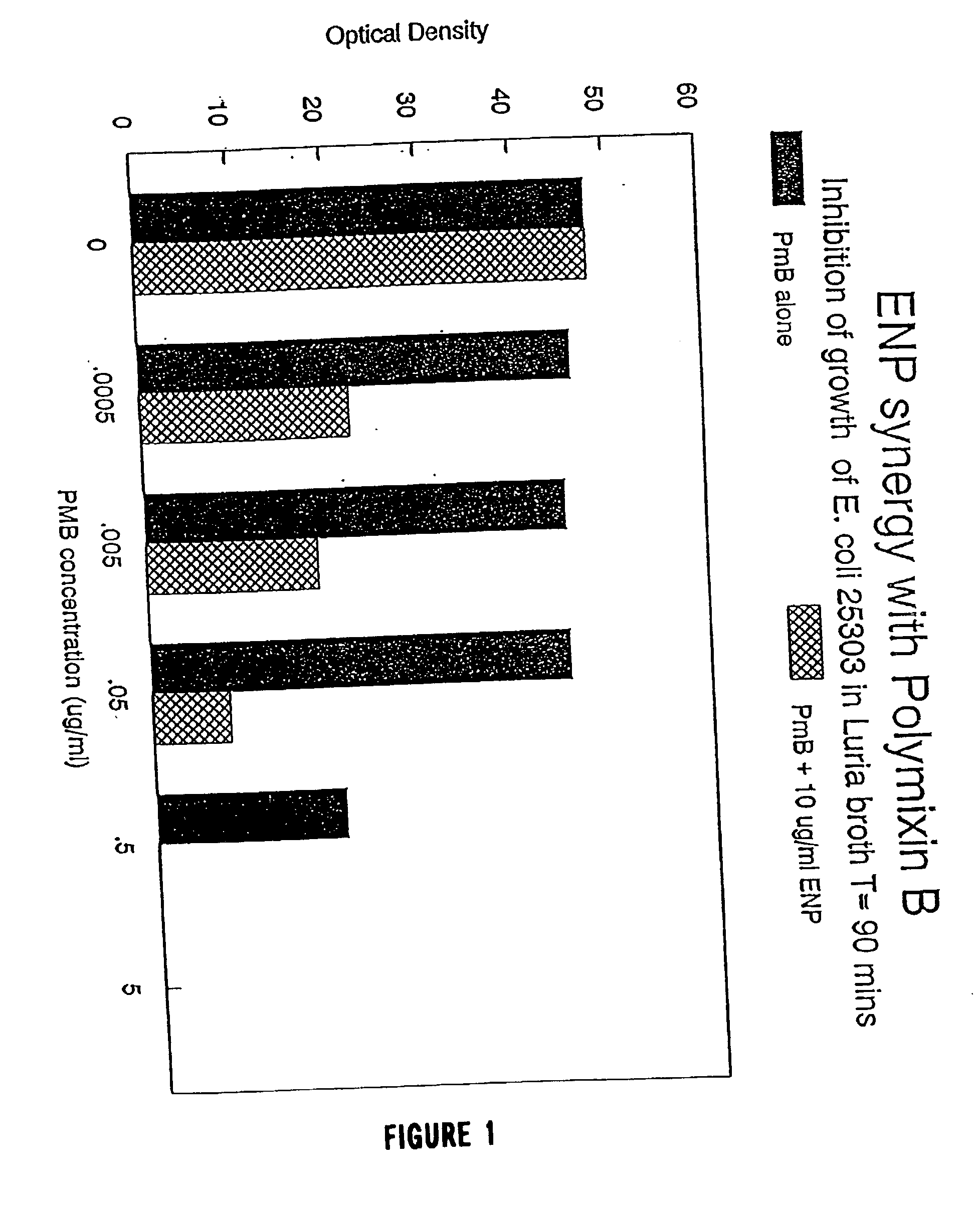
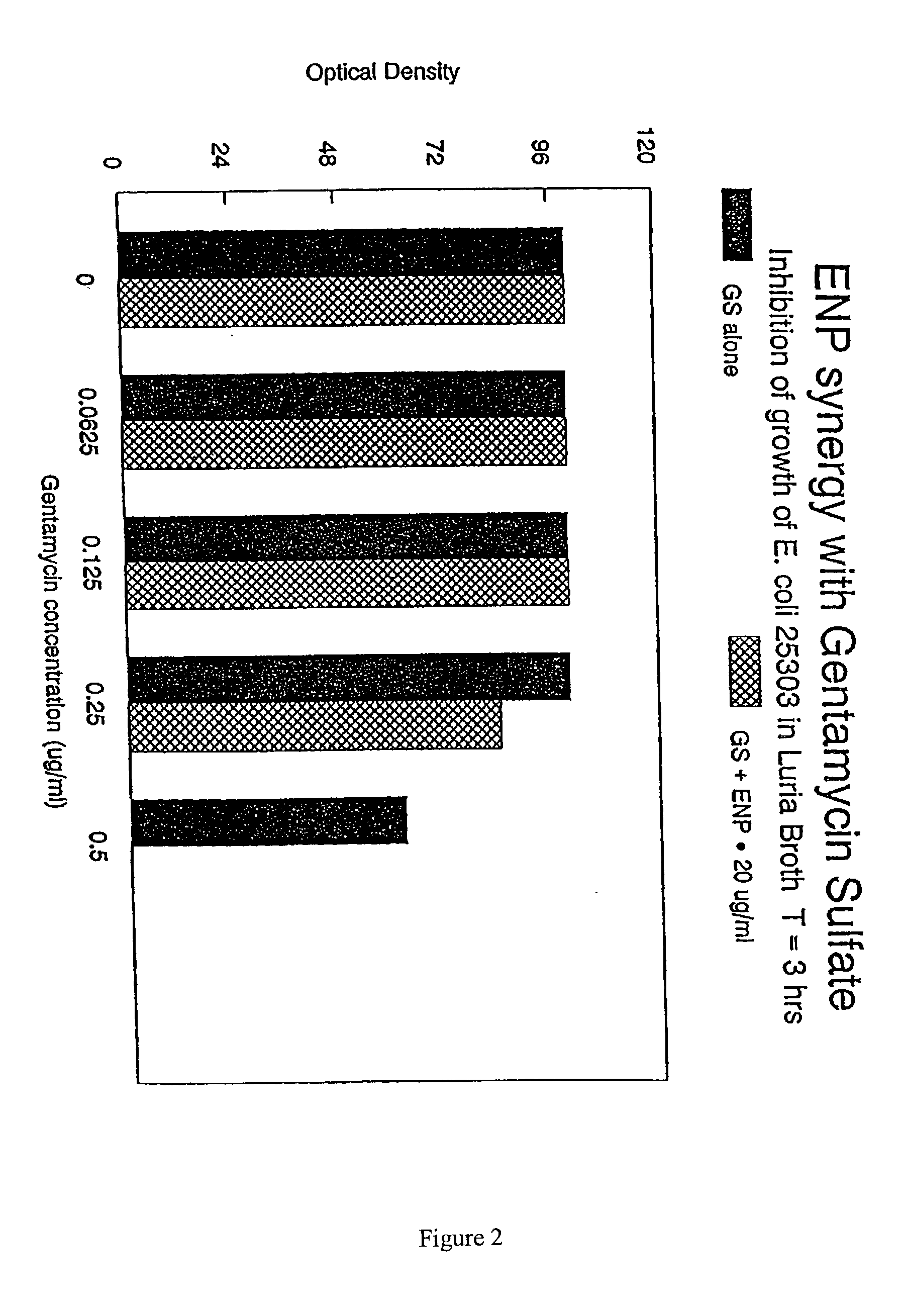
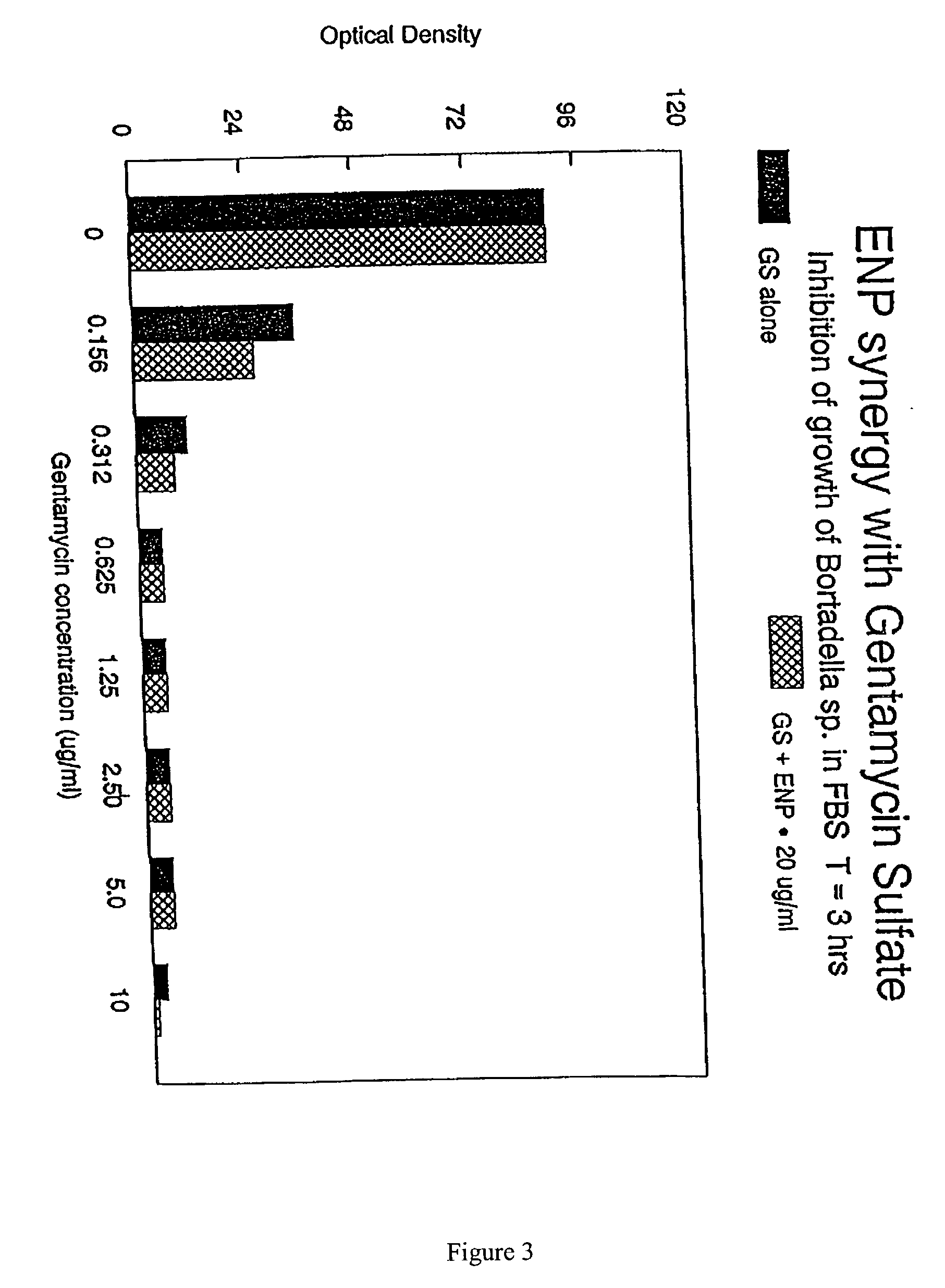
The present invention relates to the use of endotoxin neutralizing protein (ENP) and derivatives thereof as stand alone microbial inhibitors or as synergistic enhancers of antibiotics and preservatives. Compositions comprising ENP or alone or in combination with an antibiotic may be used to prevent or treat gram-negative bacterial infections, endotoxemia, septic shock, gram-positive bacterial infections, yeast infections and fungal infections. Compositions comprising ENP alone or in combination with a conventional preservative may be used as antimicrobial preservative in cosmetic or personal care preparations.
Owner:ASSOCS OF CAPE COD
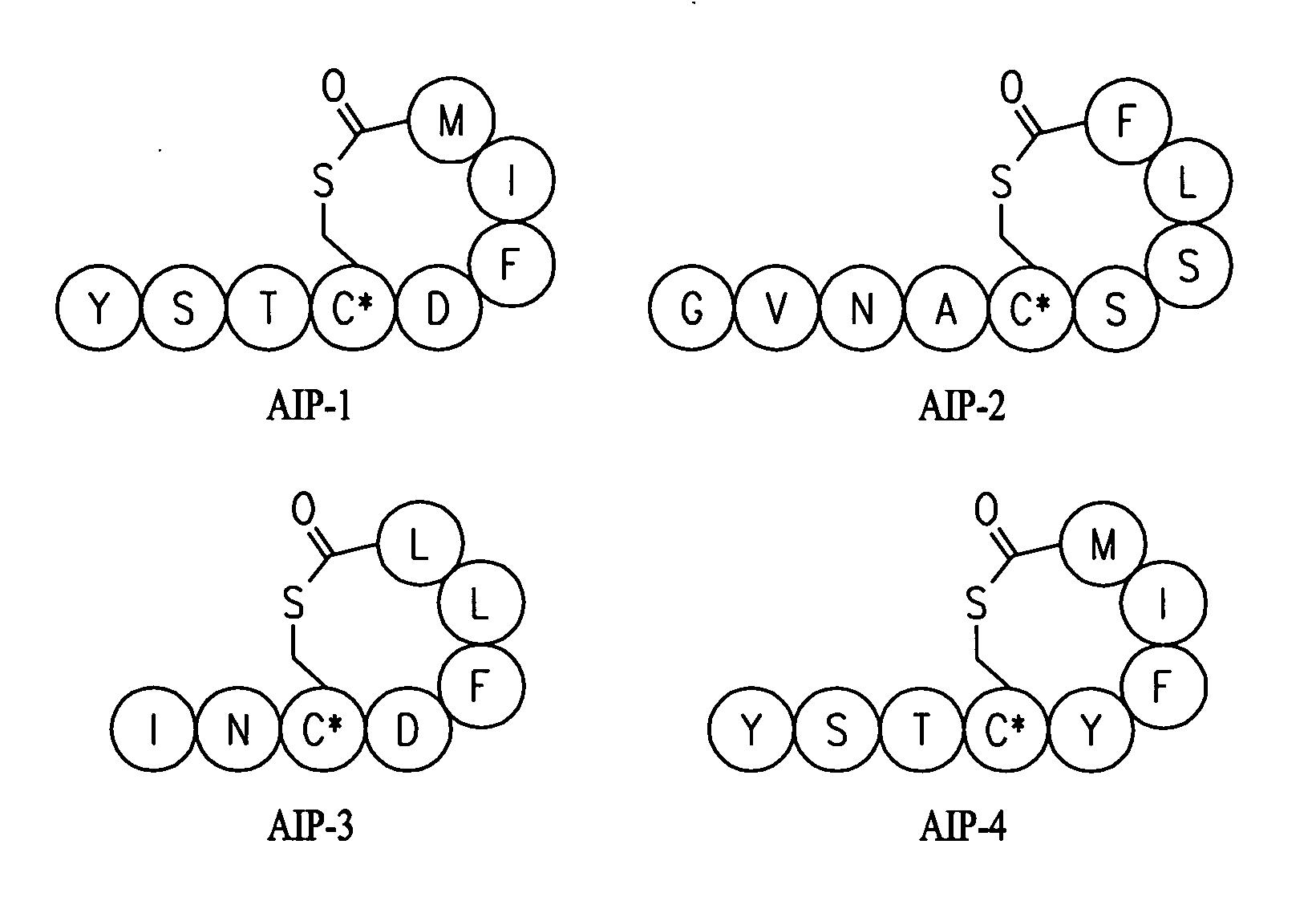
Antibody-mediated disruption of quorum sensing in bacteria
ActiveUS20100291093A1Suppress bacterial pathogenicityInhibition of pathogenicityAntibacterial agentsBiocideDiseaseBacteroides
The invention provides an immunogenic molecular entity, a supramolecular assembly, and an antibody that can be used to inhibit Gram-positive bacterial quorum sensing, prevent infection or development of a disease condition associated with a Gram-positive bacterial infection. The invention also provides methods of inhibiting Gram-positive bacterial quorum sensing, and methods of preventing infection or development of a disease condition associated with a Gram-positive bacterial infection.
