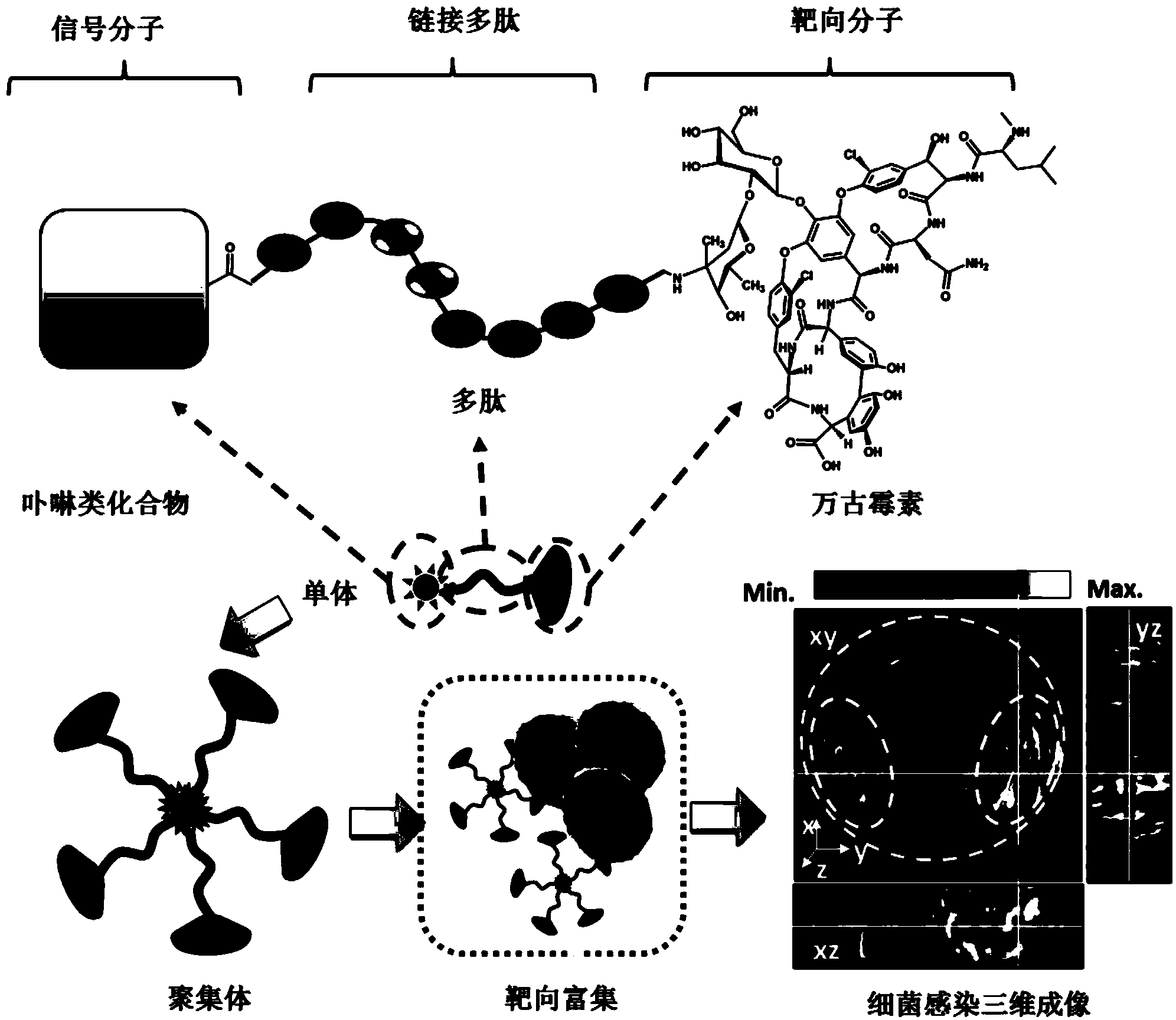
Targeting opto-acoustic contrast agent as well as preparation method and application thereof
A photoacoustic contrast, targeting technology, applied in the field of imaging diagnostic drugs, can solve the problem of non-specific targeting, and achieve the effect of good targeting specificity and broad clinical application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
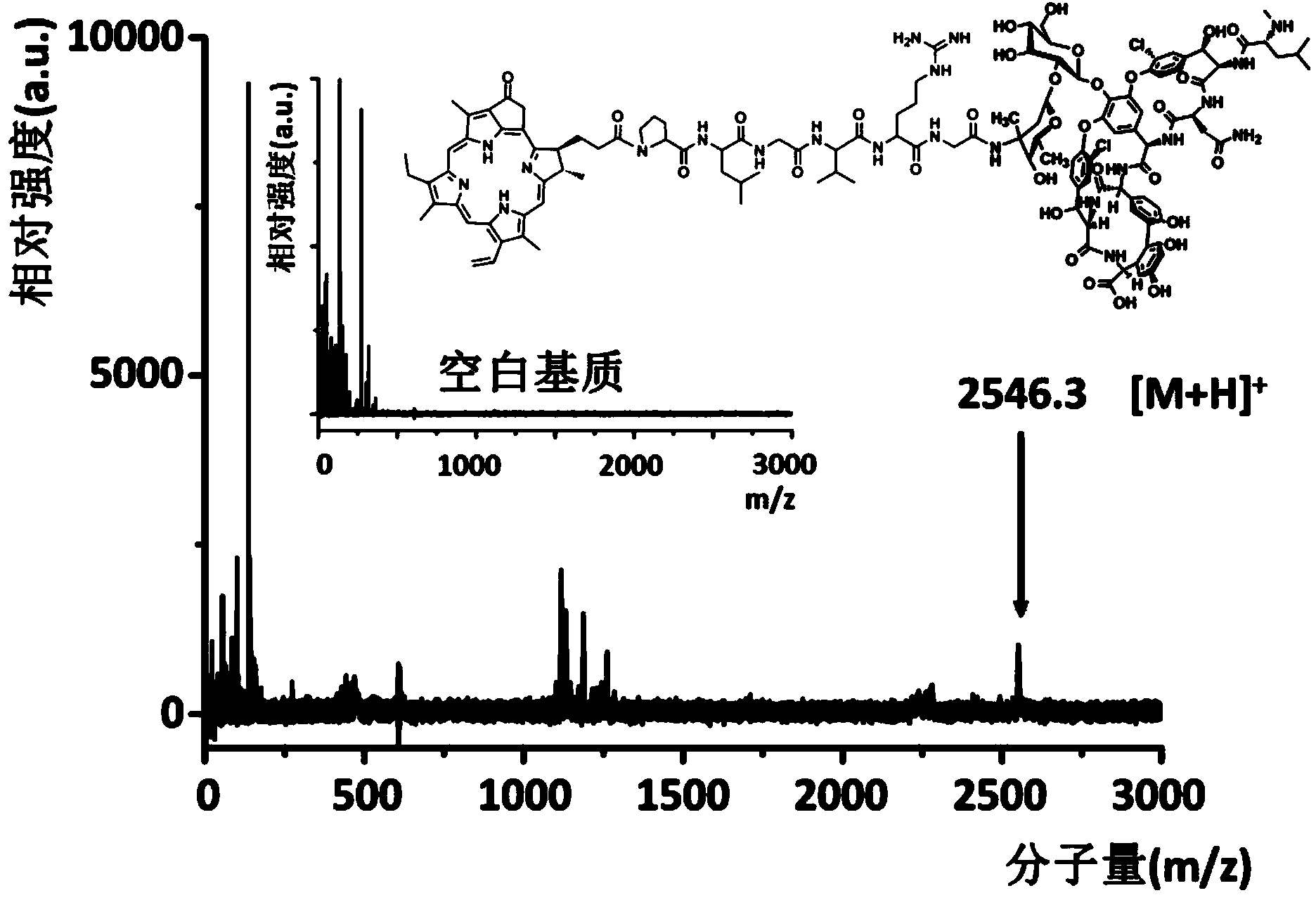
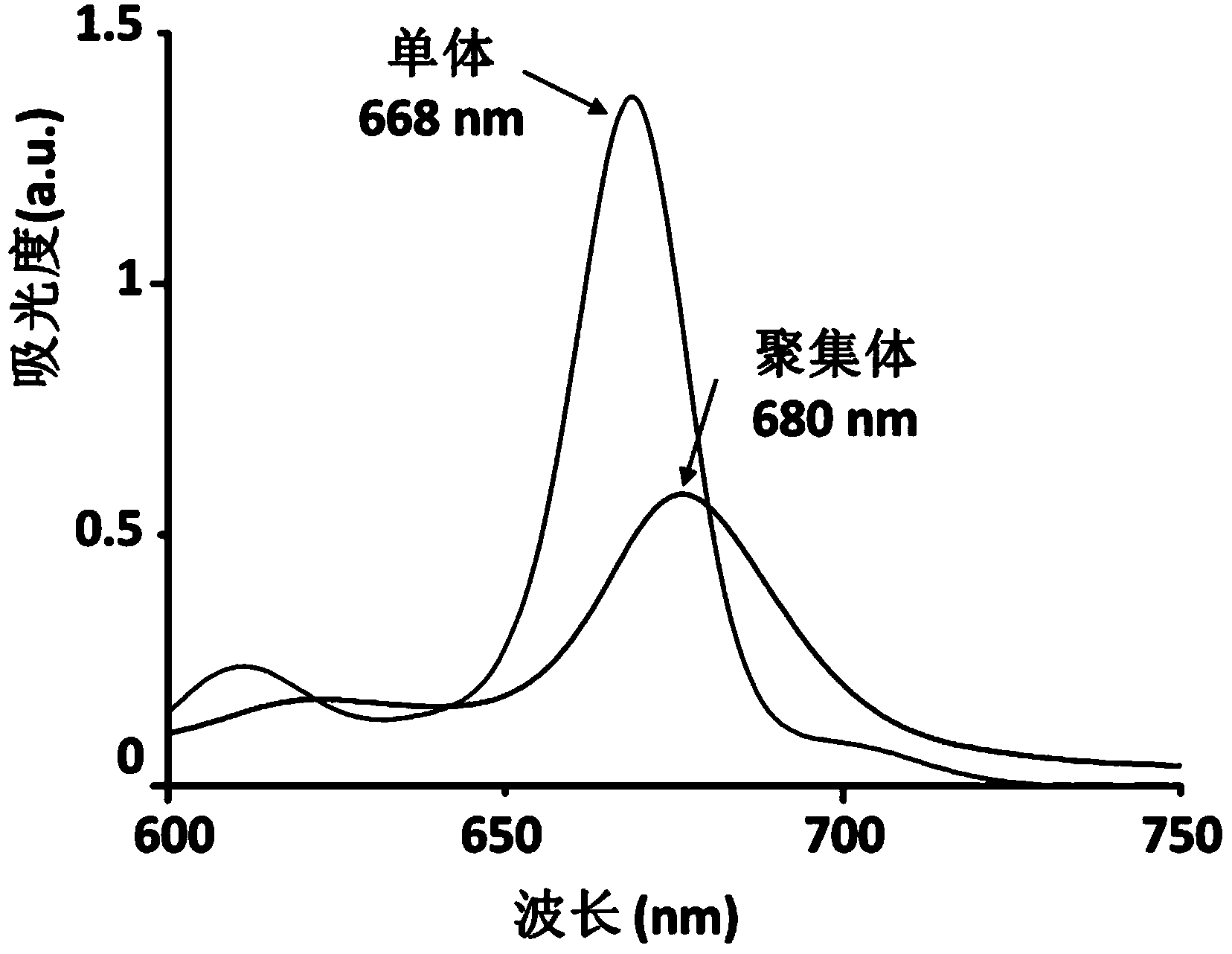
[0061] Example 1 Preparation of targeted photoacoustic contrast agent of the present invention
[0062] Using pyropheophytin a as the photoacoustic signal molecule and the polypeptide with the sequence Pro-Lys-Gly-Val-Arg-Gly as the connecting polypeptide, the synthesis of the targeted photoacoustic contrast agent of the present invention is carried out according to the following steps:
[0063] (1) Synthesis of Linked Peptides
[0064] Synthesis of linked peptides by Fmoc solid-phase synthesis:
