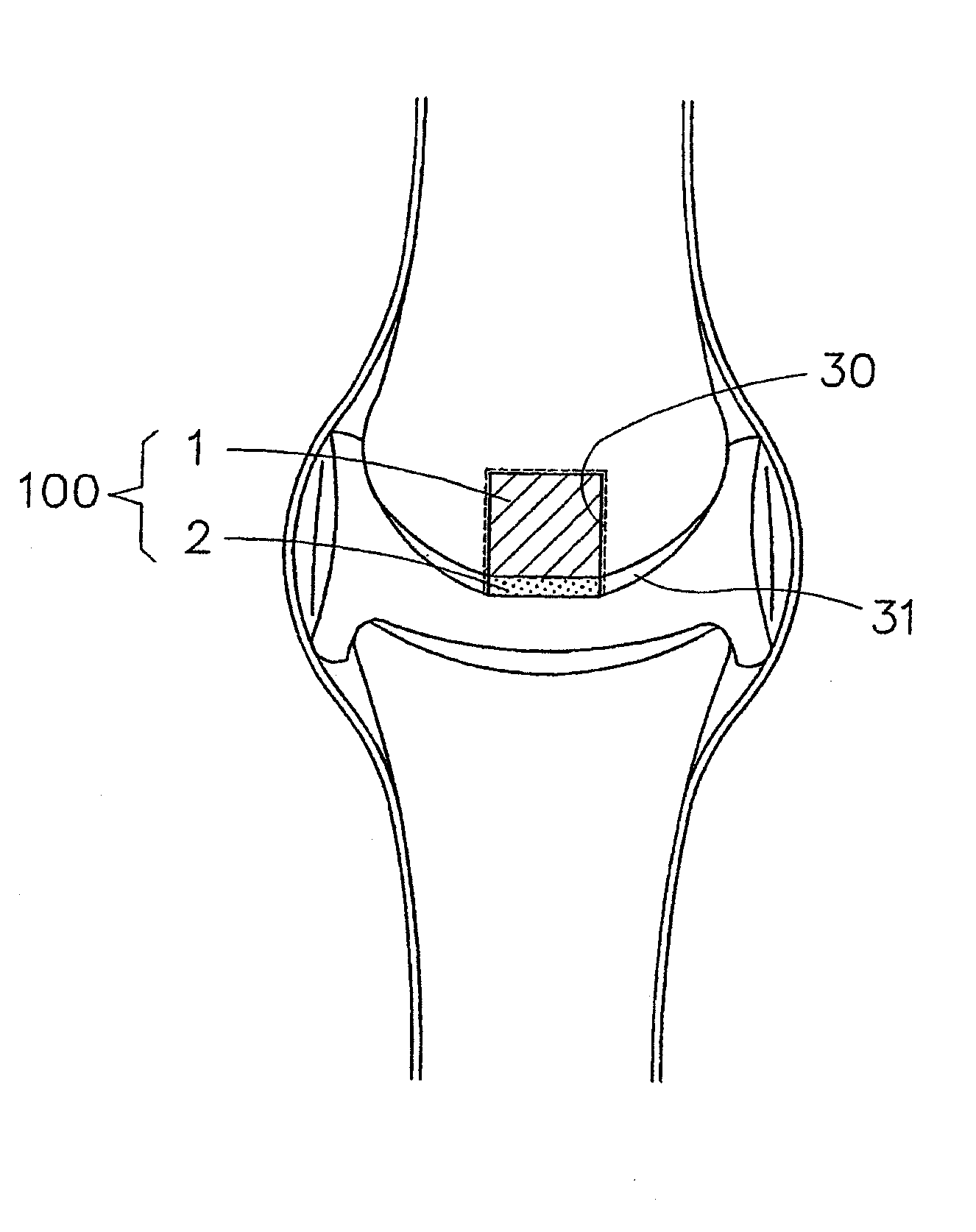
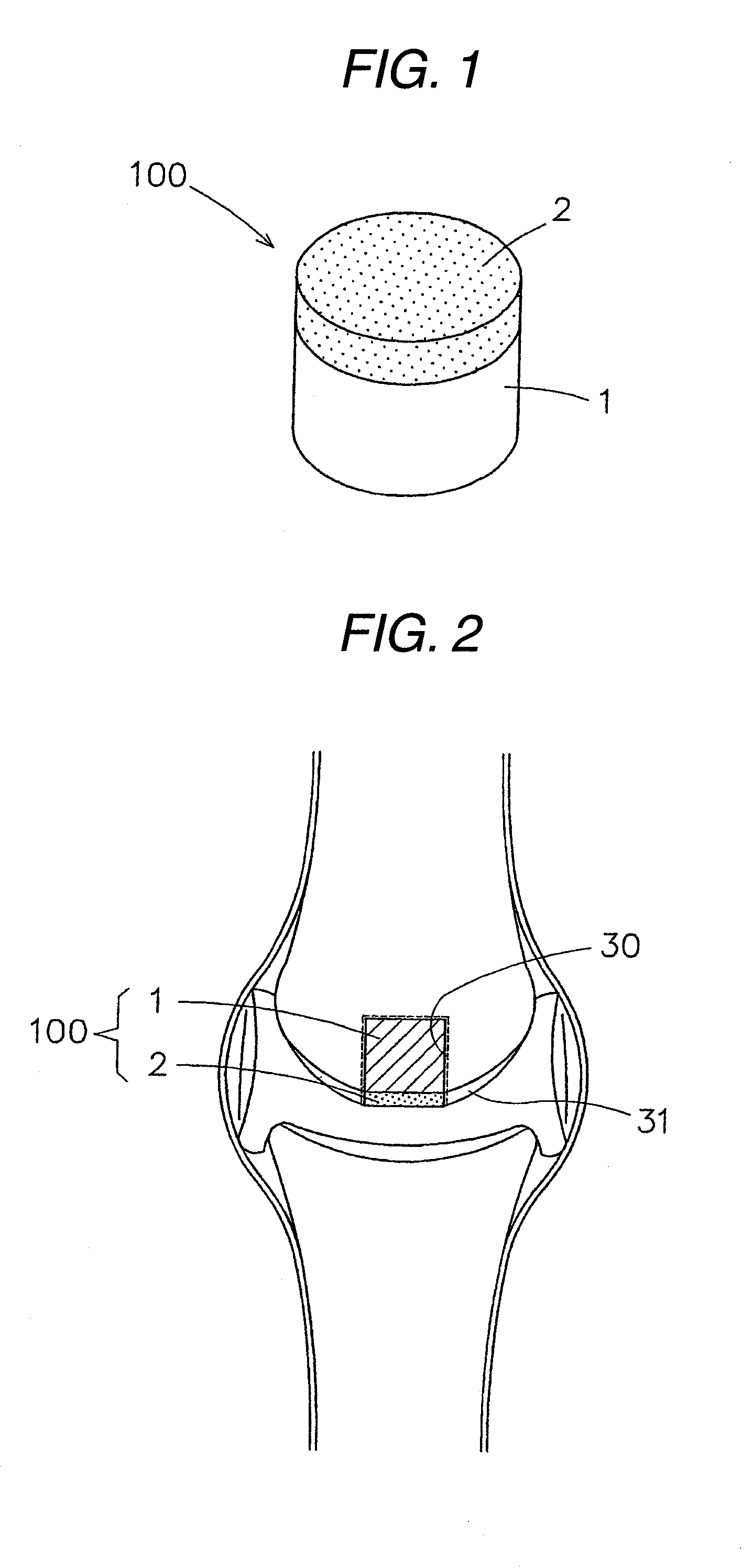
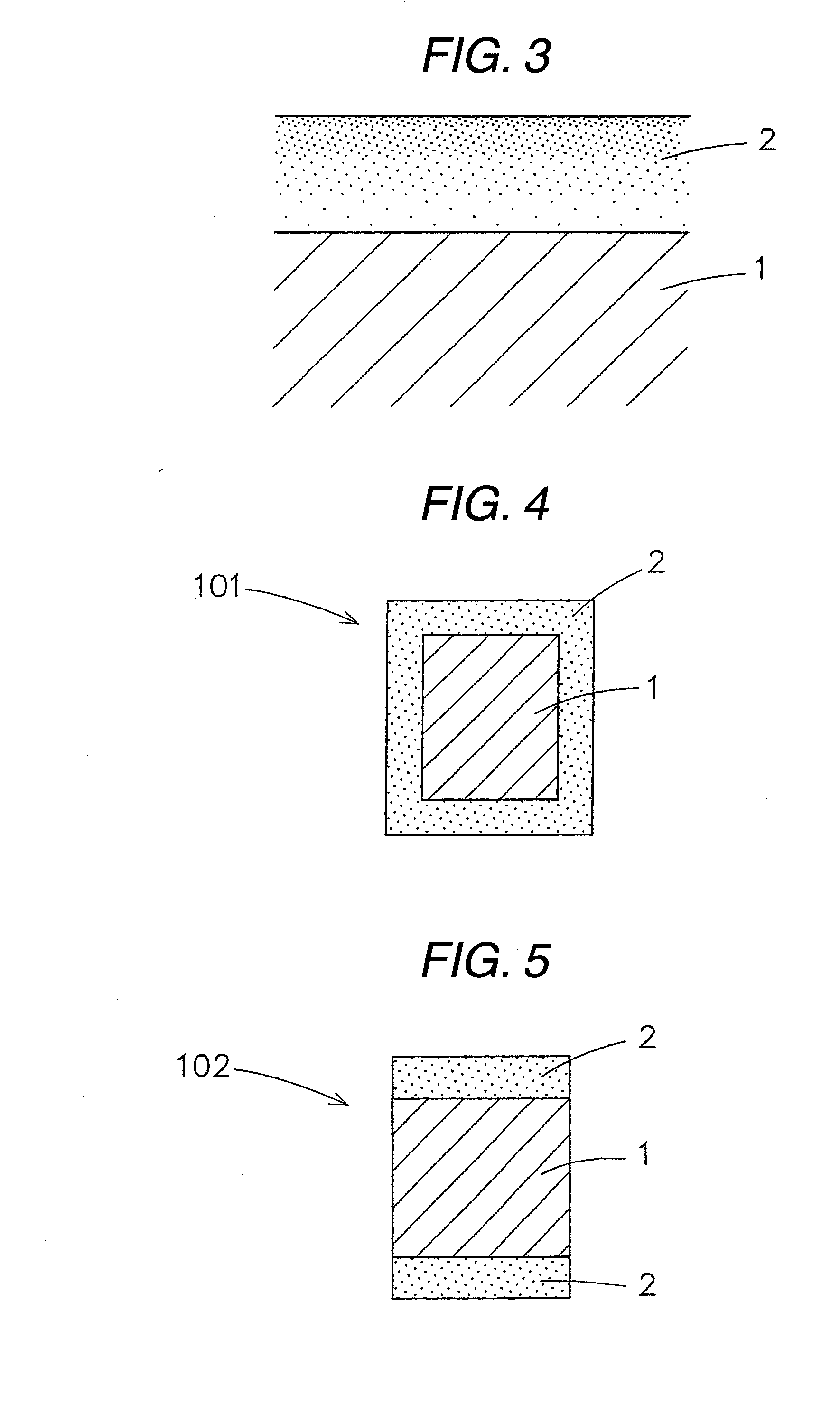