Owner:THE SCRIPPS RES INST
Dalbavancin compositions for treatment of bacterial infections
ActiveUS20050004050A1Reduce adverse side effectsAntibacterial agentsBiocideDalbavancinGram-positive bacterial infections
The invention provides methods and compositions for treatment of bacterial infections. Methods of the invention include administration of dalbavancin formulations for treatment of a bacterial infection, in particular a Gram-positive bacterial infection of skin and soft tissue. Dosing regimes include once weekly administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection.
Owner:VICURON PHARM INC
Dalbavancin compositions for treatment of bacterial infections
InactiveUS7119061B2Reduce adverse side effectsAntibacterial agentsBiocideDalbavancinGram-positive bacterial infections
The invention provides methods and compositions for treatment of bacterial infections. Methods of the invention include administration of dalbavancin formulations for treatment of a bacterial infection, in particular a Gram-positive bacterial infection of skin and soft tissue. Dosing regimes include once weekly administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection.
Owner:APTALIS PHARMA
Screen for Anti-infective compounds
InactiveUS20080026389A1Useful measurementSugar derivativesMicrobiological testing/measurementBiological bodyDisinfectant
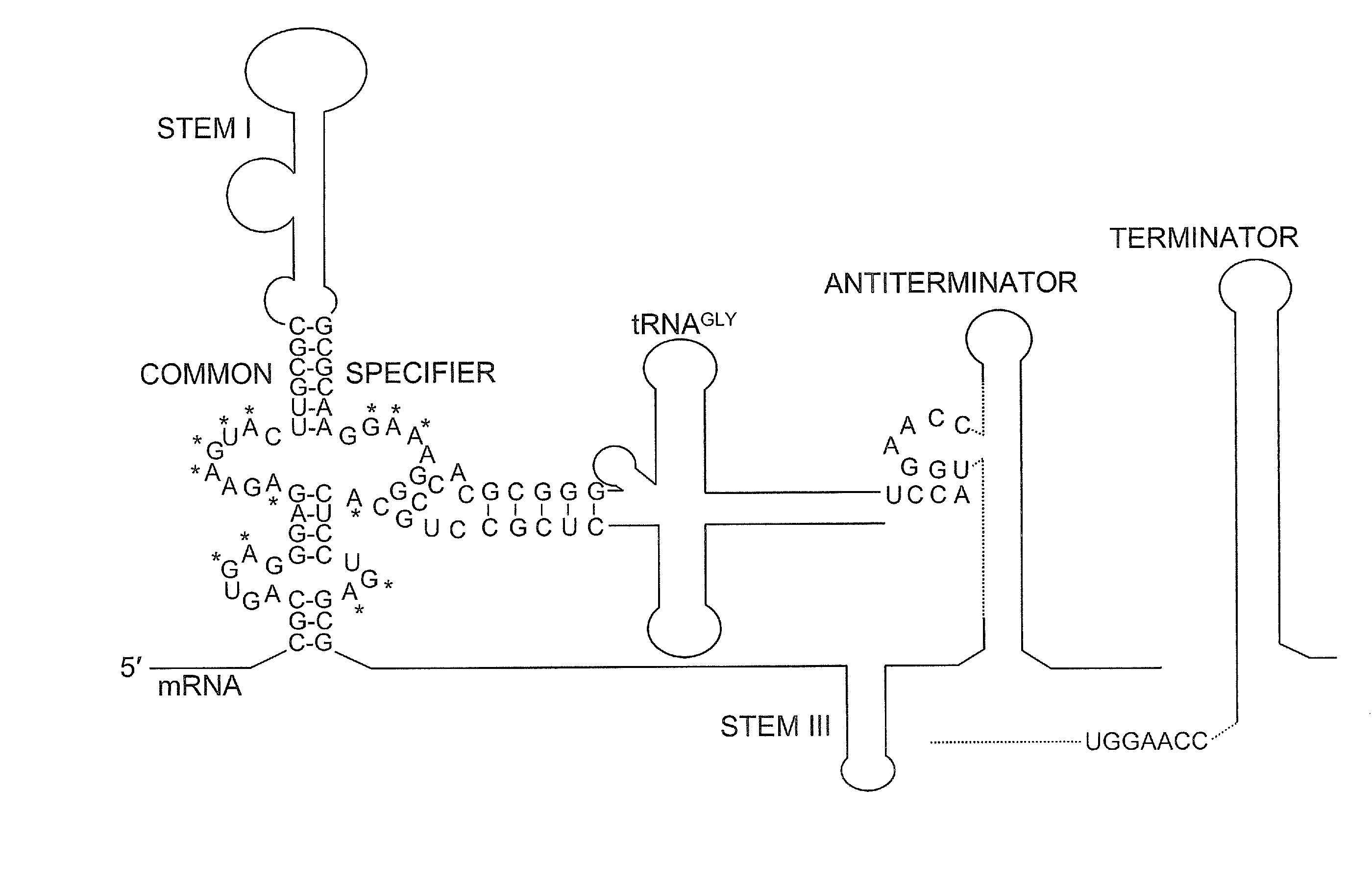
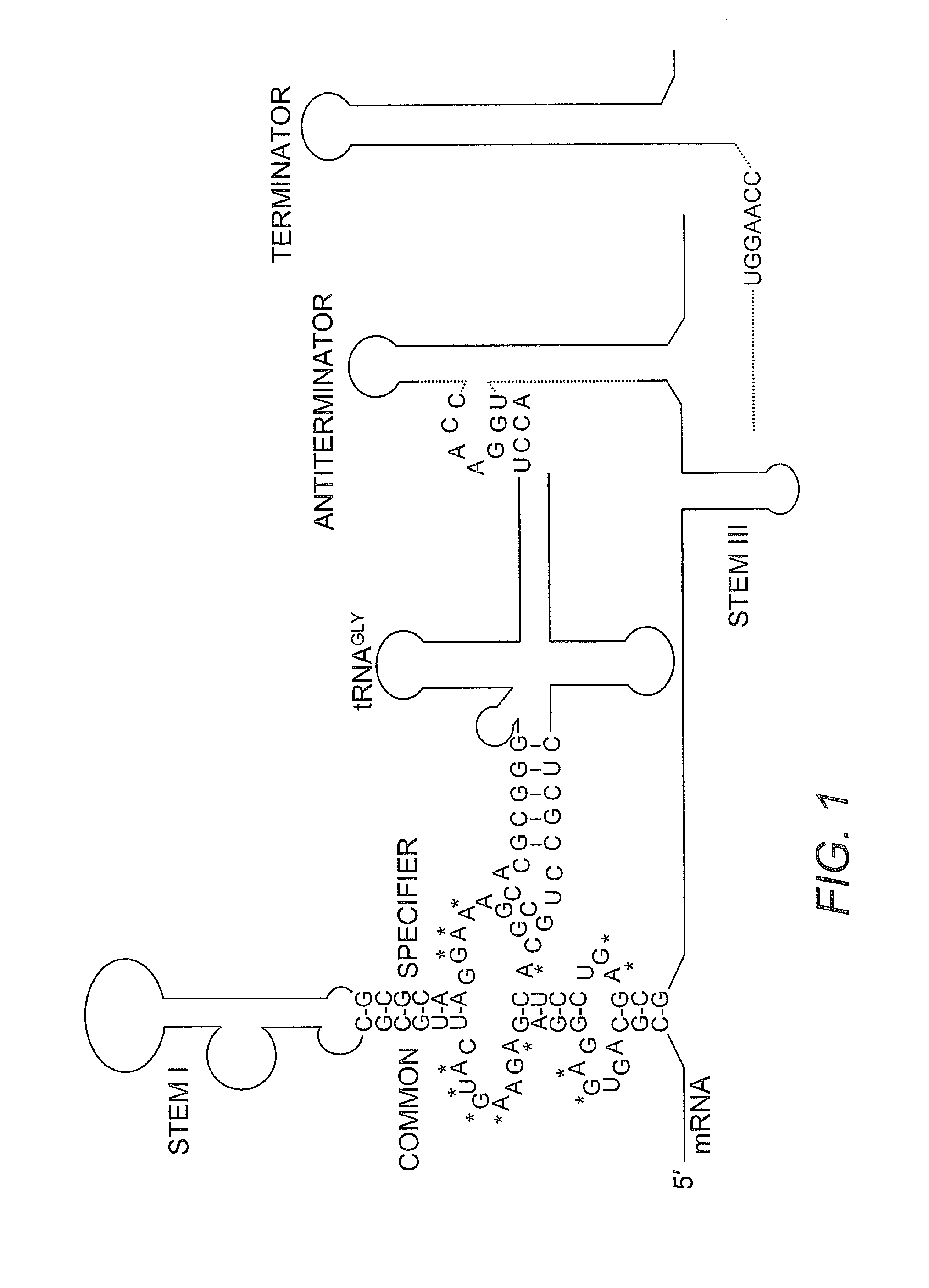
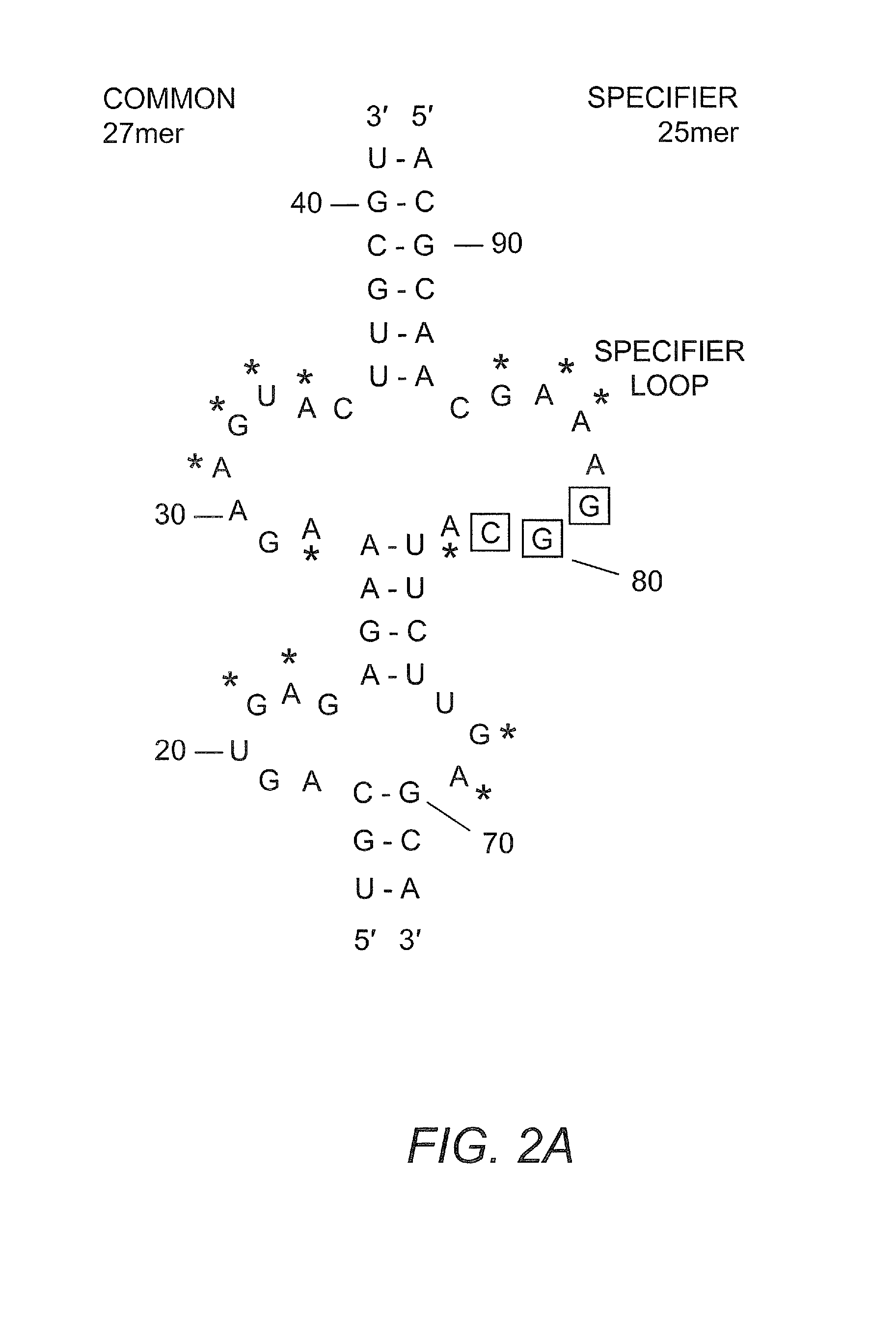
The present invention provides methods and compositions for identifying compounds useful as therapeutics and disinfectants. Such compounds would be particularly useful in treating Gram-positive bacterial infections. Such therapeutics would target the interaction of a tRNA molecule with an mRNA molecule, interrupting translation of the mRNA molecule and thus interfering with gene expression for a target gene critical to the survival of these organisms.
