Application of crizotinib in preparation of medicines capable of resisting gram-positive bacteria
An anti-Gram-positive, Gram-positive technology, applied in the field of biomedicine, can solve the problem of no crizotinib to inhibit bacteria, etc., and achieve the effect of reliable pharmacological safety, growth inhibition and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
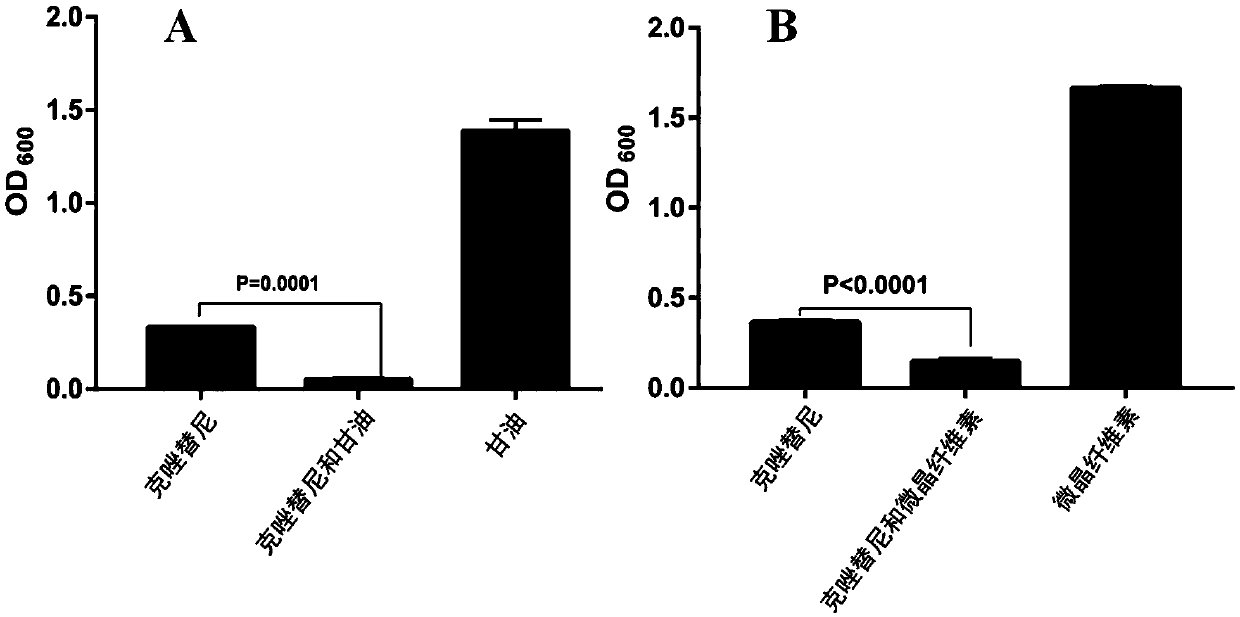
[0039] The antibacterial spectrum research of embodiment 1 crizotinib
[0040] This example studies the antibacterial spectrum of crizotinib.