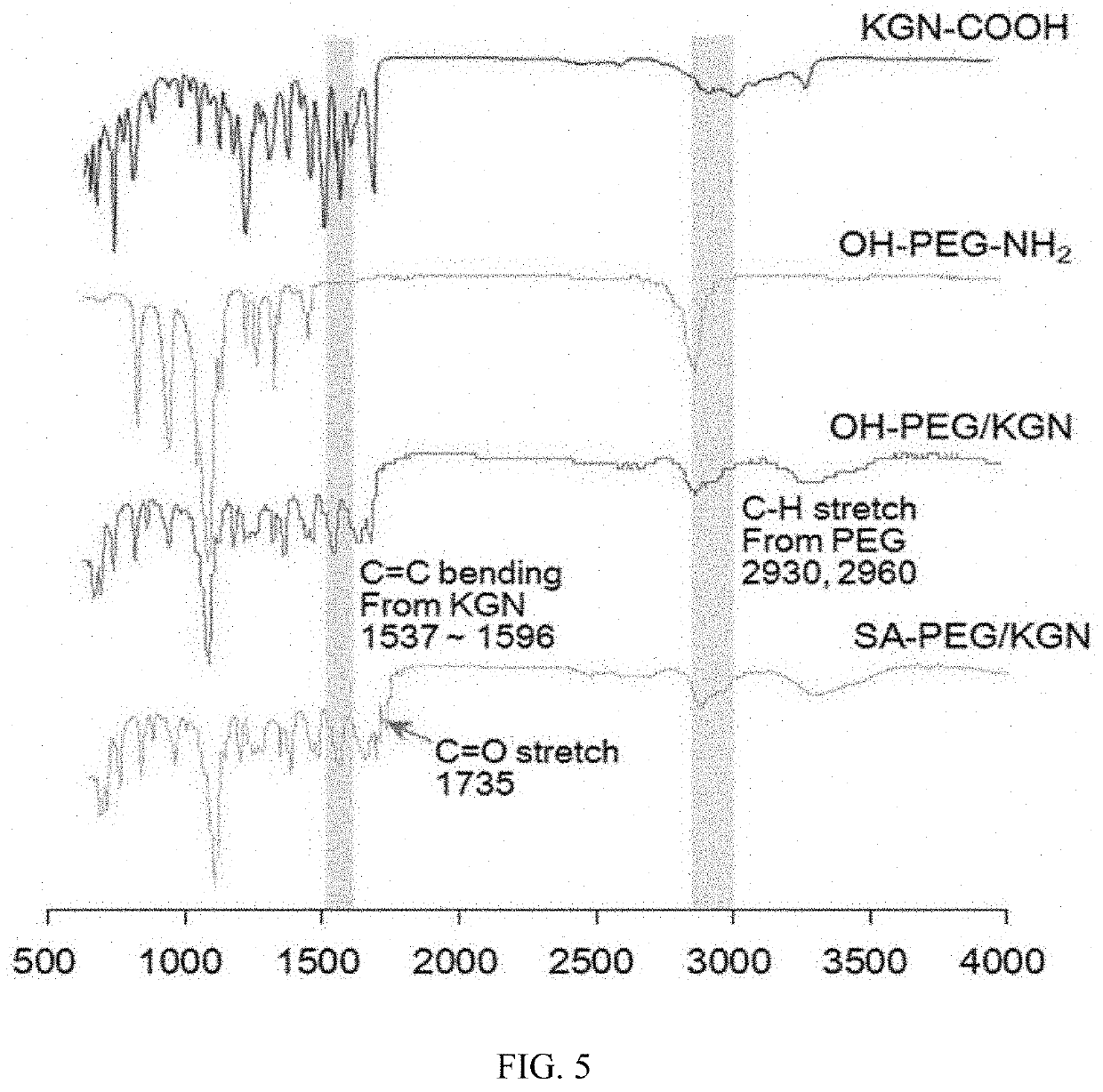
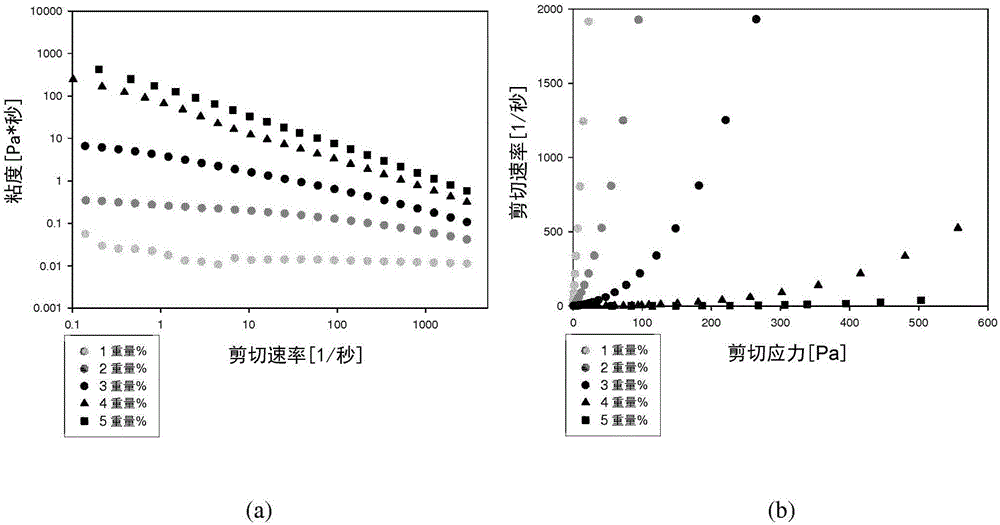
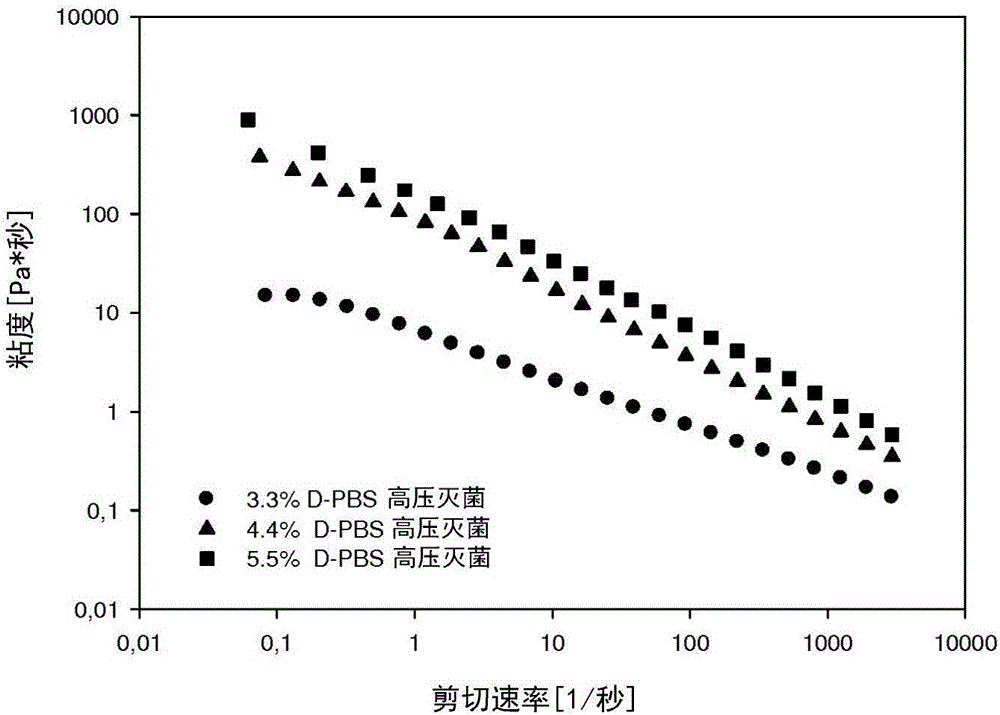