Owner:NORTH CAROLINA STATE UNIV
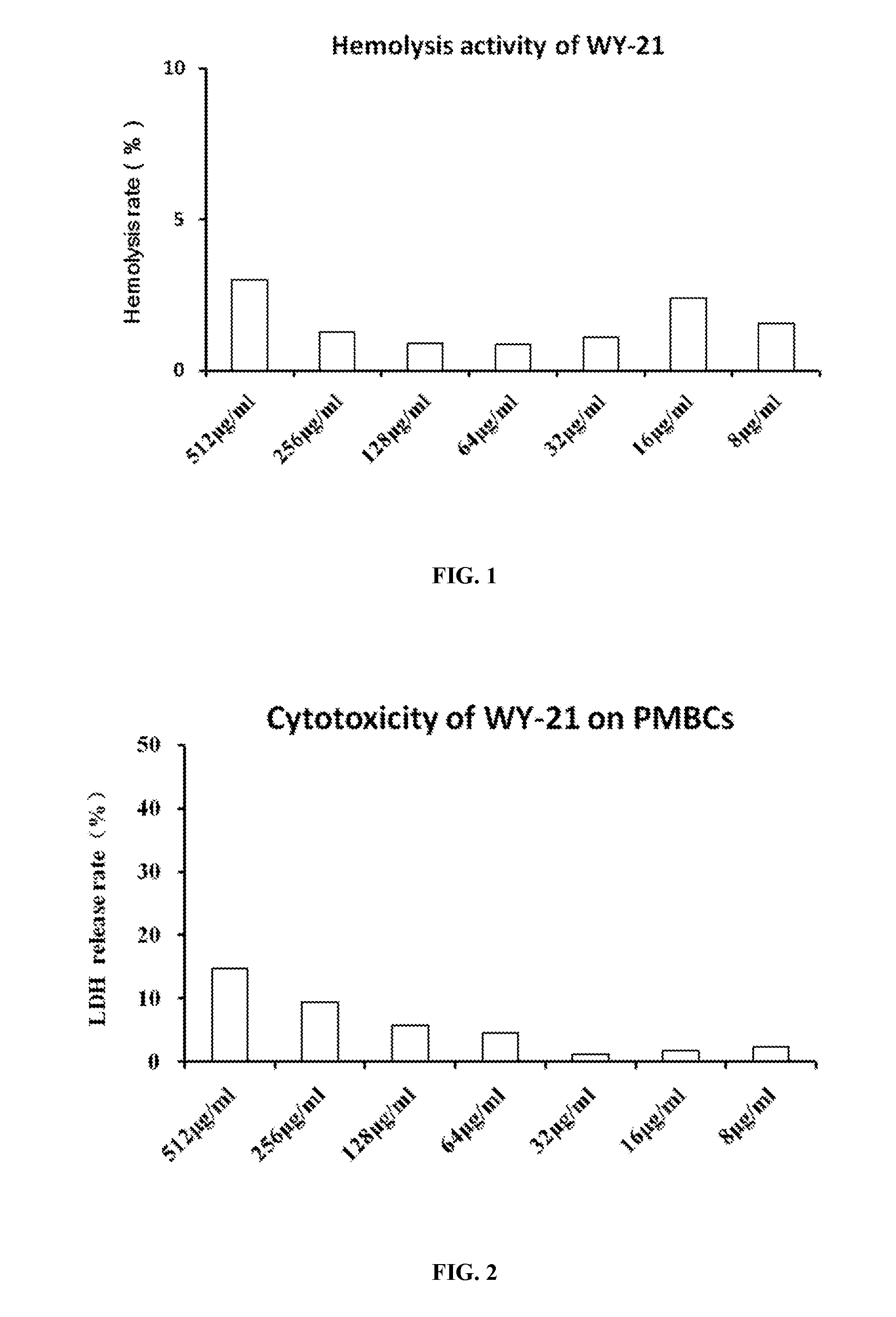
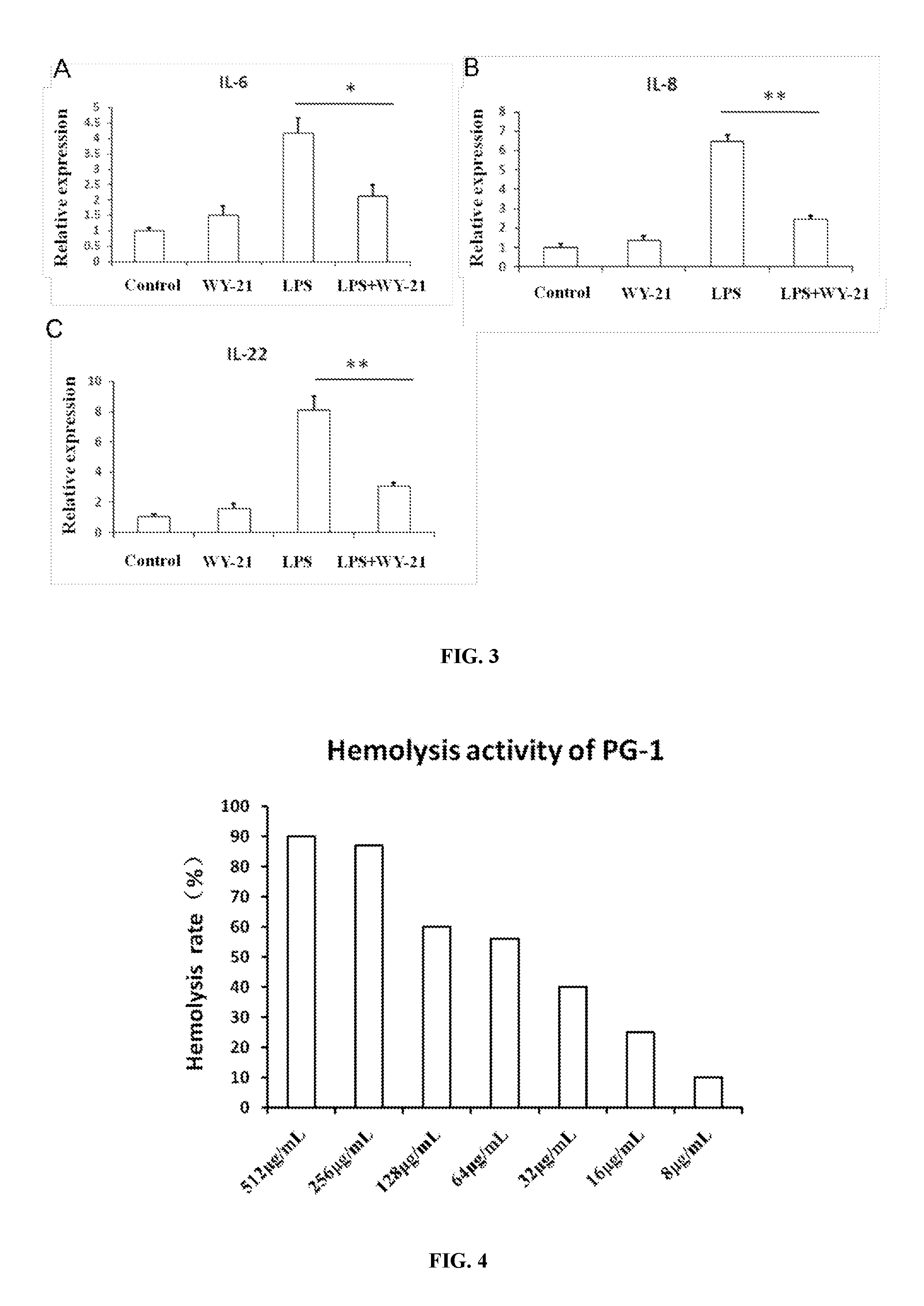
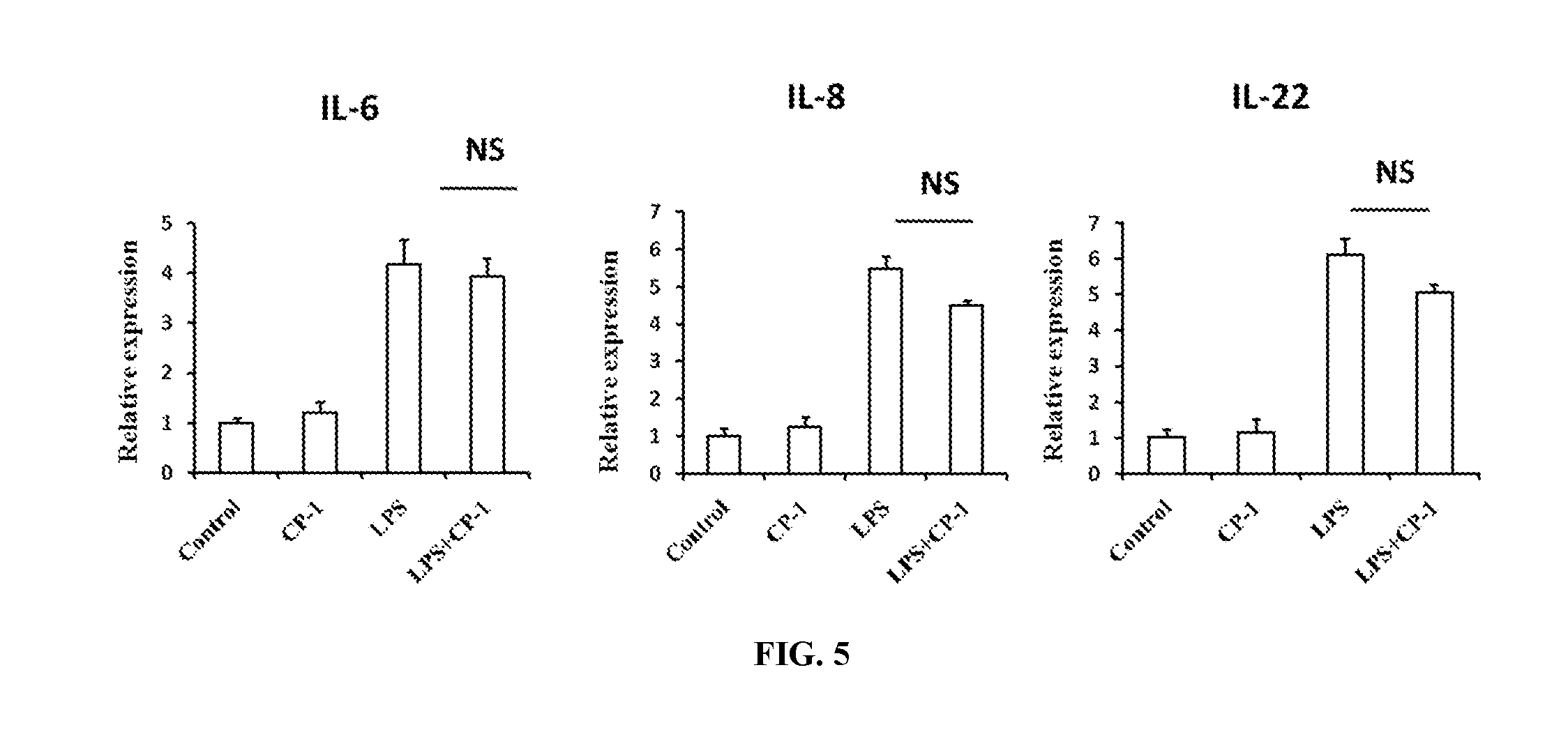
Antimicrobial peptide wy-21 and application thereof
InactiveUS20160257718A1Improve stabilityEasy to synthesizeAntibacterial agentsPeptide/protein ingredientsGramGram-positive bacterial infections
An antimicrobial peptide WY-21 has amino acid sequence of Val-Lys-Phe-Phe-Arg-Lys-Leu-Lys-Lys-Ser-Val-Lys-Glu-Lys-Ile-Gly-Lys-Glu-Phe-Lys-Arg. The antimicrobial peptide WY-21 can be used as broad-spectrum antibacterial agents for the treatment of Gram-negative or Gram-positive bacterial infection. It can also be applied for reducing immune system regulated inflammation.
Owner:ZHEJIANG UNIV
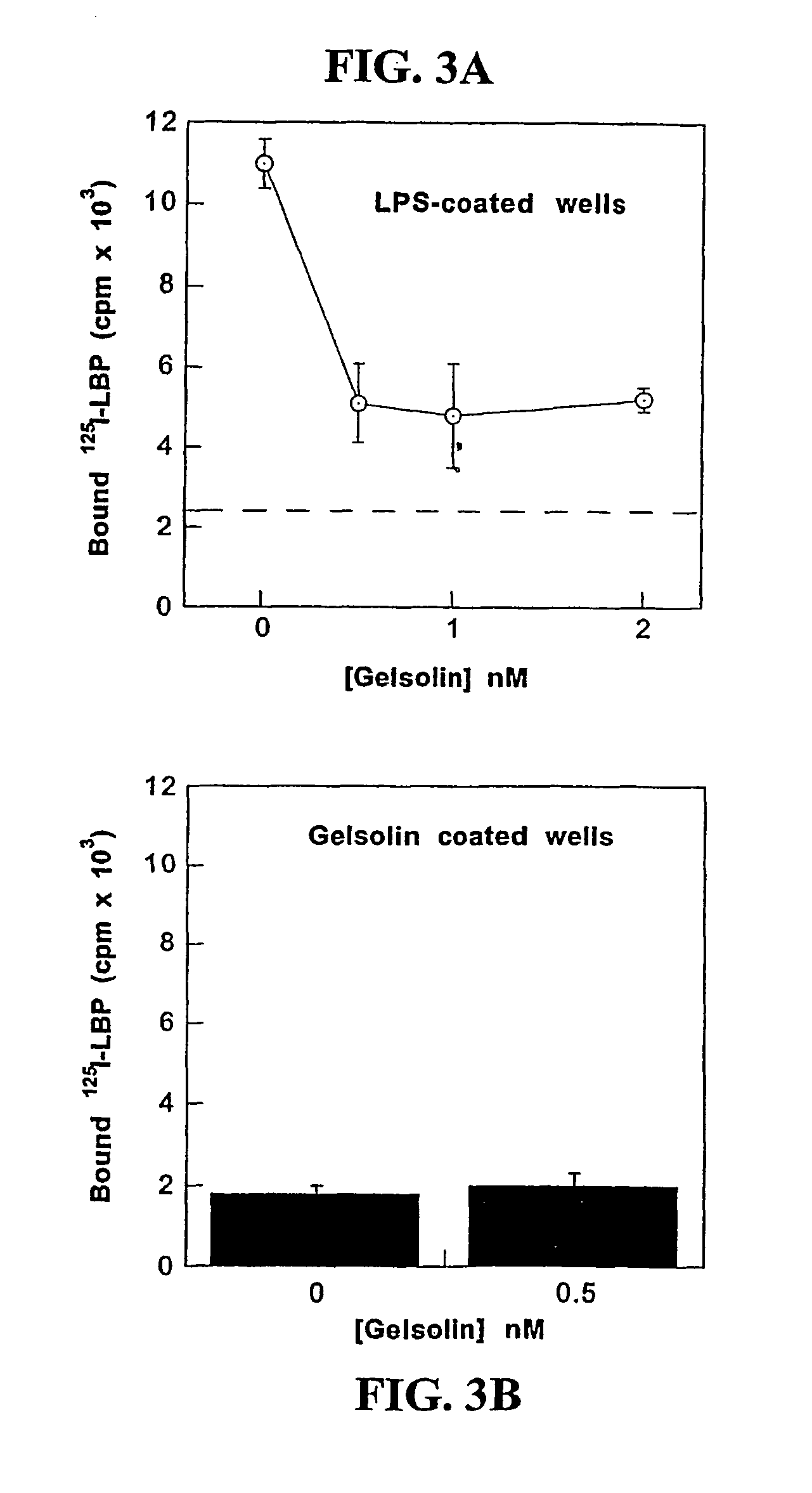
Methods of using gelsolin to treat or prevent bacterial sepsis
ActiveUS9408891B2Prevented irreversible tissue damageMicrovascular dysfunction is avertedFibrinogenPeptide/protein ingredientsBacteroidesGram-positive bacterial infections
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods for treatment of bacterial infections in impaired renal patients
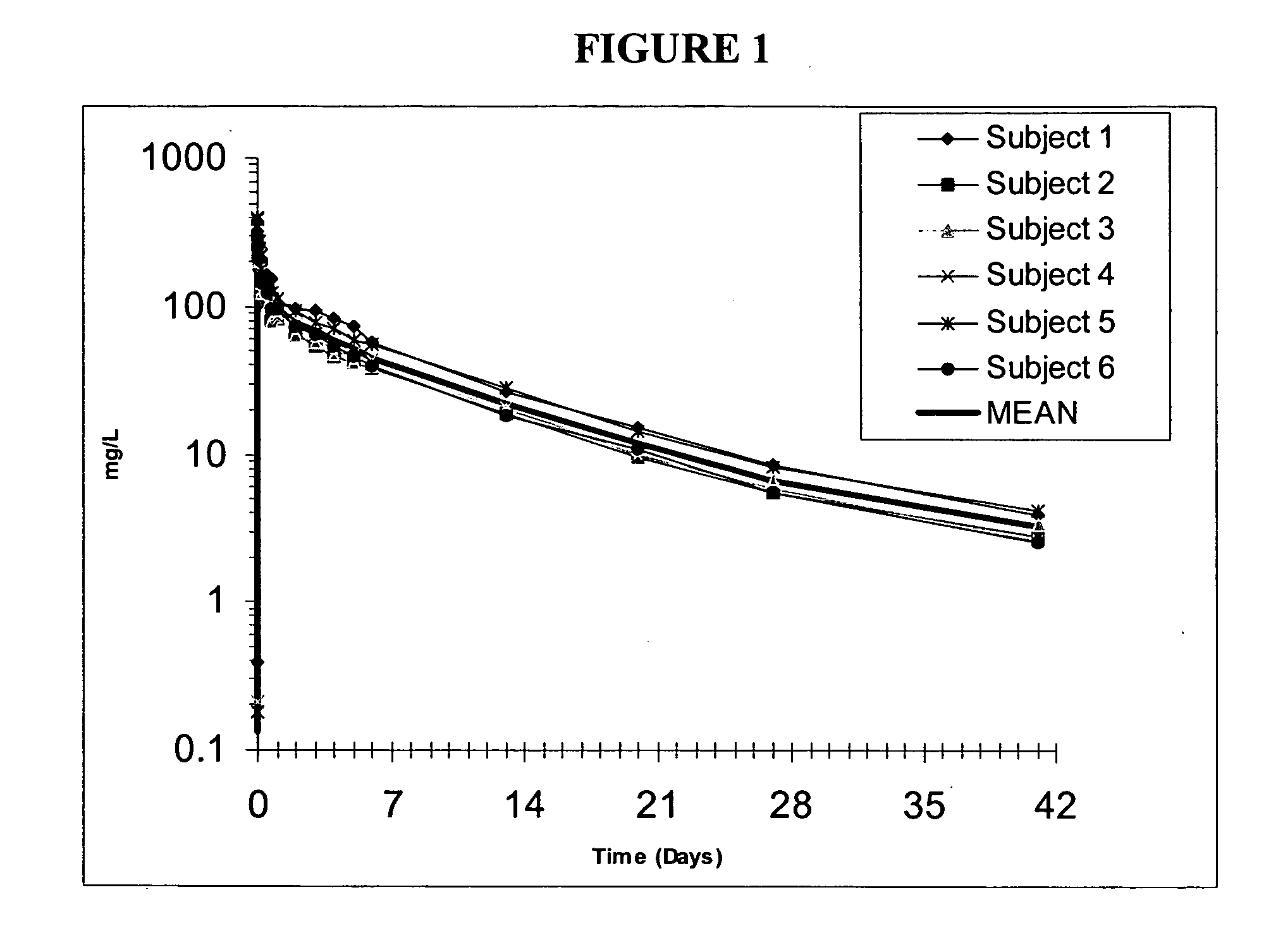
The invention provides methods for treatment of bacterial infections in impaired renal patients. Methods of the invention include administration of a therapeutically effective dose of dalbavancin for treatment of a bacterial infection, in particular a Gram-positive bacterial infection of skin and soft tissue.
