Dry powder vancomycin compositions and associated methods
A vancomycin and composition technology, applied in the field of dry powder, can solve the problems of patient burden, inappropriate dose, no vancomycin dry powder, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
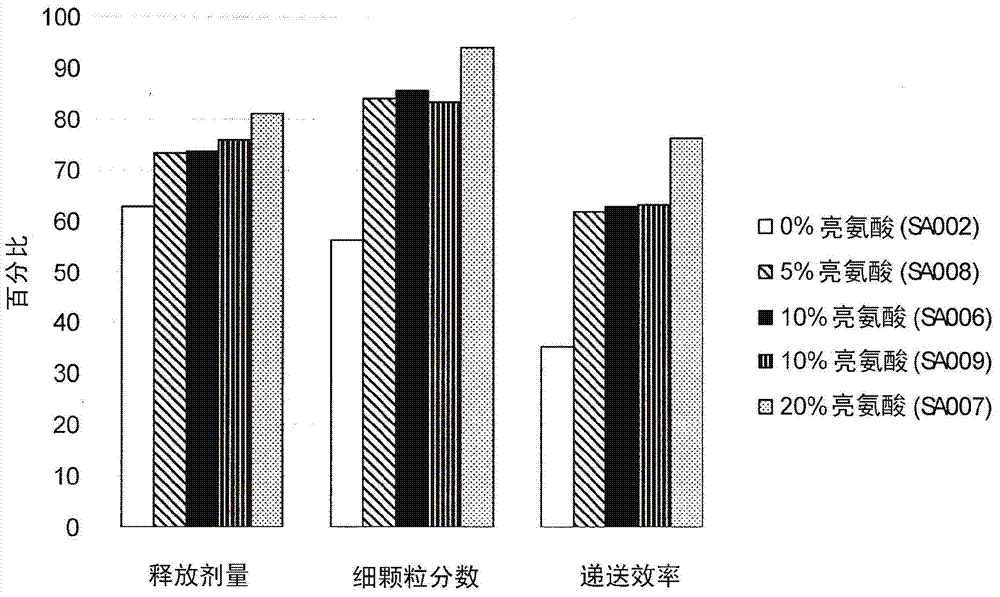
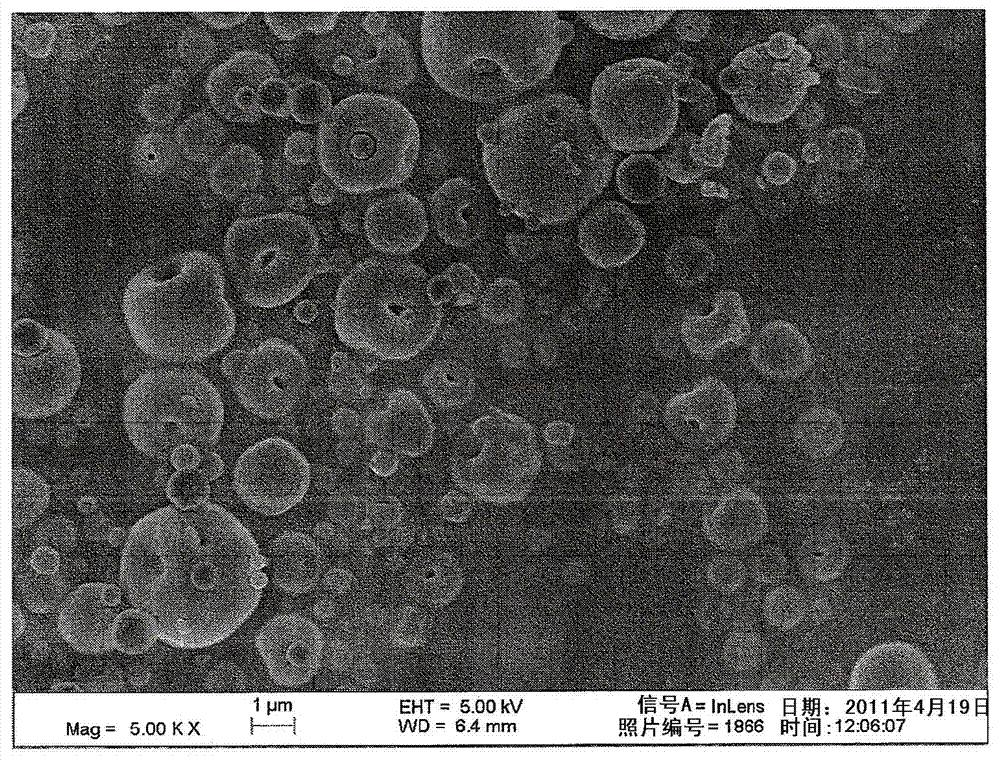
[0054] Dry powder vancomycin compositions were manufactured in high yields (75-95%) and in two different batch sizes (1 g and 20 g) without loss of purity using a Buchi laboratory scale spray dryer. These powders showed very high delivery efficiency and lot-to-lot consistency.