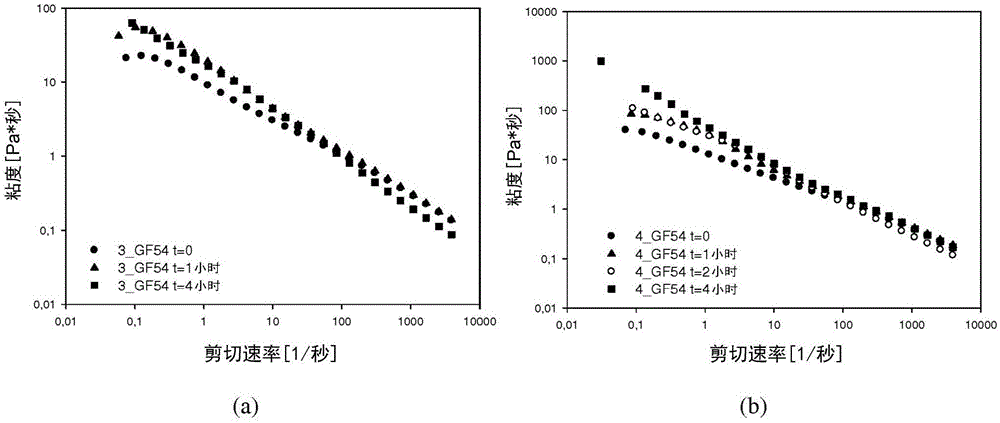
Patents
Literature
84 results about "Cartilage disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Condition in which there is a deviation from or interruption of the normal structure or function of cartilage, the non-vascular form of connective tissue composed of chondrocytes embedded in a matrix of type II collagen and chondroitin sulfate.