Owner:VICURON PHARM INC
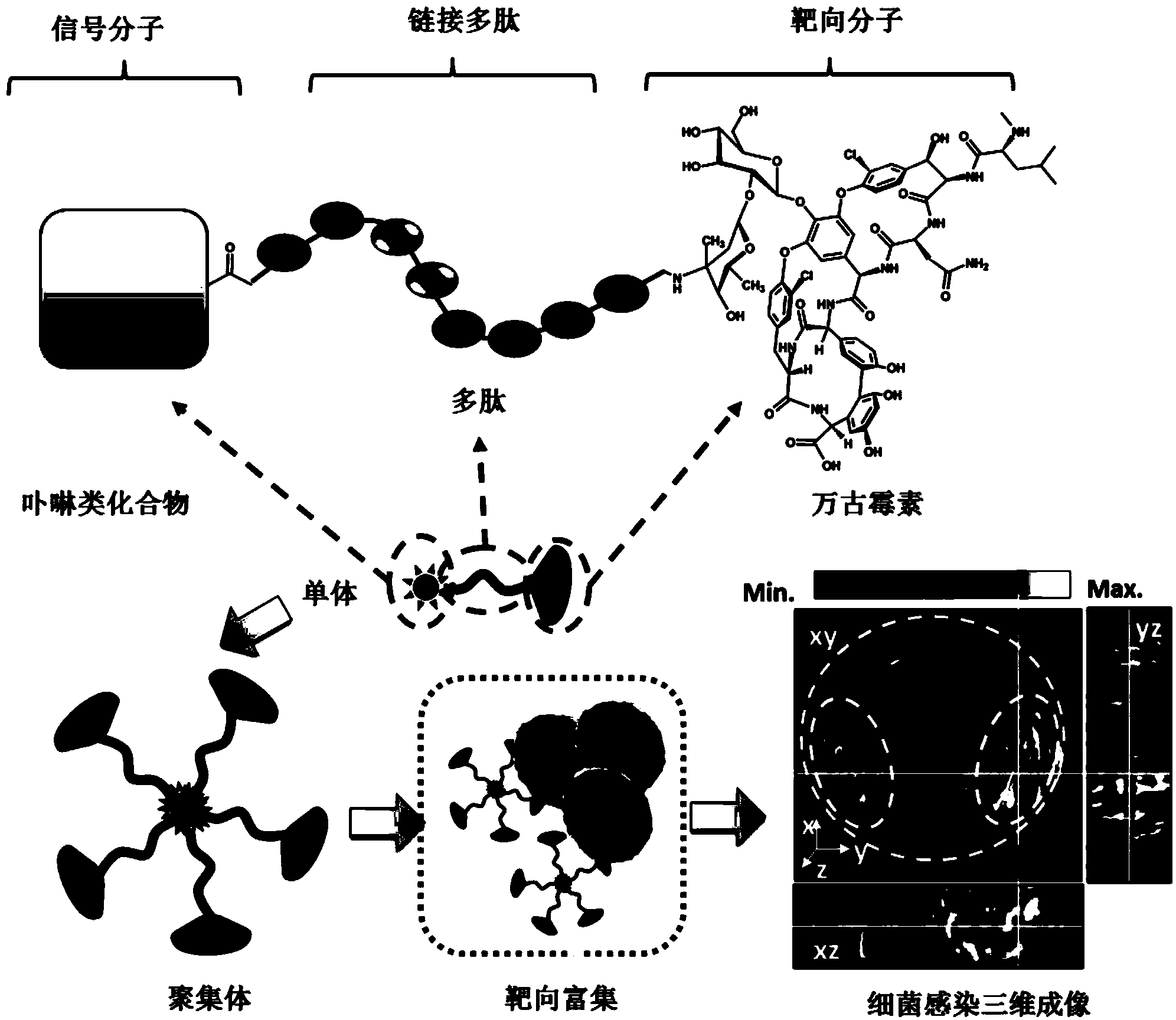
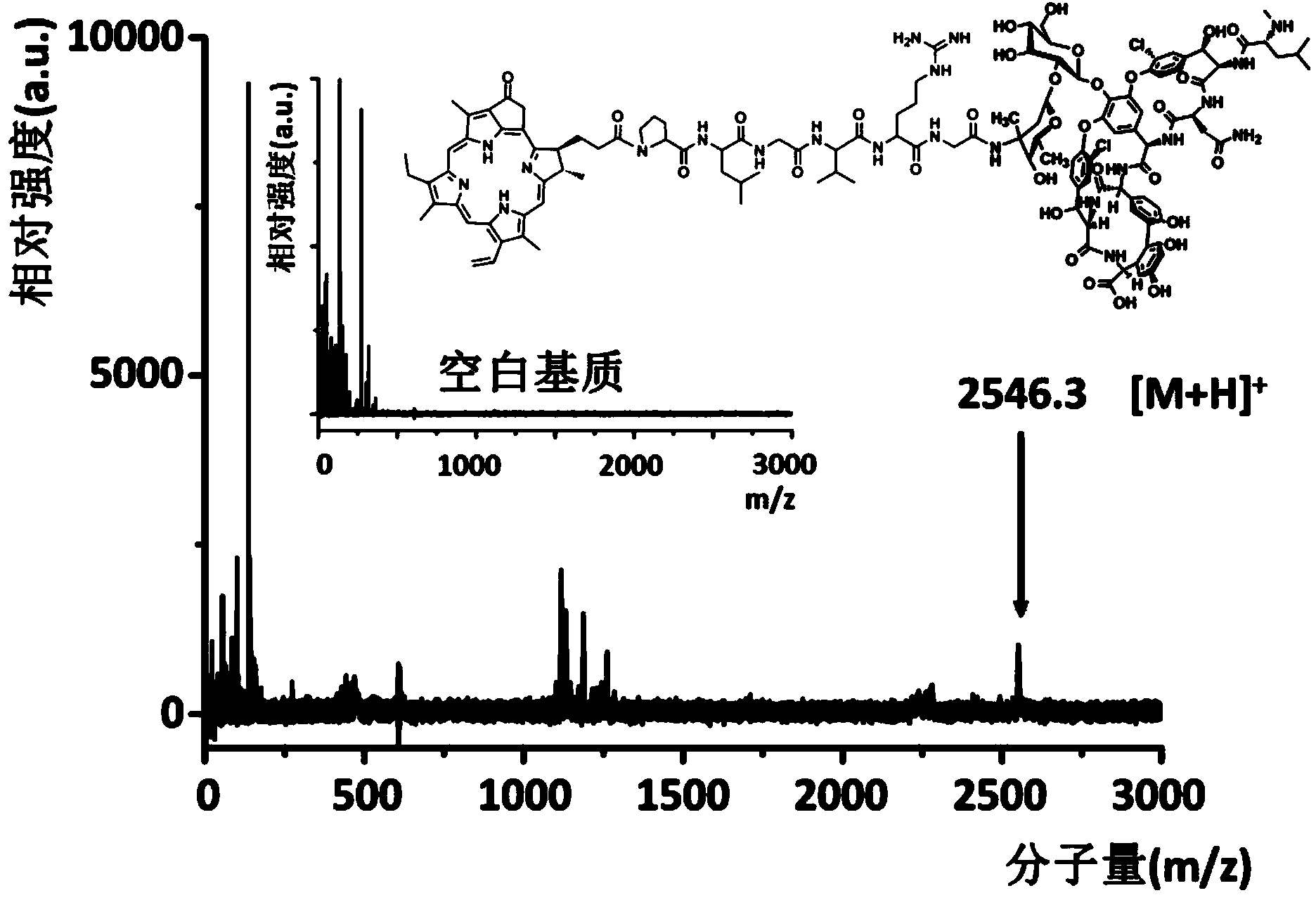
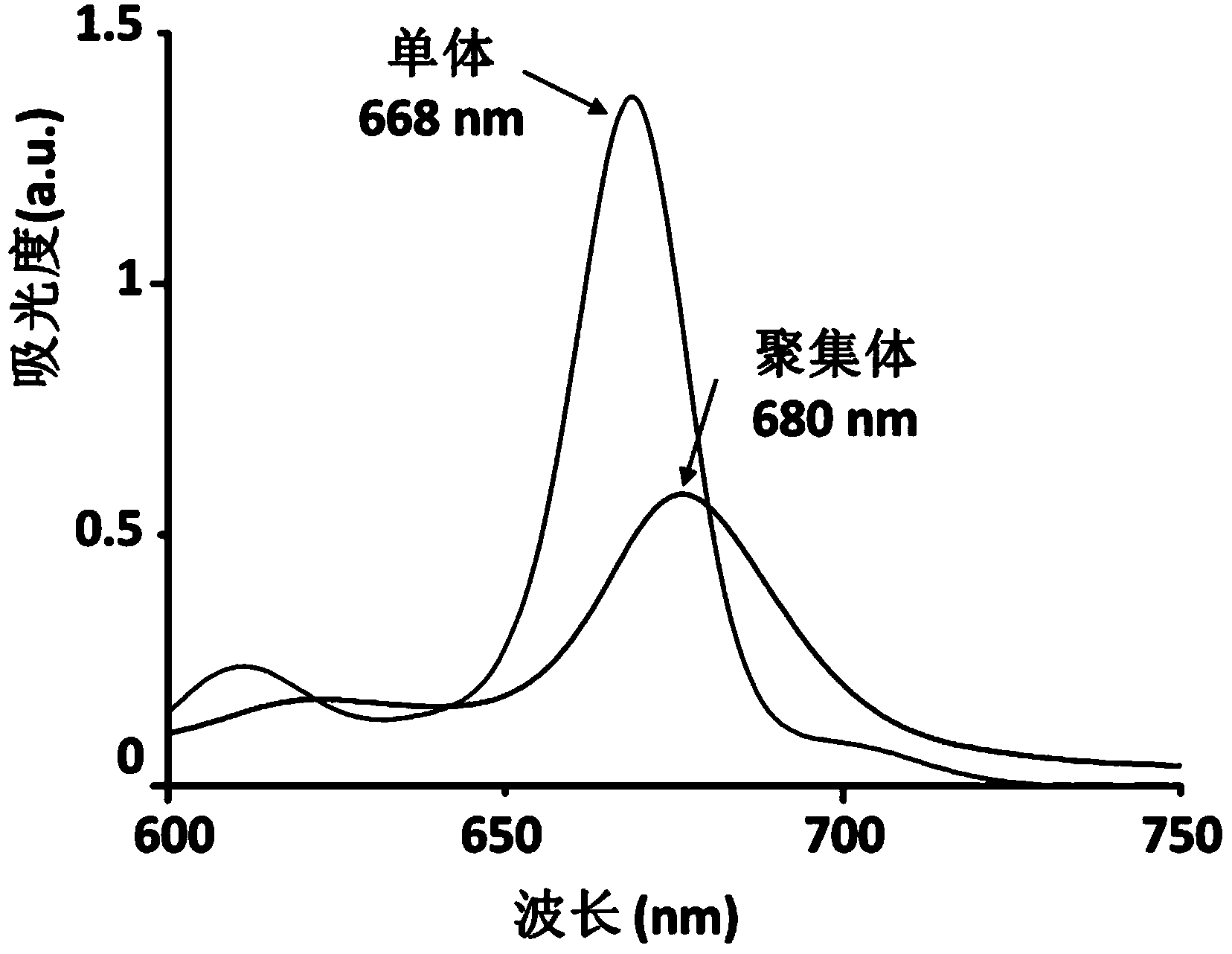
Targeting opto-acoustic contrast agent as well as preparation method and application thereof
ActiveCN103961722AWith UV absorption red shiftSuitable for parentsIn-vivo testing preparationsSolubilitySignalling molecules
The invention discloses a targeting opto-acoustic contrast agent. The targeting opto-acoustic contrast agent sequentially comprises a fat-soluble opto-acoustic signal molecule, a connecting polypeptide and a water-soluble targeting molecule. The targeting opto-acoustic contrast agent disclosed by the invention is an amphipathic molecule, can form a stable aggregate with good water solubility and has the characteristics of ultraviolet absorption red shift, fluorescence quenching and opto-acoustic signal enhancement. The targeting opto-acoustic contrast agent can be enriched at an inflammation part infected by gram-positive bacteria in vivo in a targeting peculiar manner, realizes the defection of gram-positive bacteria infection in vivo and has very good clinical application prospect.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
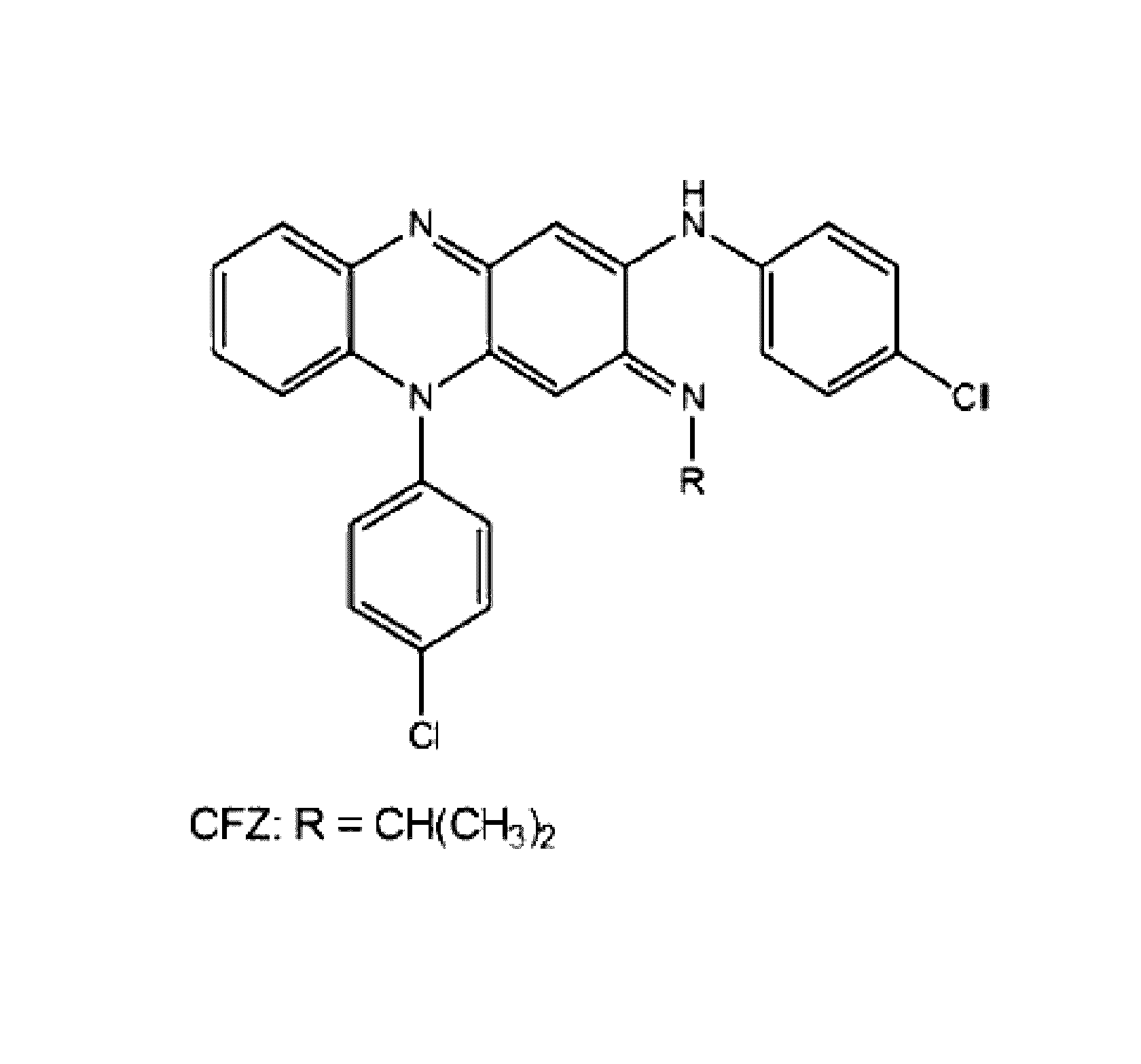
Therapeutic agents
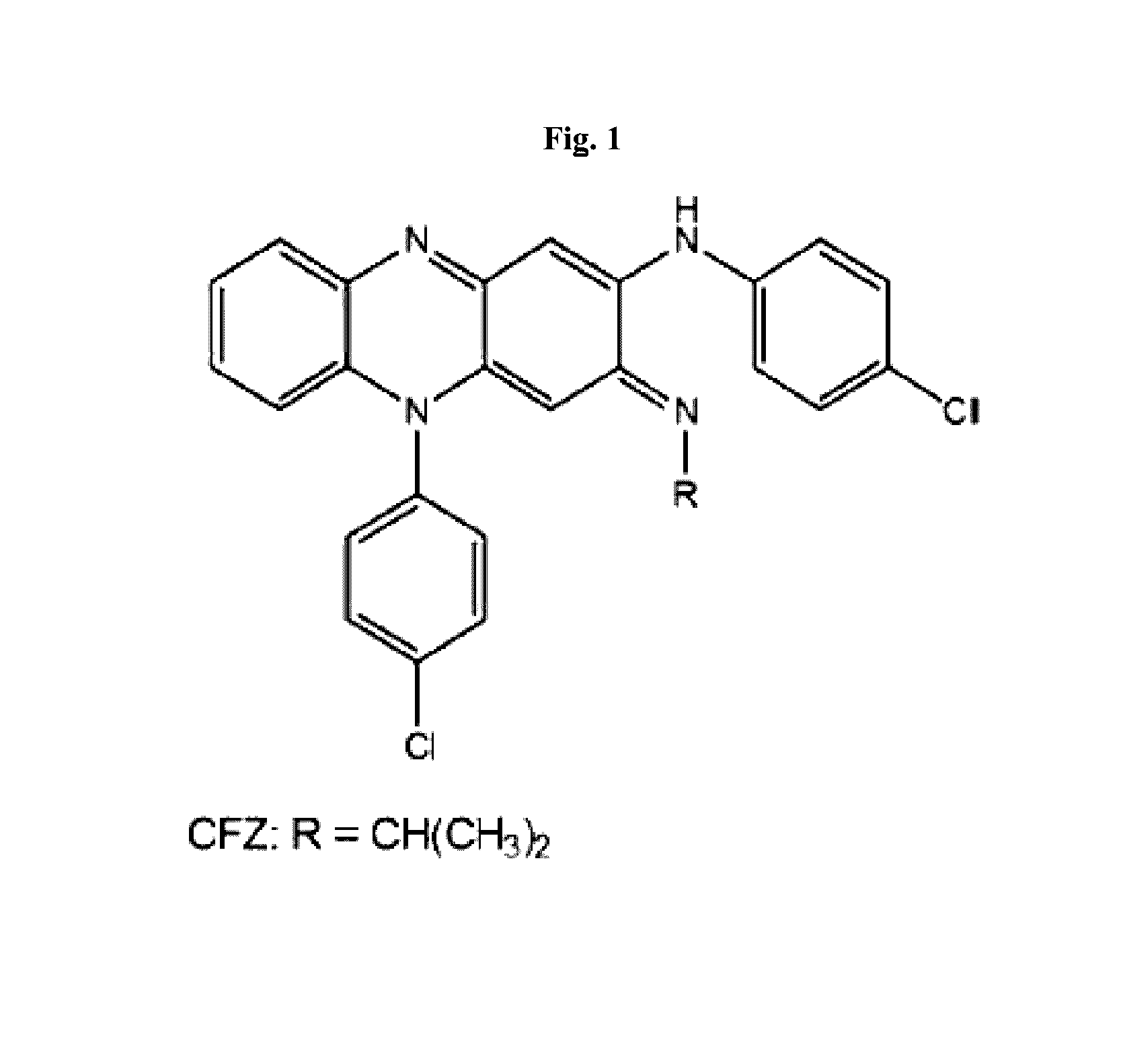
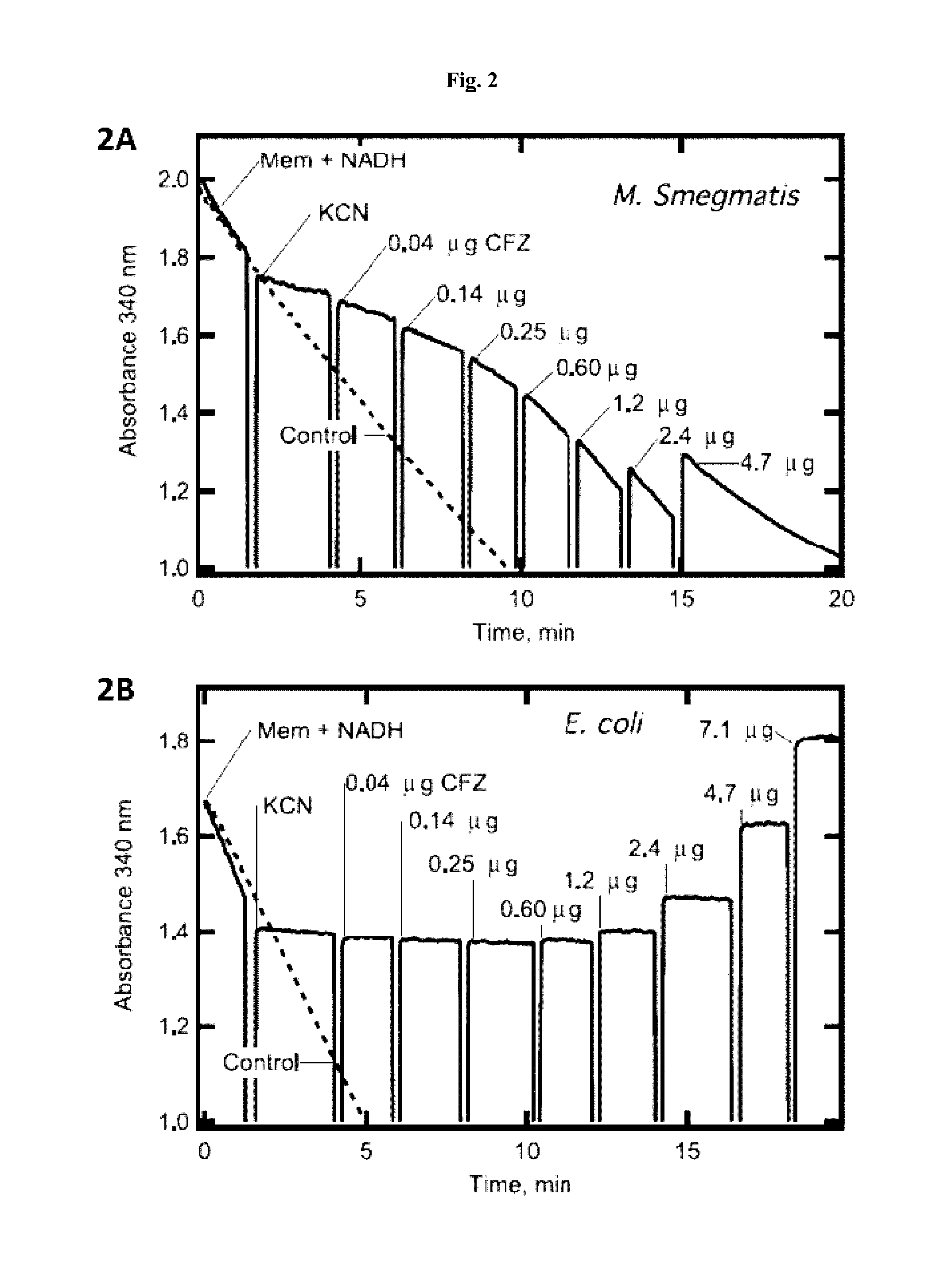
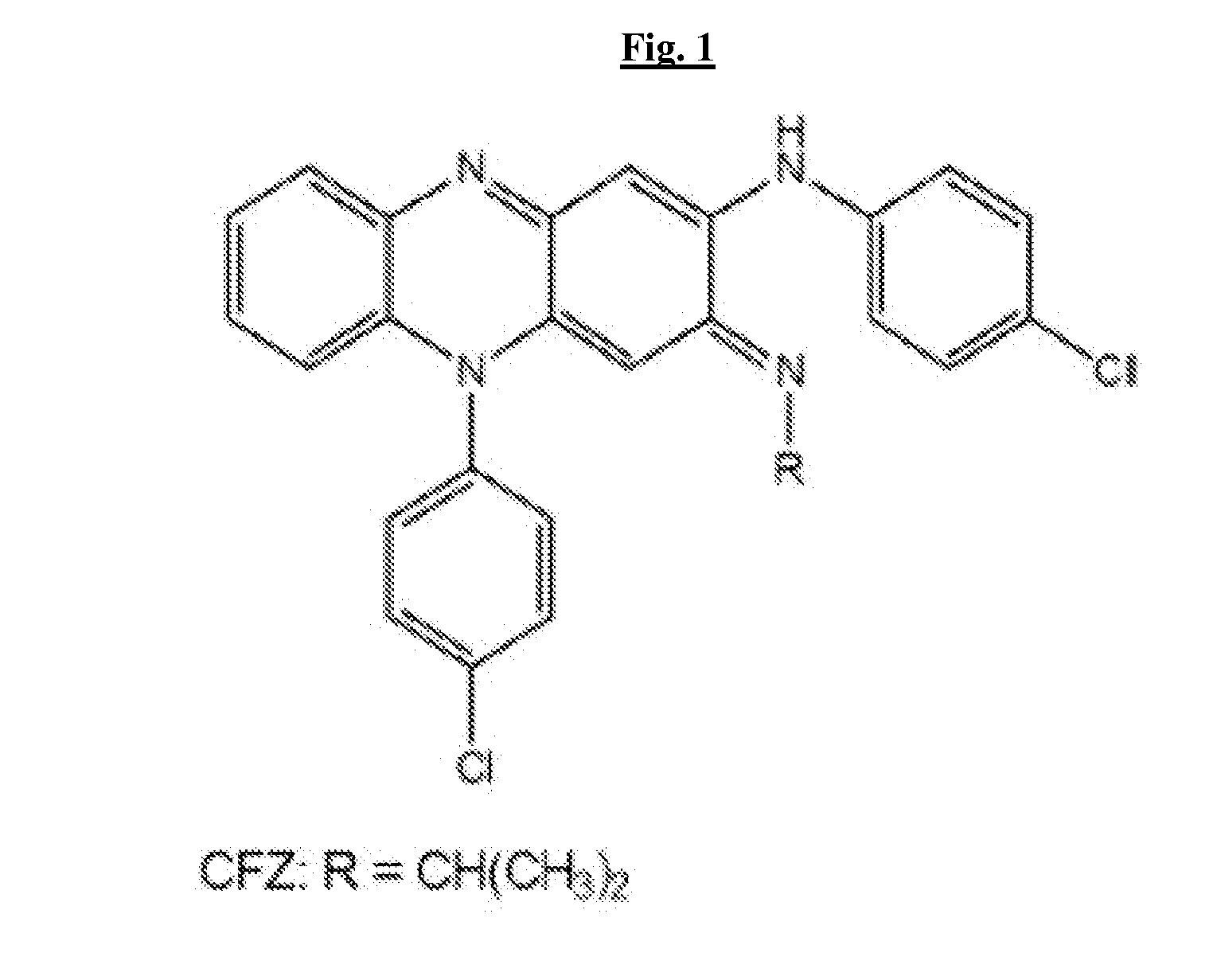
