Compositions and methods for treating gram positive bacterial infection in a mammalian subject
A Gram-positive, bacterial infection technology, applied in the direction of botanical equipment and methods, drug combinations, chemical instruments and methods, etc., can solve the problem that the role of antibacterial defense has not yet been established in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] Methods for making other antibodies and antibody fragments are known in the art. For example, although F v two domains of fragment V L and V H Encoded by two separate genes, they can be separated by V through recombinant methods or synthetic linkers. L and V H Domains pair to form single protein chains of monovalent molecules (termed single-chain F v (scF v ); See Bird et al., Science 242:423-426,1988; Huston et al., Proc.Natl.Acad.Sci.USA 85:5879-5883,1988; Colcher et al., Ann.NY Acad.Sci.880: 263-80, 1999; and Reiter, Clin. Cancer Res. 2:245-52, 1996).
[0059] Techniques for preparing single-chain antibodies are described in U.S. Pat. Nos. 4,946,778 and 4,704,692. Such single chain antibodies are encompassed within the term "antigen-binding fragment" of an antibody. These antibody fragments can be obtained by conventional techniques known to those of ordinary skill in the art, and the fragments can be screened for use by screening intact antibodies. In addit...
Embodiment 1
[0312] Flake: a visible phenotypic variant with associated immunodeficiency
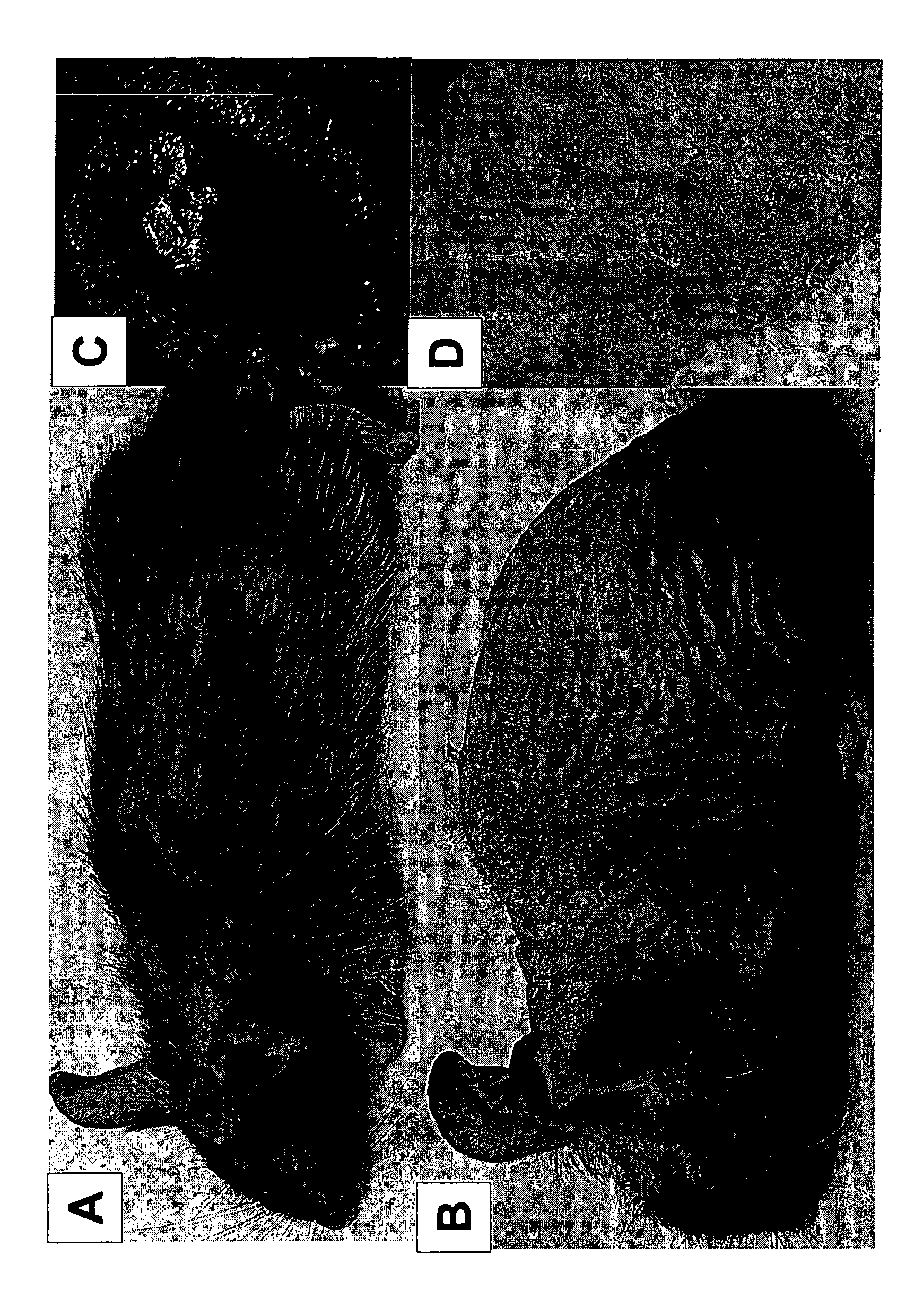
[0313] ENU-induced germline mutations were used in an effort to identify genes required for general immune function. A total of 20,792F1 and 33,202F3 animals were screened for visible and immune phenotypes. Among them, a recessive mutation known as "flake" (flk) was found to cause progressive hair loss and chronic exfoliative dermatitis. These features appear at weaning age and are more pronounced in older animals ( figure 1 ). Visible disruption of epidermal integrity and spontaneous skin infections requiring antibiotic treatment prompted us to examine the integrity of innate immune function in these mice.