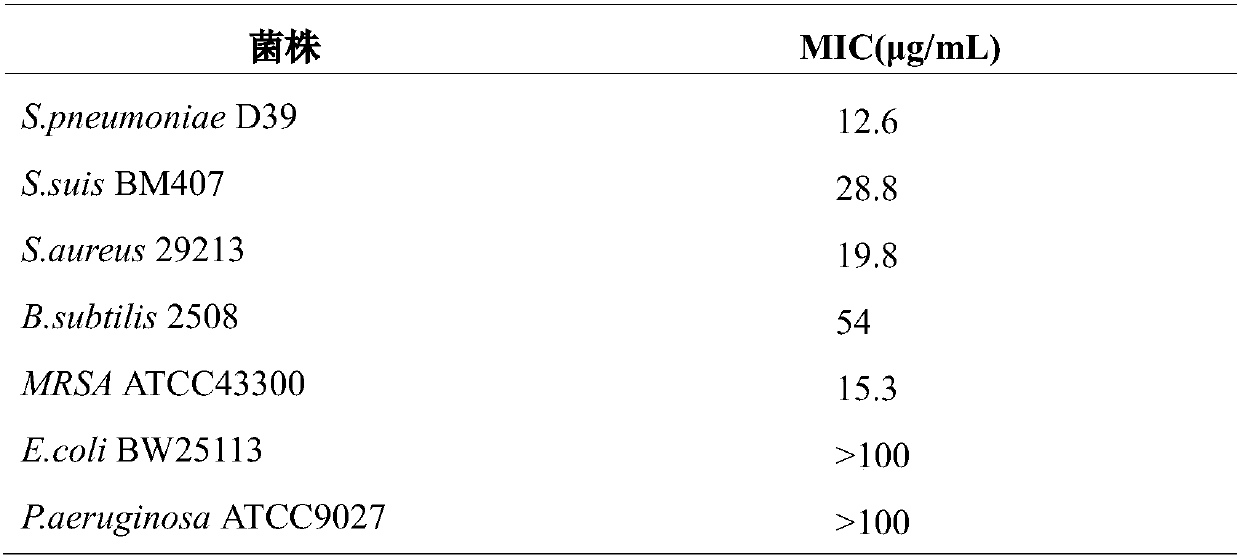
[0041]Tested strains: Streptococcus pneumoniae (S.pneumoniae D39) was purchased from China Type Culture Collection; Streptococcus suis (S.suis BM407), Staphylococcus aureus (S.aureus 29213), MRSA (S.aureus ATCC43300) Bacillus subtilis (B.subtilis 2508) was purchased from the Microbial Culture Collection Center of China; Bacillus subtilis (B.subtilis 2508) was purchased from the Guangdong Provincial Microbial Culture Collection Center; The bacterium (P.aeruginosa ATCC9027) was obtained from the American Type Culture Collection, and the minimum inhibitory concentration (MIC) of the model was determined (three single clones of each strain).
[0042] 1. Experimental steps
[0043] Streptococcus pneumoniae was inoculated into 0.5% THYE liquid medium for overnight activation according to the inoculum amount of 5%, and then transferred to f...
Embodiment 2
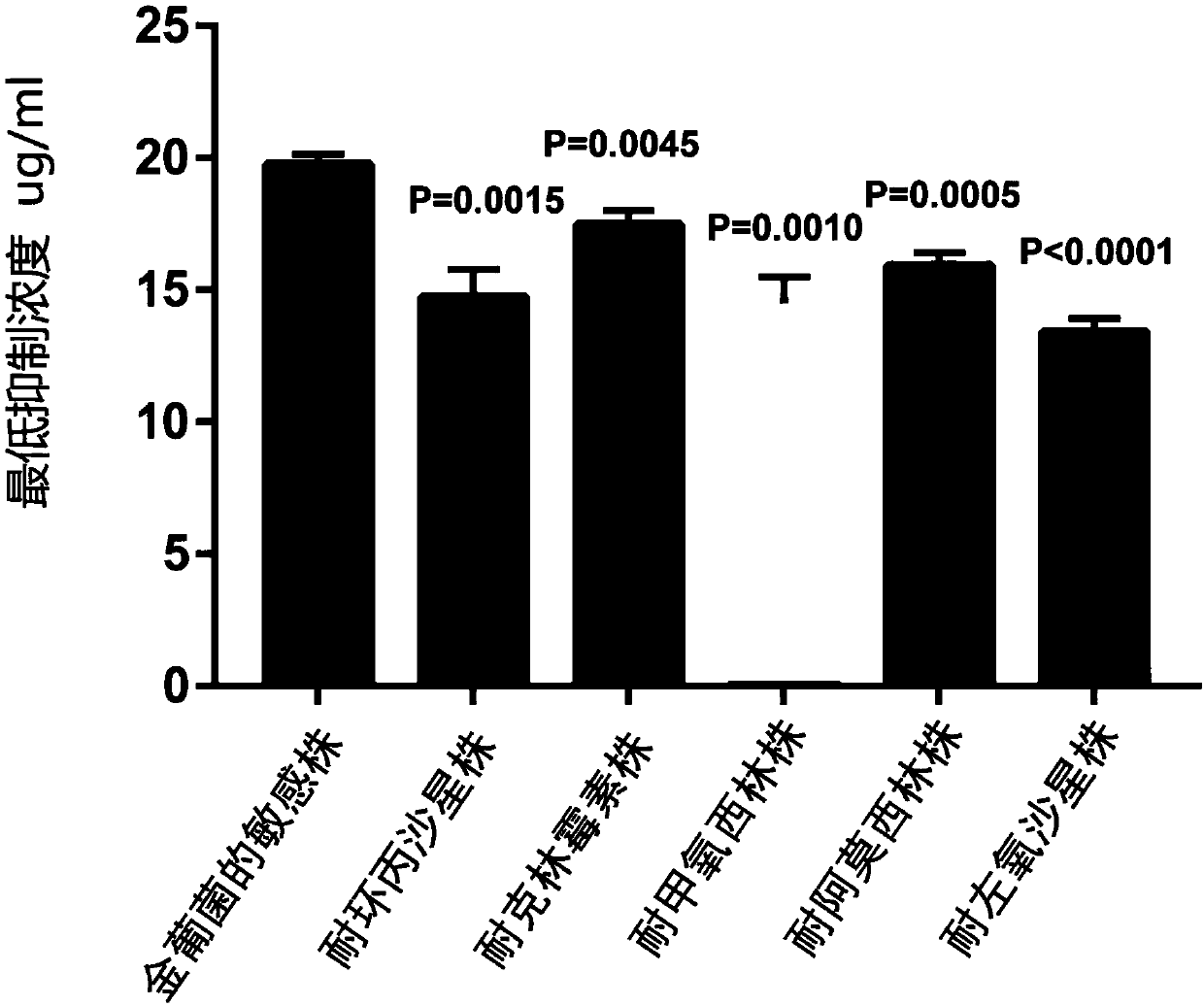
[0051] Embodiment 2 crizotinib is measured to Staphylococcus aureus minimum inhibitory concentration (MIC)
[0052] The tested strains of this embodiment: Staphylococcus aureus methicillin-resistant strain MRSA (ATCC43300) and the sensitive strain of Staphylococcus aureus (29213) are from the General Microbiology Center of China Committee for Culture Collection of Microorganisms; Staphylococcus aureus single Nike Linmycin strain (29213-K), Staphylococcus aureus single-resistant gentamicin strain (29213-G), Staphylococcus aureus single-resistant ciprofloxacin strain (29213-C), Staphylococcus aureus single-resistant Amoxicillin (29213-A), Staphylococcus aureus single-resistant levofloxacin strain (29213-L) is domesticated from a sensitive strain of Staphylococcus aureus (29213).