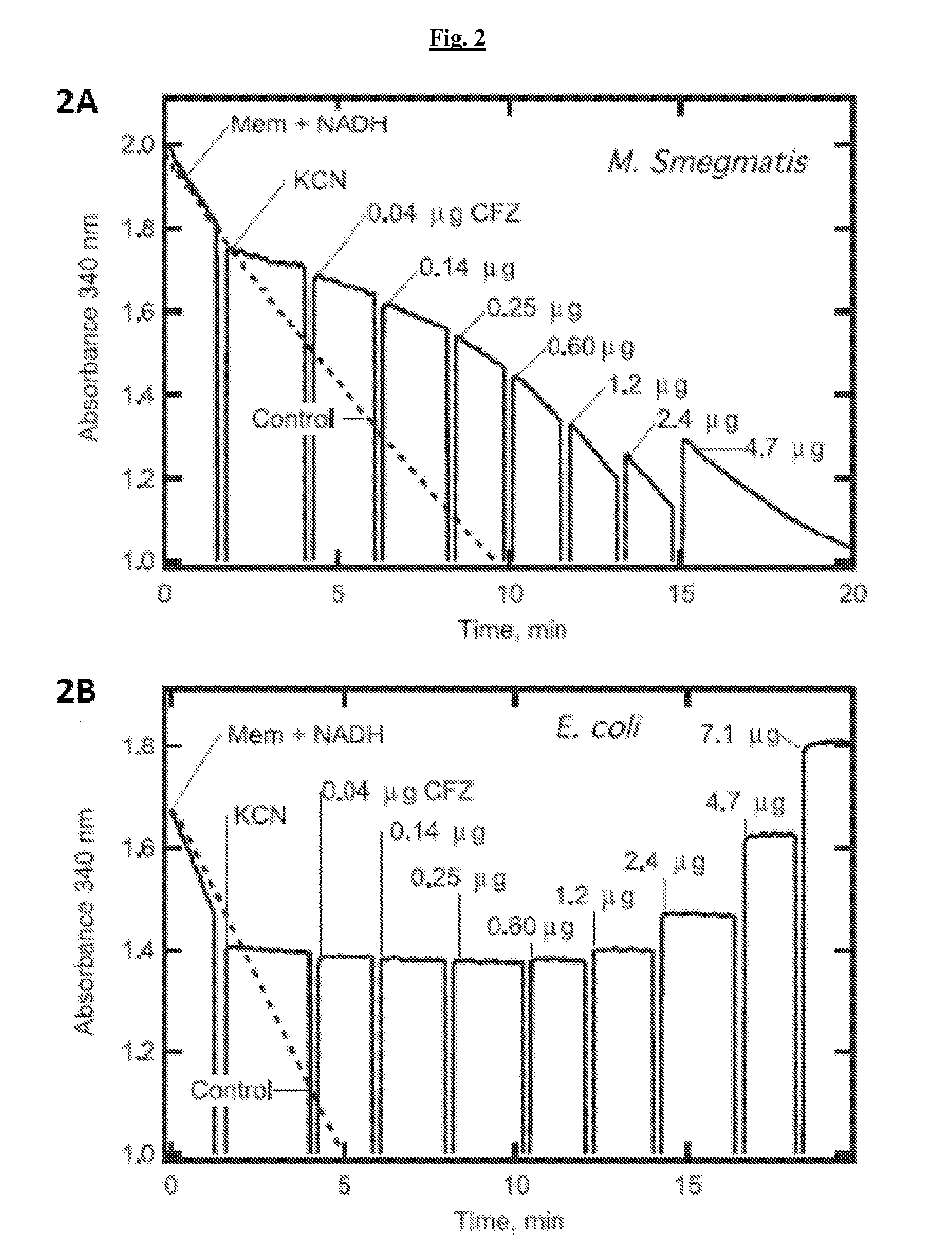
The present invention includes a method of treating or preventing a disease or disorder such as a mycobacterial infection, a Gram-positive bacterium infection, a yeast infection, an inflammatory condition, an auto-immune disorder and / or a proliferative disorder in a subject in need thereof. The method comprises administering to the subject a therapeutically effective amount of at least one compound of the invention, which includes clofazimine derivatives.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and methods for treating gram positive bacterial infection in a mammalian subject
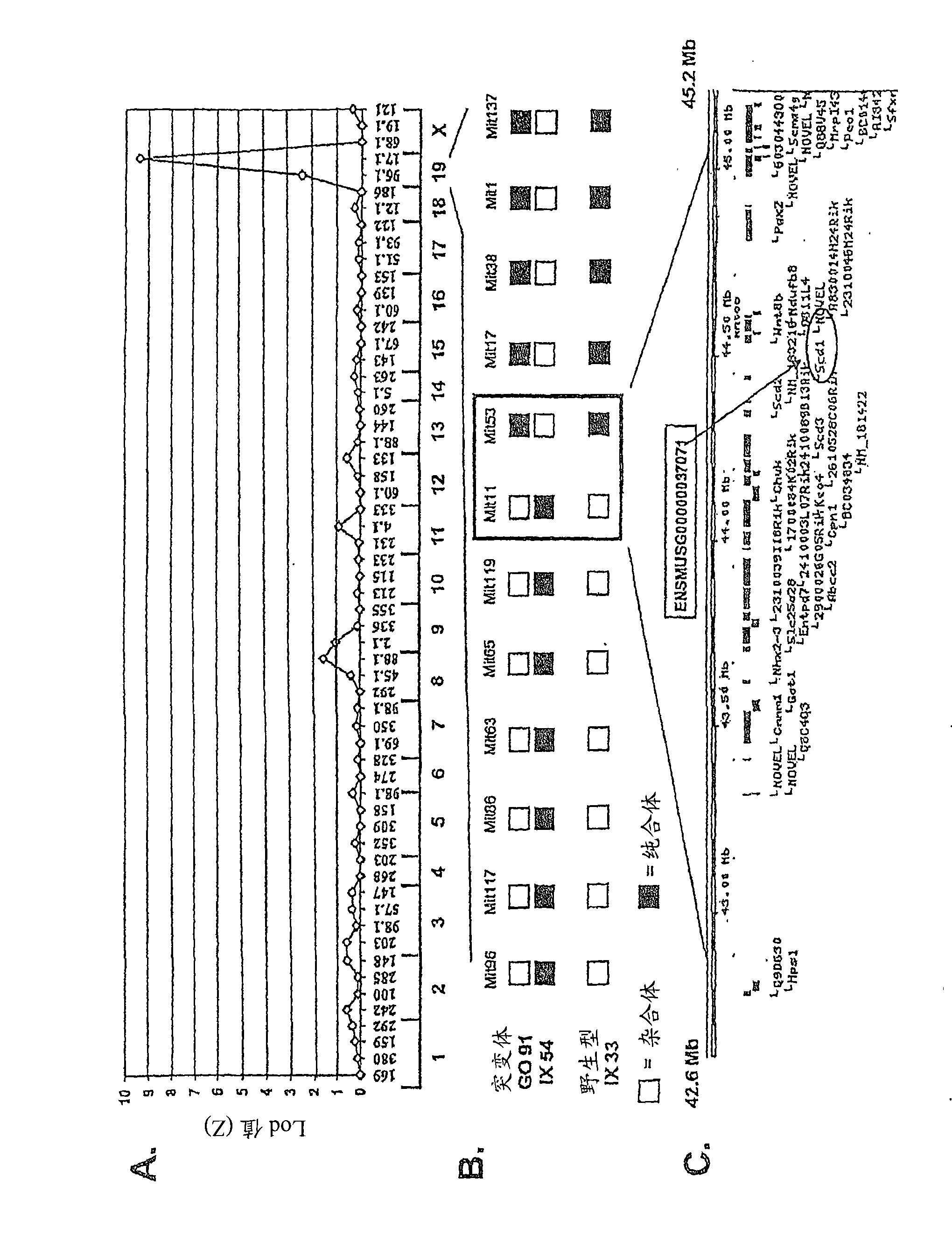
Compositions and methods are provided for treating Gram positive bacterial infection in a mammalian subject. Compositions and methods are further provided for treating Gram positive bacterial skin infection in the mammalian subject. Compositions and methods are provided that comprise administering to the mammalian subject an effective amount of a compound that activates Scd1 gene expression or activates Scd1 gene product.
Owner:THE SCRIPPS RES INST
Application of crizotinib in preparation of medicines capable of resisting gram-positive bacteria
ActiveCN110652512ABroad spectrum antibacterialGrowth inhibitionAntibacterial agentsOrganic active ingredientsGram-positive bacterial infectionsAnti neoplastic