[0314] figure 1 Visible phenotypes observed in flake mutant mice are shown. A. 6 week old mouse B. 8 month old mouse C. Ocular infection in 8 month old mouse D. Magnification of mouse in B to highlight severe dermatitis.
Embodiment 2
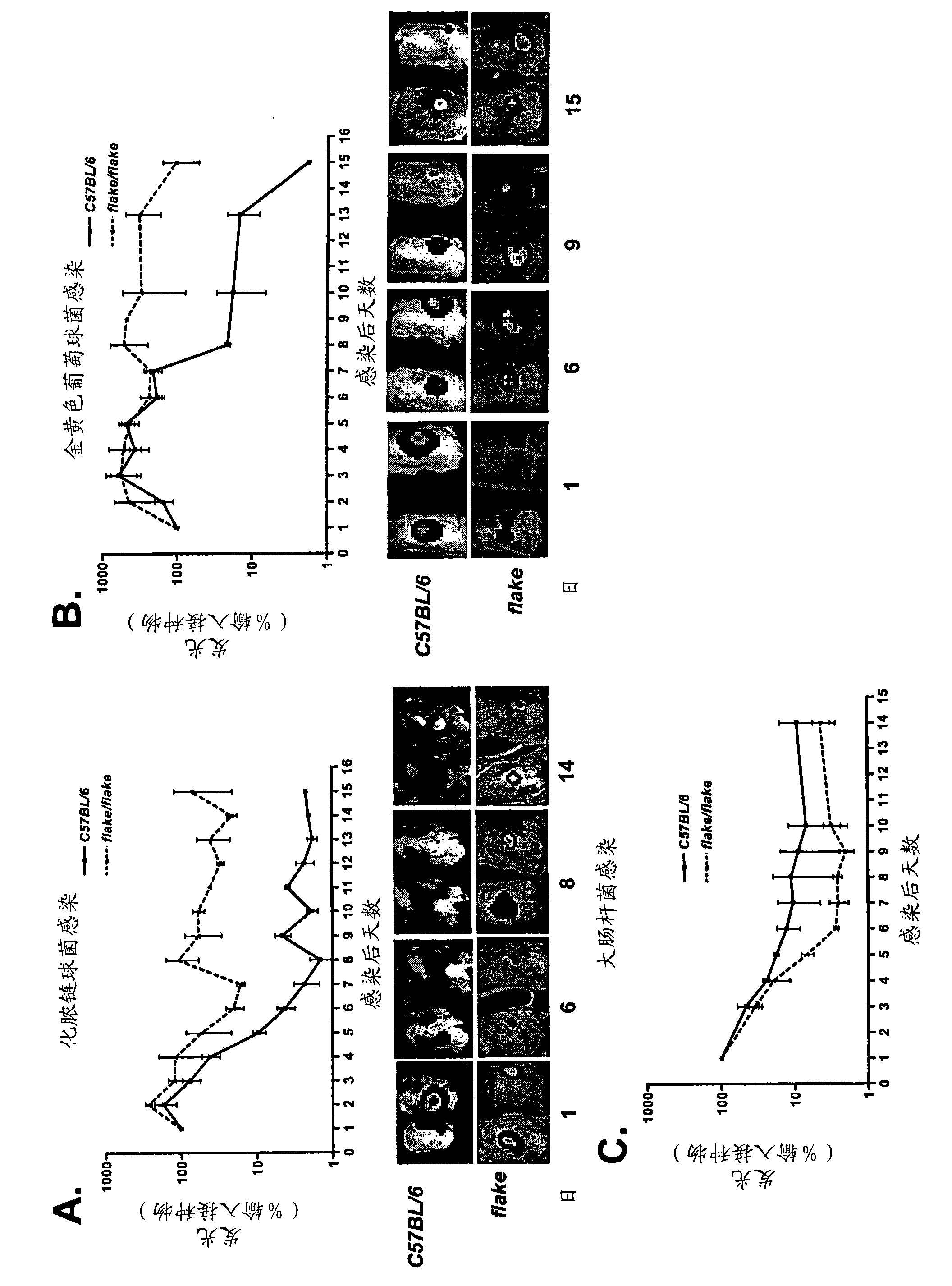
[0316] Persistent Streptococcus pyogenes and Staphylococcus aureus skin infections in flk / flk mutant mice
[0317] mutant mice
[0318] The gram-positive cocci Streptococcus pyogenes and Staphylococcus aureus are the main pathogens of impetigo, cellulitis and wound infections in humans. Guay, Expert. Opin. Pharmacother. 4:1259-1275, 2003; Hedrick, Paediatr. Drugs 1:35-46, 2003. Experimental full-thickness skin infection in murine models can be reliably established by direct subcutaneous injection with fine-pitch needles, thereby overcoming the need for trauma and poor infectivity and reproducibility associated with patch vaccination. Bunce et al., Infect. Immun. 60:2636-2640, 1992; Kraft et al., Infect. Immun. 52:707-713, 1986; Nizet et al., Nature 414:454-457, 2001.
[0319] Luminescently labeled S. pyogenes and S. aureus and E. coli strains, each constitutively expressing the bacterial luciferase gene operon from Achromobacter, can be used. Kuklin et al., Antimicrob Agent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com