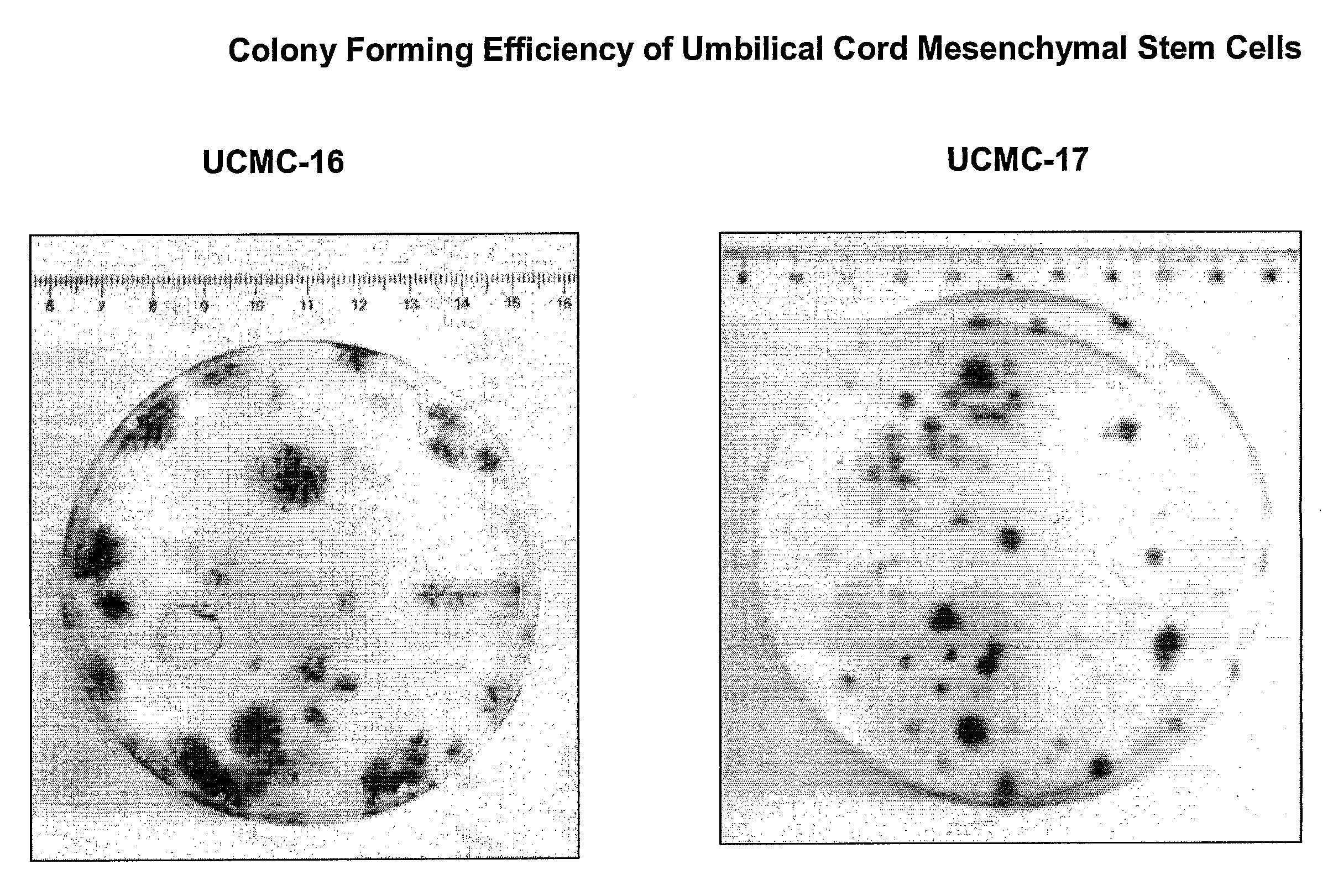
Isolation and Cultivation of Stem/Progenitor Cells From the Amniotic Membrane of Umbilical Cord and Uses of Cells Differentiated Therefrom
The present invention relates to a skin equivalent and a method for producing the same, wherein the skin equivalent comprises a scaffold and stem / progenitor cells isolated from the amniotic membrane of umbilical cord. These stem / progenitor cells may be mesenchymal (UCMC) and / or epithelial (UCEC) stem cells, which may then be further differentiated to fibroblast and keratinocytes. Further described is a method for isolating stem / progenitor cells from the amniotic membrane of umbilical cord, wherein the method comprises separating the amniotic membrane from the other components of the umbilical cord in vitro, culturing the amniotic membrane tissue under conditions allowing cell proliferation, and isolating the stem / progenitor cells from the tissue cultures. The invention also refers to therapeutic uses of these skin equivalents. Another aspect of the invention relates to the generation of a mucin-producing cell using stem / progenitor cells obtained from the amniotic membrane of umbilical cord and therapeutic uses of such mucin-producing cells. In yet another aspect, the invention relates to a method for generating an insulin-producing cell using stem / progenitor cells isolated from the amniotic membrane of umbilical cord and therapeutic uses thereof. The invention further refers to a method of treating a bone or cartilage disorder using UCMC. Furthermore, the invention refers to a method of generating a dopamin and tyrosin hydroxylase as well as a HLA-G and hepatocytes using UCMC and / or UCEC. The present invention also refers to a method of inducing proliferation of aged keratinocytes using UCMC.