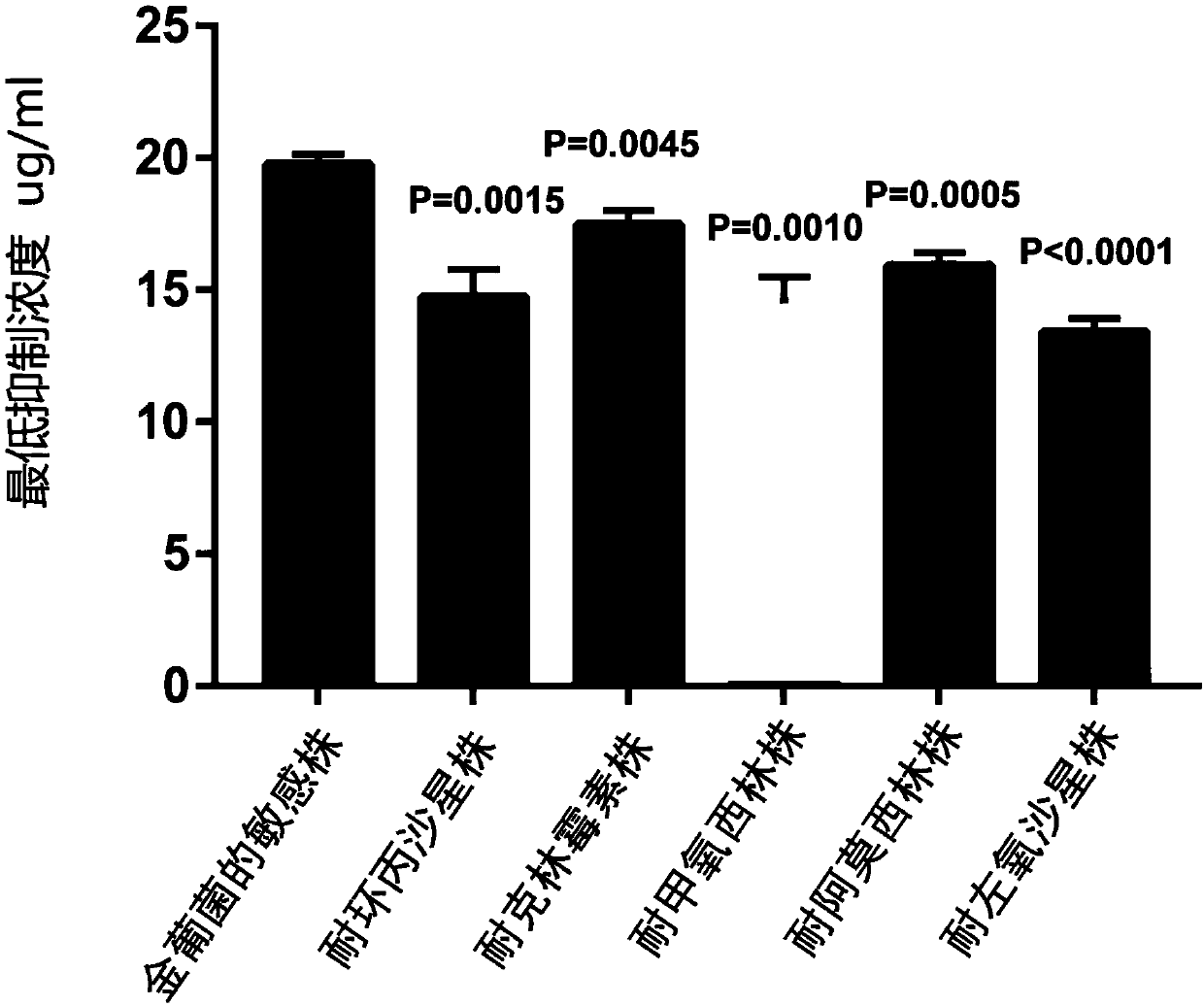
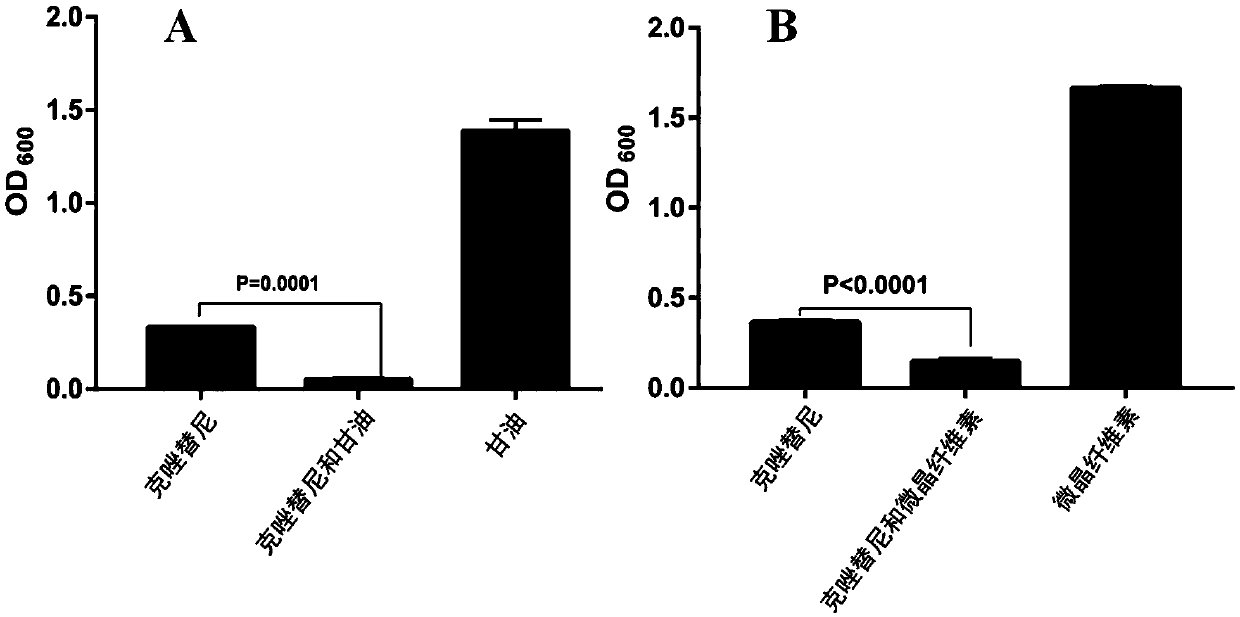
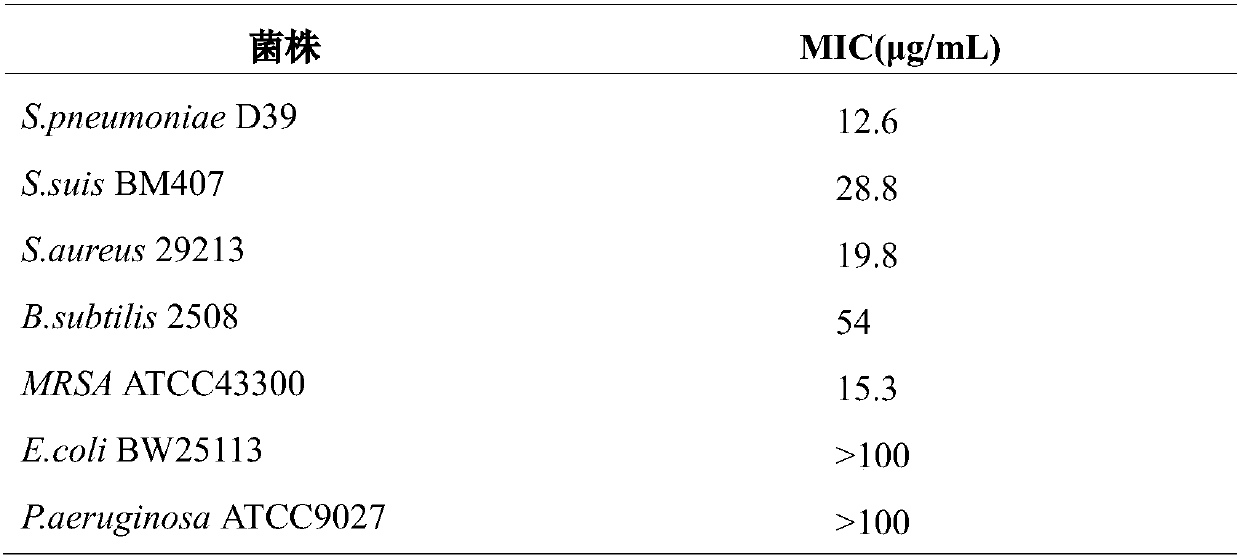
The invention provides application of crizotinib in preparation of medicines capable of resisting gram-positive bacteria. For the first time, it is proposed that an anti-tumor drug, namely the clozatinib, can effectively inhibit gram-positive bacteria; and the clozatinib is particularly significant in the effect of inhibiting growth of single drug resistant strains and clinical multi-drug-resistant strains, as well as wide in anti-bacterial spectrum. Thus, application range of the crizotinib is expanded, so that the crizotinib has the potential to be developed into anti-bacterial medicines. The clozatinib is a drug which has been approved for marketing by drug regulatory authorities in many countries around the world, so that no more toxicological experiment is required due to more reliable pharmacological safety; and thus, the clozatinib is excellent in development and utilization prospects. The application of the crizotinib in preparation of the medicines capable of resisting the gram-positive bacteria is of important practical significance to treatment of gram-positive bacterial infection (and especially for drug-resistant bacterial infection), so that a new drug choice is provided for clinical treatment of the gram-positive bacterial infection.