[0065] A Wang resin with a modification density of 0.35 mM was selected for synthesis, in which the N-terminal of the first amino acid (glycine) was protected by Fmoc, and the C-terminal was fixed on the resin. Use 20% (v / v) hexahydropyridine in DMF to remove the Fmoc protection at the N-terminal, and then use the ninhydrin test to test the deprotection result. Then the carboxyl group of the next amino acid was treated with 0.4M 4-methylmorpholine (NMM) and 0.4M benzotriazole-N,N,N...
Embodiment 2
[0074] Example 2 Specific bacterial targeting of the photoacoustic contrast agent of the present invention
[0075] Select Staphylococcus aureus and Staphylococcus epidermidis as model strains of Gram-positive bacteria, Pseudomonas aeruginosa, Serratia, Escherichia coli and Proteus as model strains of Gram-negative bacteria, and the concentration was 1×10 8 CFU bacterial culture solution and 40 μM of the photoacoustic contrast agent of the present invention prepared in Example 1 were co-incubated for 30 minutes in equal volume, and the contrast agent without targeted adsorption was washed with PBS, and the isolated bacterial cells were sampled and observed by laser confocal microscope Specific targeted enrichment of Gram-positive bacteria by photoacoustic contrast agents.
[0076] The result is as Image 6 as shown, Image 6 The results show that the photoacoustic contrast agent of the invention has a specific targeted enrichment effect on Staphylococcus aureus and Staphyloc...
Embodiment 3
[0077] Example 3 Imaging of bacterial infection in vivo by photoacoustic contrast agent of the present invention
[0078] Select 6-8 week old BALB / c mice and inject 1×10 8 CFU Staphylococcus aureus culture solution for 48 hours to form a mouse hind leg myositis model. Inject 200 μL of a photoacoustic contrast agent with a concentration of 200 μM through the tail vein, and select time points of 1, 4, 8, 12 and 24 hours to observe the imaging of myositis infection at different time points, and construct a 3D model of muscle infection in mice. The result is as Figure 7 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com