[0053] Among them, Staphylococcus aureus single-resistant clindamycin strain (29213-K), Staphylococcus aureus single-resistant gentamycin strain (29213-G), Staphylococcus aureus single-resistant ciprof...
Embodiment 3
[0063] The minimum inhibitory concentration (MIC) determination of embodiment 3 crizotinib to clinically isolated multidrug-resistant Staphylococcus aureus, Enterococcus faecalis, Staphylococcus hemolyticus
[0064] The tested bacterial strain of this embodiment is clinically isolated multi-drug resistant Staphylococcus aureus (168272, 168023, 166471, 166534, 166138, 168293, 900624, 168205, 179634, 179148, 179475, 179459, 178425, 178524, 178360) , Enterococcus faecalis (179521), and Staphylococcus hemolyticus (179595), from the Laboratory Department of Southern Medical University. The above numbers are the system numbers of the corresponding strains in the Laboratory Department of Southern Medical University. The drug resistance of related strains is shown in Table 3.
[0065] Table 3 Drug resistance of related strains (MIC unit: μg / mL)
[0066]
[0067]
[0068] 1. Experimental steps
[0069] The clinically isolated multi-drug-resistant Staphylococcus aureus, Enterococ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Minimum inhibitory concentration | aaaaa | aaaaa |
| Minimum inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com