Owner:JINAN UNIVERSITY
Use of platelets in treating infections
PendingUS20210100846A1Reduce the amount requiredAntibacterial agentsPowder deliveryProtozoaEbola virus
Provided herein in some embodiments is a method of treating a disease or condition such as an antibiotic resistant bacterial infection, a gram negative bacterial infection, a gram positive bacterial infection, a fungal infection, protozoa, a hemorrhagic virus, such as Ebola or Dengue, or a non-hemorrhagic virus, such as a coronavirus, in a subject, comprising administering to the subject in need thereof an effective amount of a composition comprising platelets or platelet derivatives, such as freeze-dried platelets; and an incubating agent comprising one or more salts, a buffer, optionally a cryoprotectant, and optionally an organic solvent.
Owner:CELLPHIRE INC
Dalbavancin compositions for treatment of bacterial infections
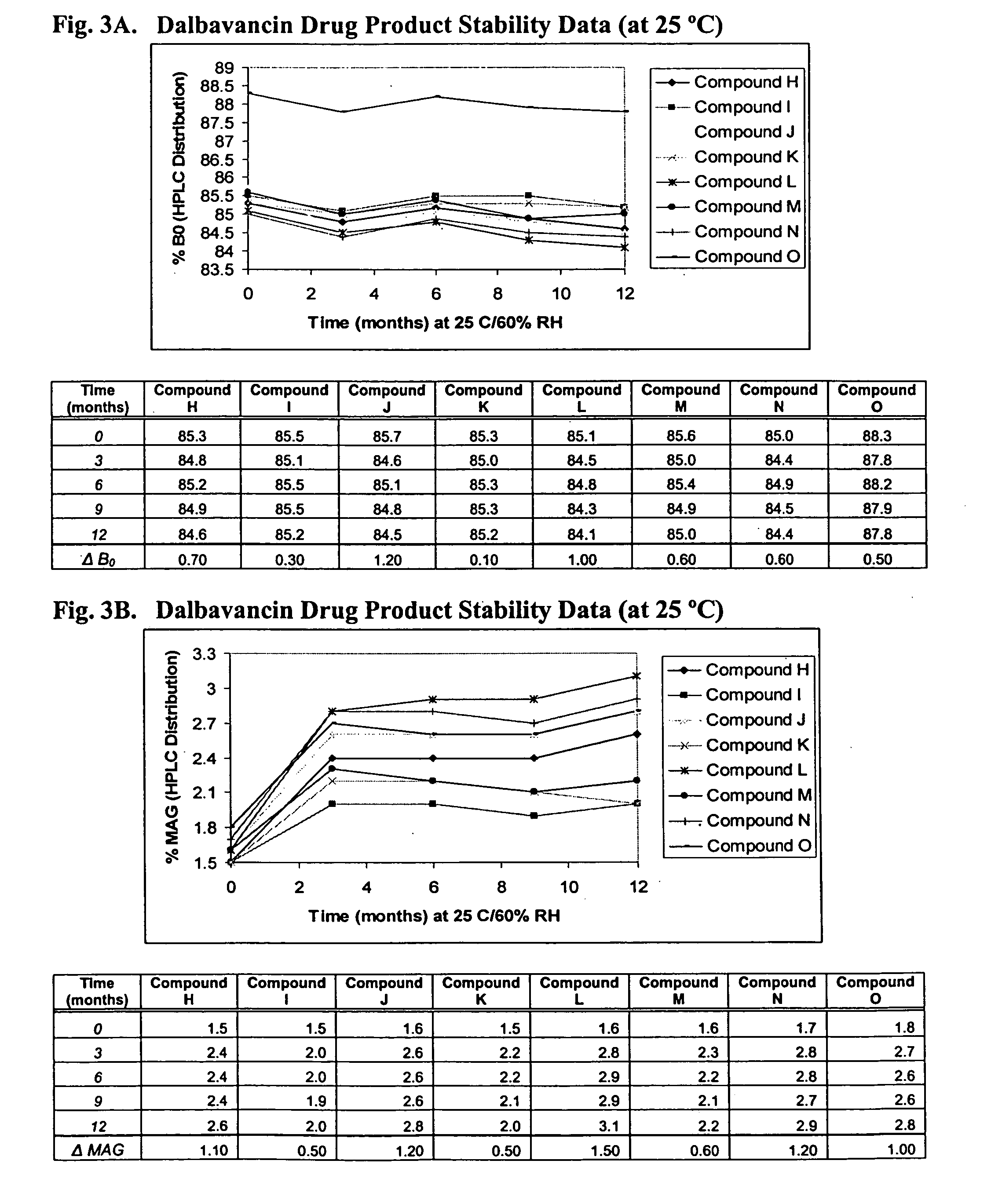
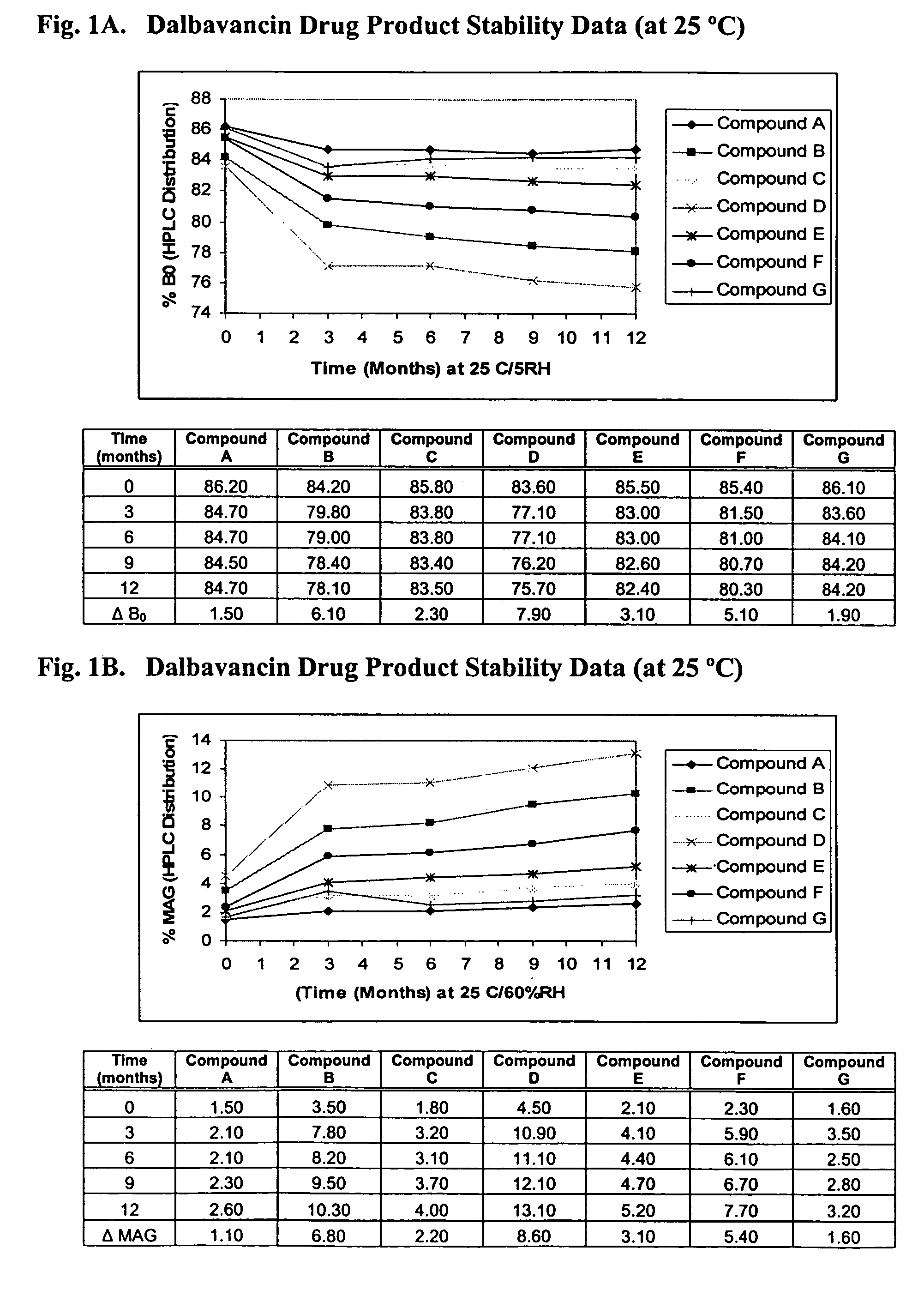
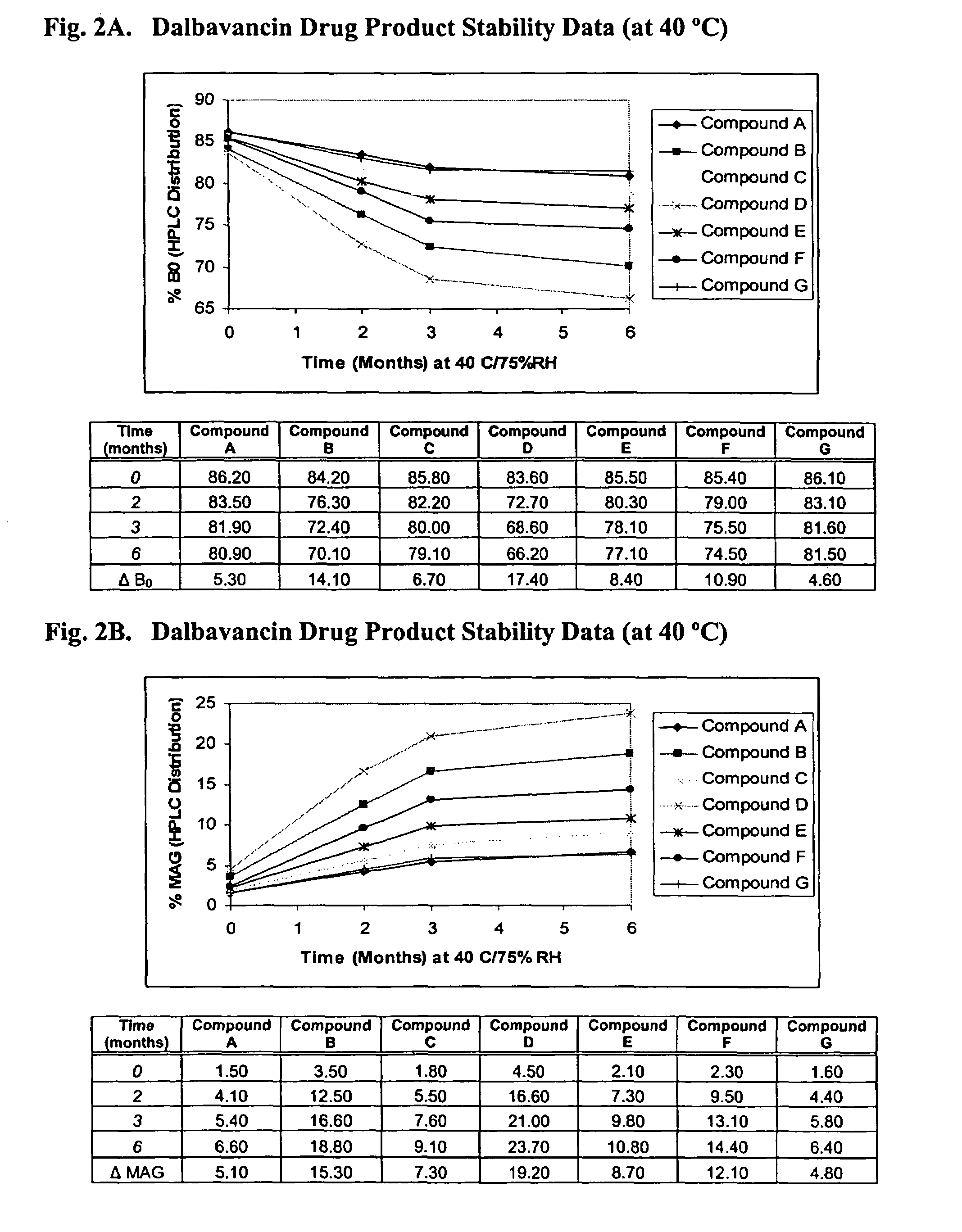
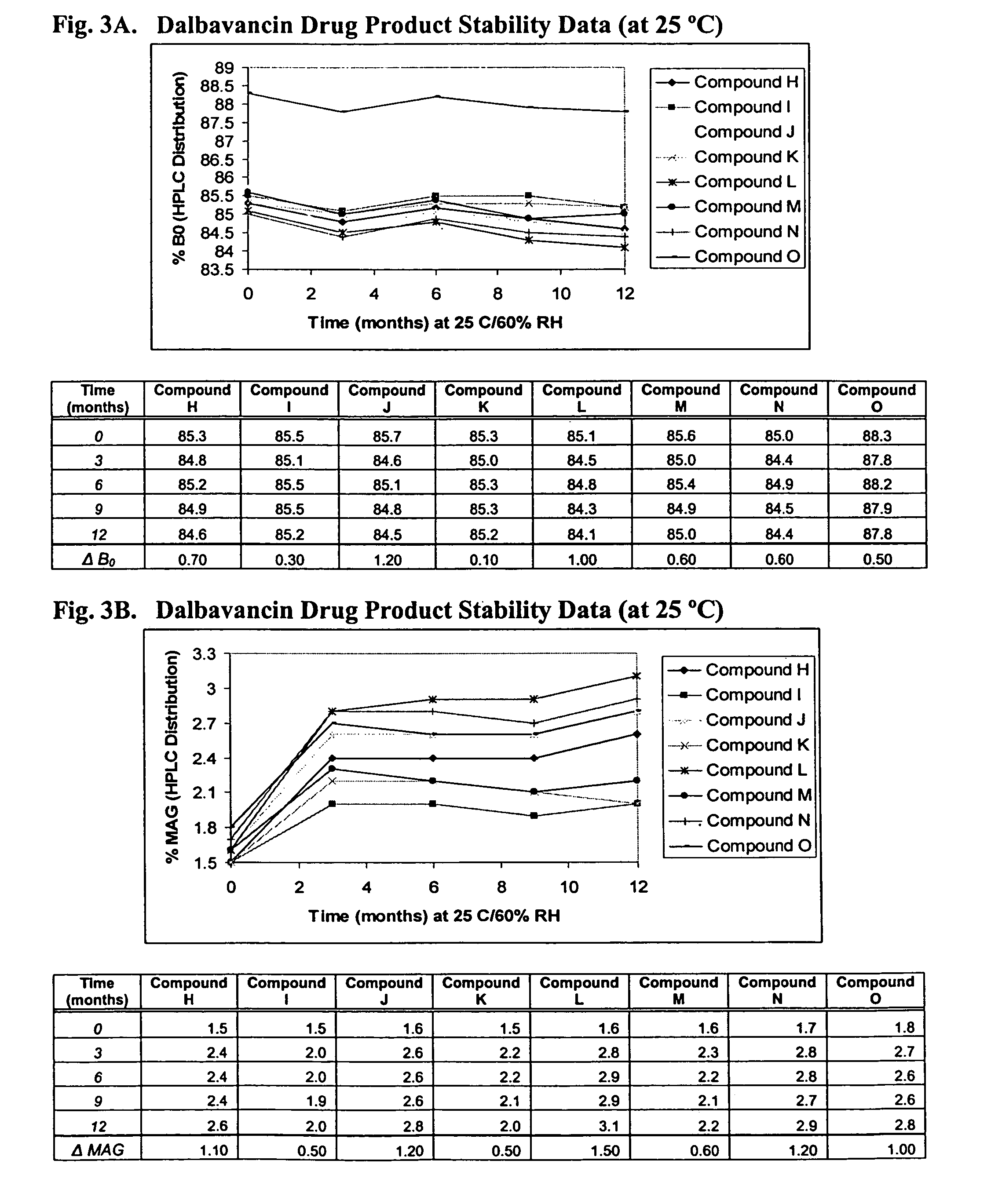
ActiveUS20090298749A1Reduce the amount requiredLow solvent contentAntibacterial agentsEsterified saccharide compoundsNephrosisRegimen
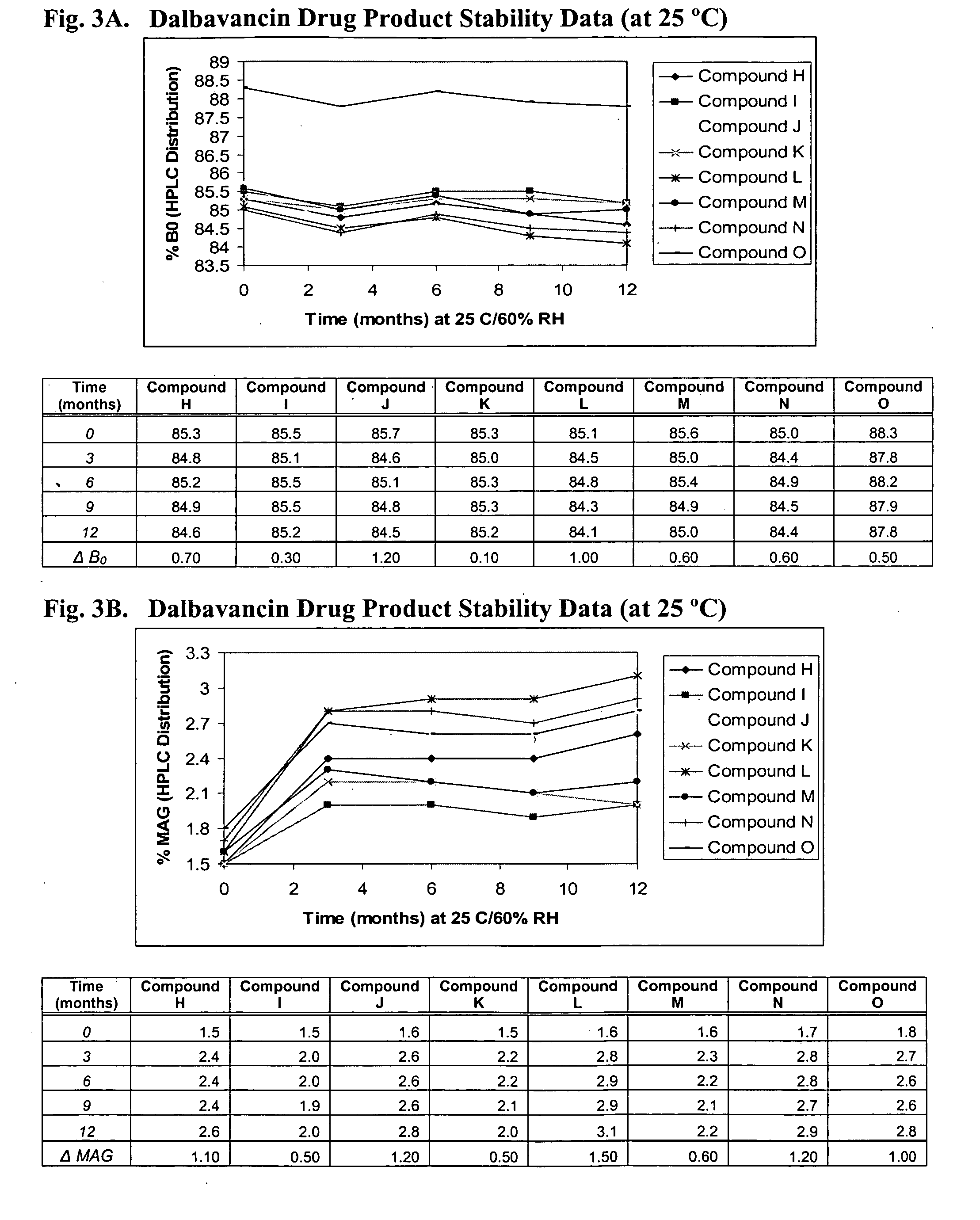
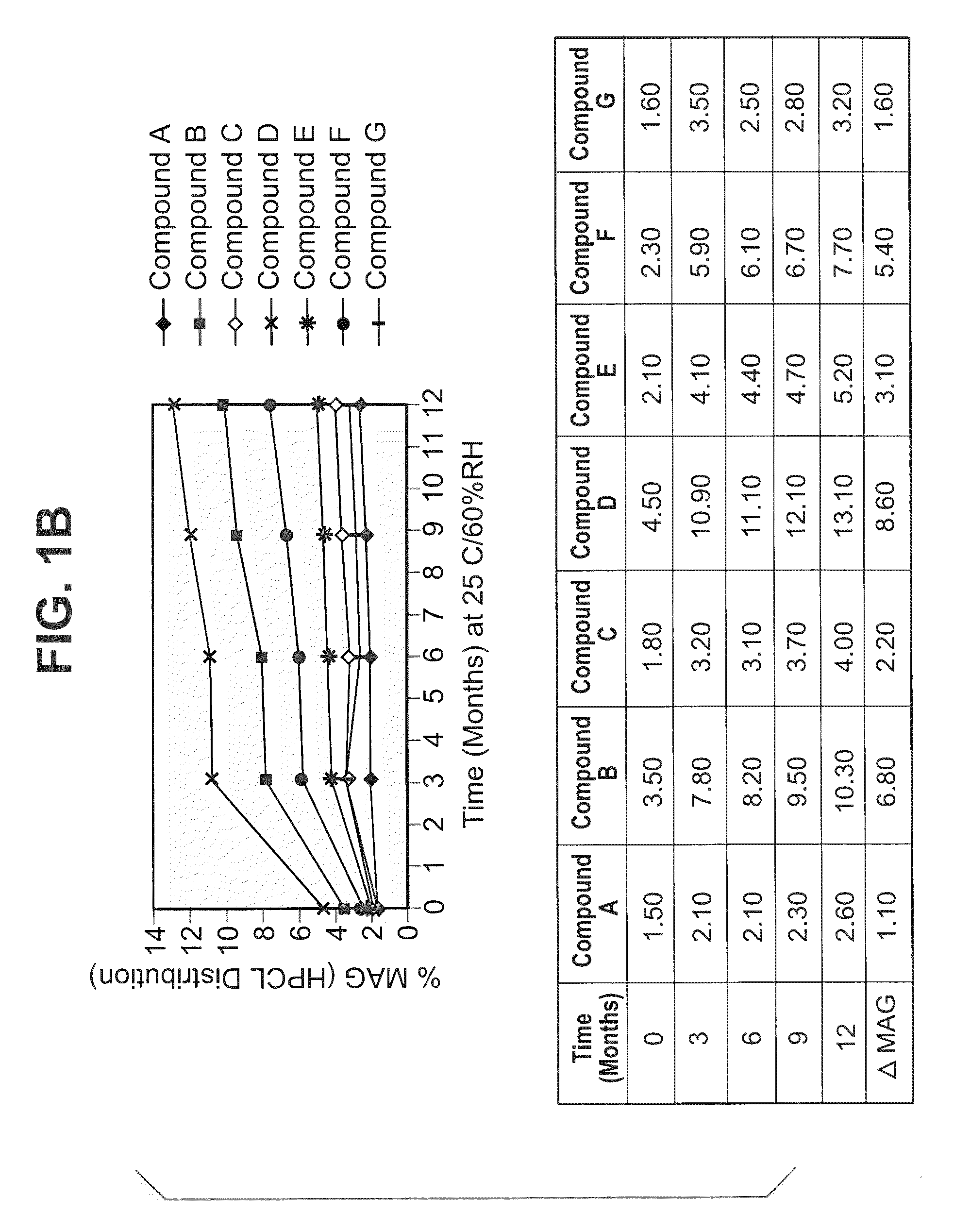
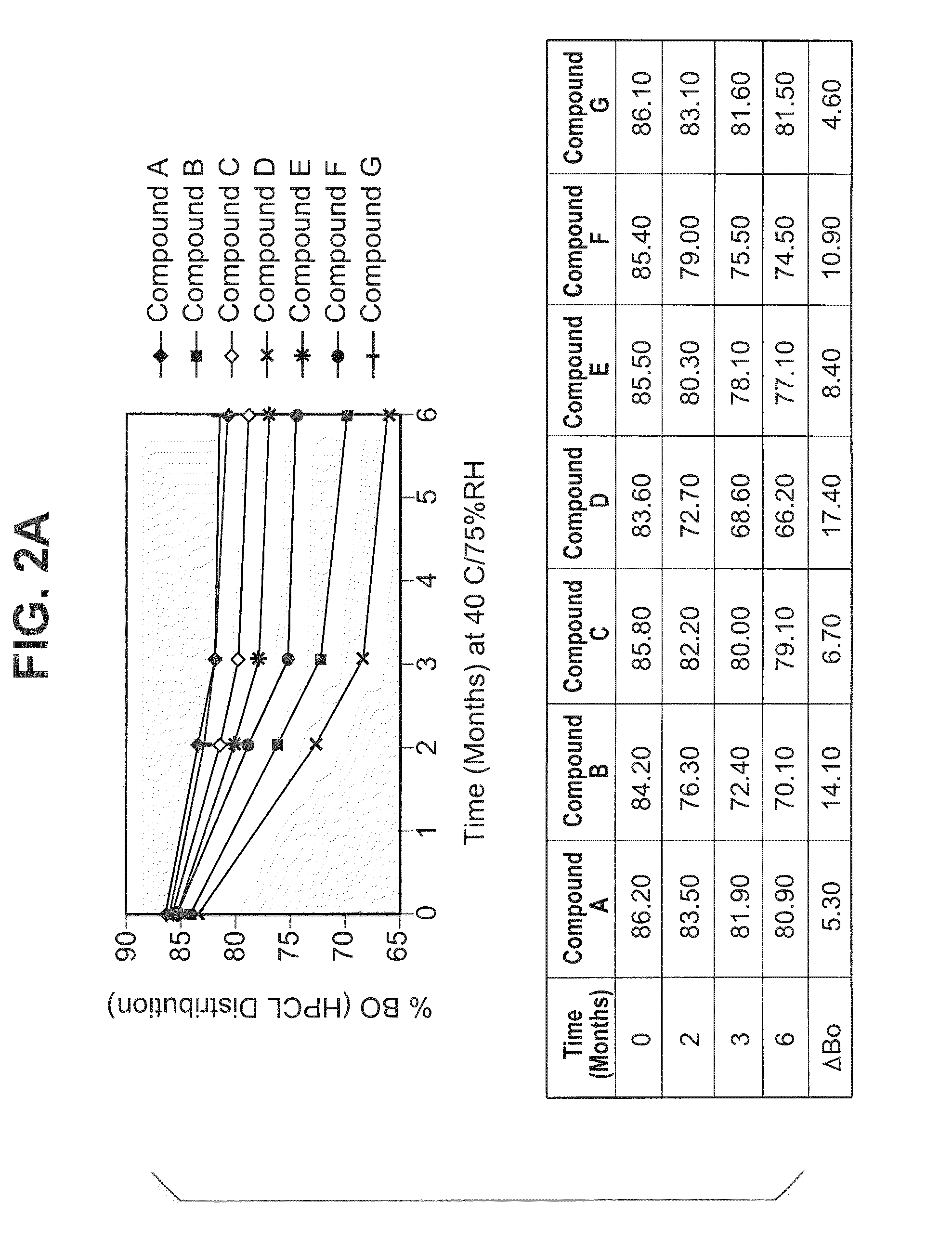
The invention provides methods and compositions for treatment of bacterial infections. The composition may be a combination of factors, which include A0, A1, B1, B2, C0, C1, isoB0, and MAG, in the presence of low level solvent. Methods of the invention include administration of dalbavancin formulations for treatment of a bacterial infection, in particular a Gram-positive bacterial infection of skin and soft tissue. Dosing regimens include multiple dose administration of dalbavancin, which often remains at therapeutic levels in the bloodstream for at least one week, providing prolonged therapeutic action against a bacterial infection. Dosing regimens for renal patients are also included.
Owner:APTALIS PHARMA
Bismuth-thiols as antiseptics for agricultural, industrial and other uses
Compositions and methods, including novel homogeneous microparticulate suspensions, are described for treating natural and artificial surfaces that contain bacterial biofilm, including unexpected synergy or enhancing effects between bismuth-thiol (BT) compounds and certain antibiotics, to provide formulations including antiseptic formulations. Previously unpredicted antibacterial properties and anti-biofilm properties of disclosed BT compounds and BT compound-plus-antibiotic combinations are also described, including preferential efficacies of certain such compositions for treating certain gram-positive bacterial infections, and distinct preferential efficacies of certain such compositions for treating certain gram-negative bacterial infections.
Owner:MICROBION
Combination of gyrase b inhibitors and protein synthesis inhibitors and uses thereof
InactiveUS20070281992A1Suppresses toxin productionReduce inflammationAntibacterial agentsBiocideBiological bodyMethicillin resistance