Owner:CELLRESEARCH CORP PTE LTD
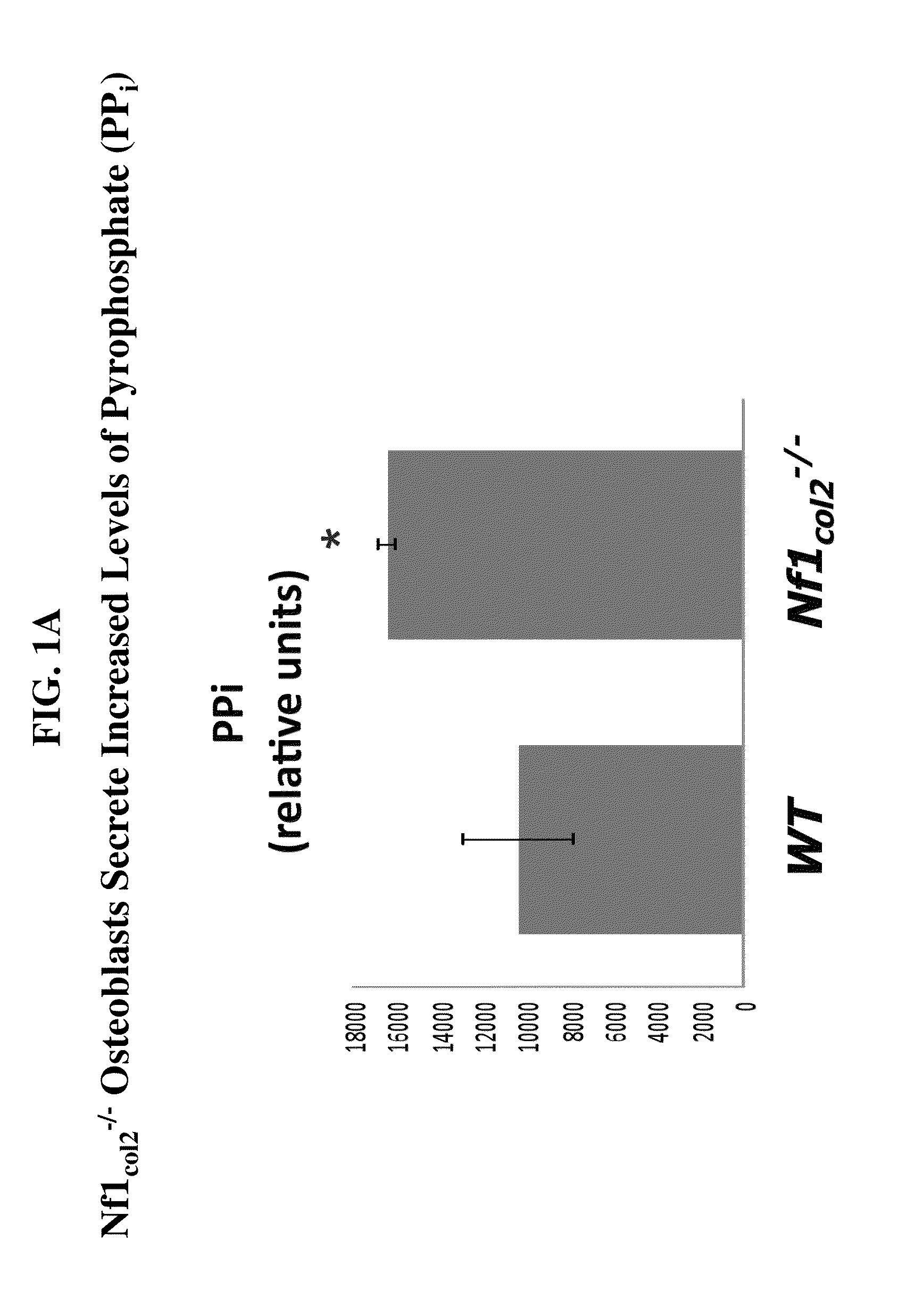