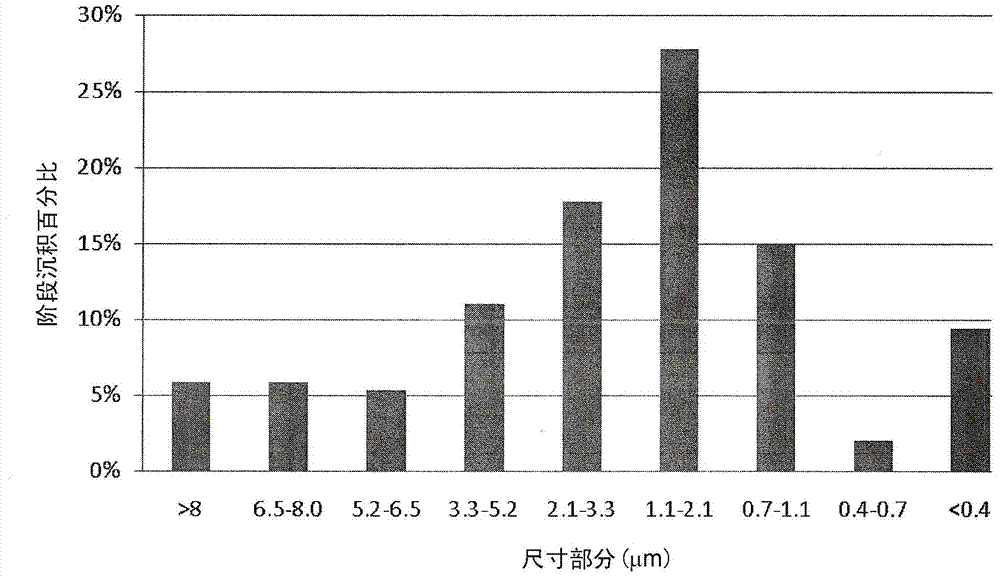
[0055] The full aerodynamic particle size distribution of the 20g batch is shown in figure 1 , the figure shows that the vast majority of the particle size distribution is smaller than 5 μm and that a large proportion (about 59%) is within the ultrafine particle fraction (3 μm) predicted for deep lung delivery.
[0056] Preliminary studies have shown that the delivered dose content uniformity of vancomycin powder readily meets the technical requirements of FDA's draft guidance on aerosol dose uniformity. In the last 10 years, this has become one of the biggest challenges facing the pulmonary drug delivery industry. The technical requirements state that no more than one out of 10 times should be outs...
Embodiment 2
[0074] As mentioned above, in some embodiments, the dry powder vancomycin composition of the present invention can be prepared by spray drying. This method has proven to be very efficient and shows excellent batch-to-batch consistency.
[0075] Table 3 below shows primary particle size and moisture content data for batch sizes ranging from 25 g to 100 g, which were prepared by spray drying. x 10 、X 50 and x 90 are the mass diameters of the particles below which 10%, 50% and 90% of the distribution falls, respectively. ND = not determined.
[0076] Also, for lot number G-11-26-1, Figure 6 The complete aerodynamic particle size distribution is shown in and shows that the vast majority of the particle size distribution is smaller than 5 μm (85%) and a large fraction (about 70%) is in the ultrafine particle fraction (<3 μm) expected for deep lung delivery. )Inside.
[0077] table 3
[0078]
[0079]
[0080] Figure 7 Shows that the delivered dose content uniformit...
Embodiment 3
[0086] Vancomycin-leucine formulations contain carbohydrate bulking agents. The formulation was subjected to a chemical stability study at 50°C for 4 weeks. Figure 8 It is shown that the addition of a carbohydrate bulking agent (eg, trehalose) can improve the chemical stability of the dry powder vancomycin composition of the present invention.
[0087] Two lots of vancomycin compositions containing 10% leucine were compared. One of the batches contained no process modifications (Lot SA010) and the other was processed under a nitrogen atmosphere and protected from light and moisture (Lot G-11-026-1). Such as Figure 9 As shown in , these modifications significantly delayed the degradation of vancomycin. Indeed, extrapolation of these data indicates that the composition is stable at room temperature for at least 2 years.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com