The present invention relates to compounds and methods of treating, preventing, or lessening the severity of drug-resistant Gram-positive bacterial infections in mammals. The compounds consist of a combination of a gyrase B inhibitor and a protein synthesis inhibitor. These compounds demonstrate antibacterial activity against drug-resistant strains of Gram-positive bacteria, and in particular, methicillin-resistant Staphylococcus aureus (MRSA). Methods for inhibiting the activity of strains of Gram-positive bacteria and methods for treating a bacterial infection caused by such organisms are described herein.
Owner:BATTS DONALD HERMAN +1
Compositions comprising endotoxin neutralizing protein and derivatives and uses thereof
The present invention relates to the use of endotoxin neutralizing protein (ENP) and derivatives thereof as stand alone microbial inhibitors or as synergistic enhancers of antibiotics and preservatives. Compositions comprising ENP or alone or in combination with an antibiotic may be used to prevent or treat gram-negative bacterial infections, endotoxemia, septic shock, gram-positive bacterial infections, yeast infections and fungal infections.
Owner:ASSOCS OF CAPE COD
Novel therapeutic agents
The present invention includes a method of treating or preventing a disease or disorder such as a mycobacterial infection, a Gram-positive bacterium infection, a yeast infection, an inflammatory condition, an auto-immune disorder and / or a proliferative disorder in a subject in need thereof. The method comprises administering to the subject a therapeutically effective amount of at least one compound of the invention, which includes clofazimine derivatives.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
30% lincomycin hydrochloride injection and preparation method thereof
InactiveCN107028878AImprove the bactericidal effectGuaranteed sterilityAntibacterial agentsOrganic active ingredientsHigh concentrationFiltration
The invention discloses 30% lincomycin hydrochloride injection. The ratio of the 30% lincomycin hydrochloride injection by C18H34N2O6S is 10ml:3g. A preparation method of the 30% lincomycin hydrochloride injection includes the steps: adding water for injection accounting for 50% of prescription dosage; adding benzyl alcohol with the prescription dosage; stirring and dissolving the benzyl alcohol; adding lincomycin hydrochloride with the prescription dosage; dissolving the lincomycin hydrochloride; replenishing the water for injection to reach total quantity; performing fine filtration with a 0.22 micrometer filter element; sterilizing solution by circulating steam at the temperature of 100 DEG C to obtain the 30% lincomycin hydrochloride injection. The 30% lincomycin hydrochloride injection is a bacteriostatic agent and has a bactericidal effect on highly sensitive bacteria when at high concentration. By increasing the concentration, the bactericidal effect of lincomycin on the sensitive bacteria is remarkably enhanced. Asepsis of a medicine is ensured by a final bactericidal production process without affecting stability of the medicine, and efficacy is ensured. Clinically, the 30% lincomycin hydrochloride injection is commonly used for gram positive bacterial infection, particularly, infection of penicillin-resistant bacteria sensitive to the lincomycin such as staphylocosis, streptococcicosis and mastitis, and can also be used for swine treponematosis and swine enzootic pneumonia caused by mycoplasma infection.
Owner:上海公谊药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com