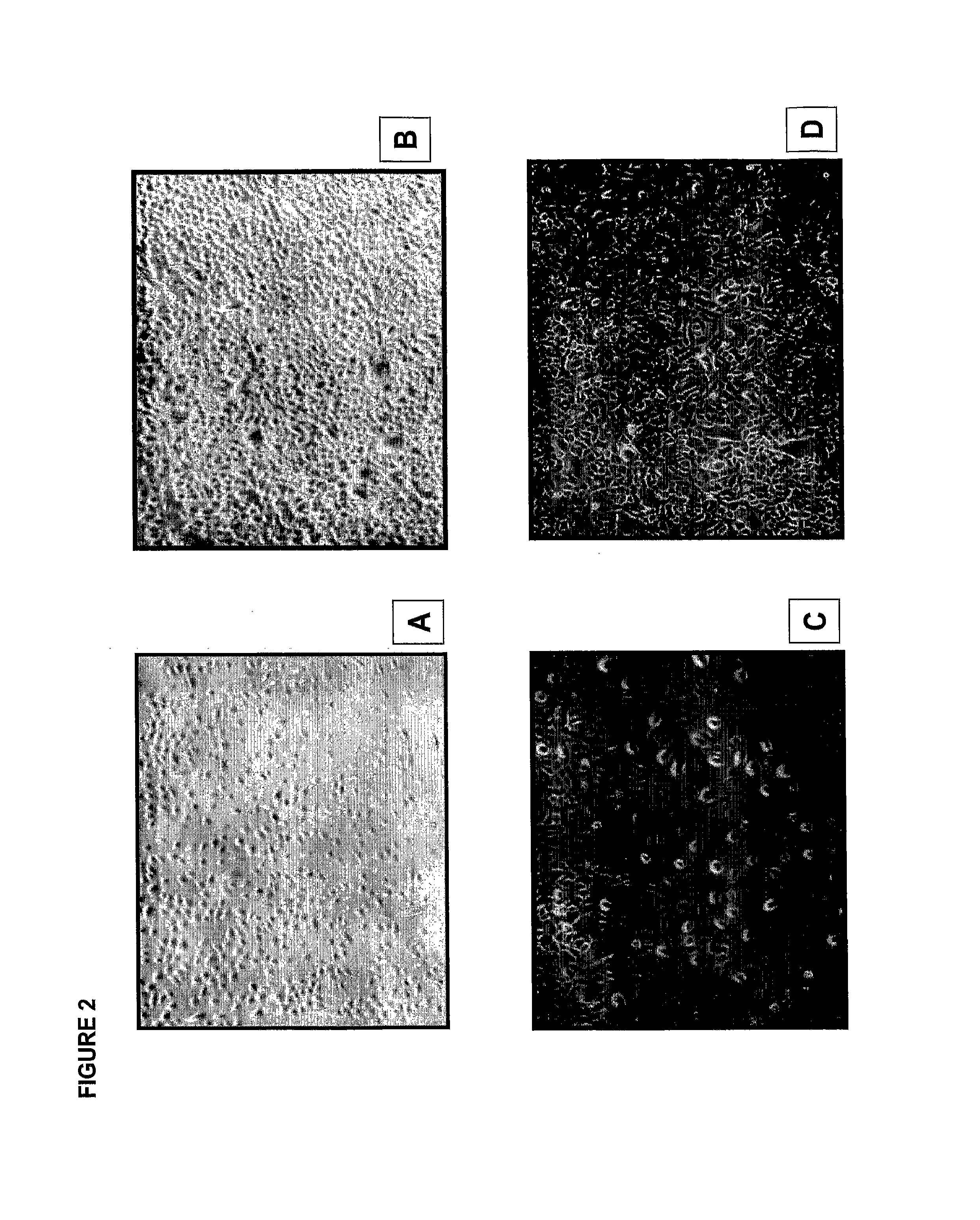
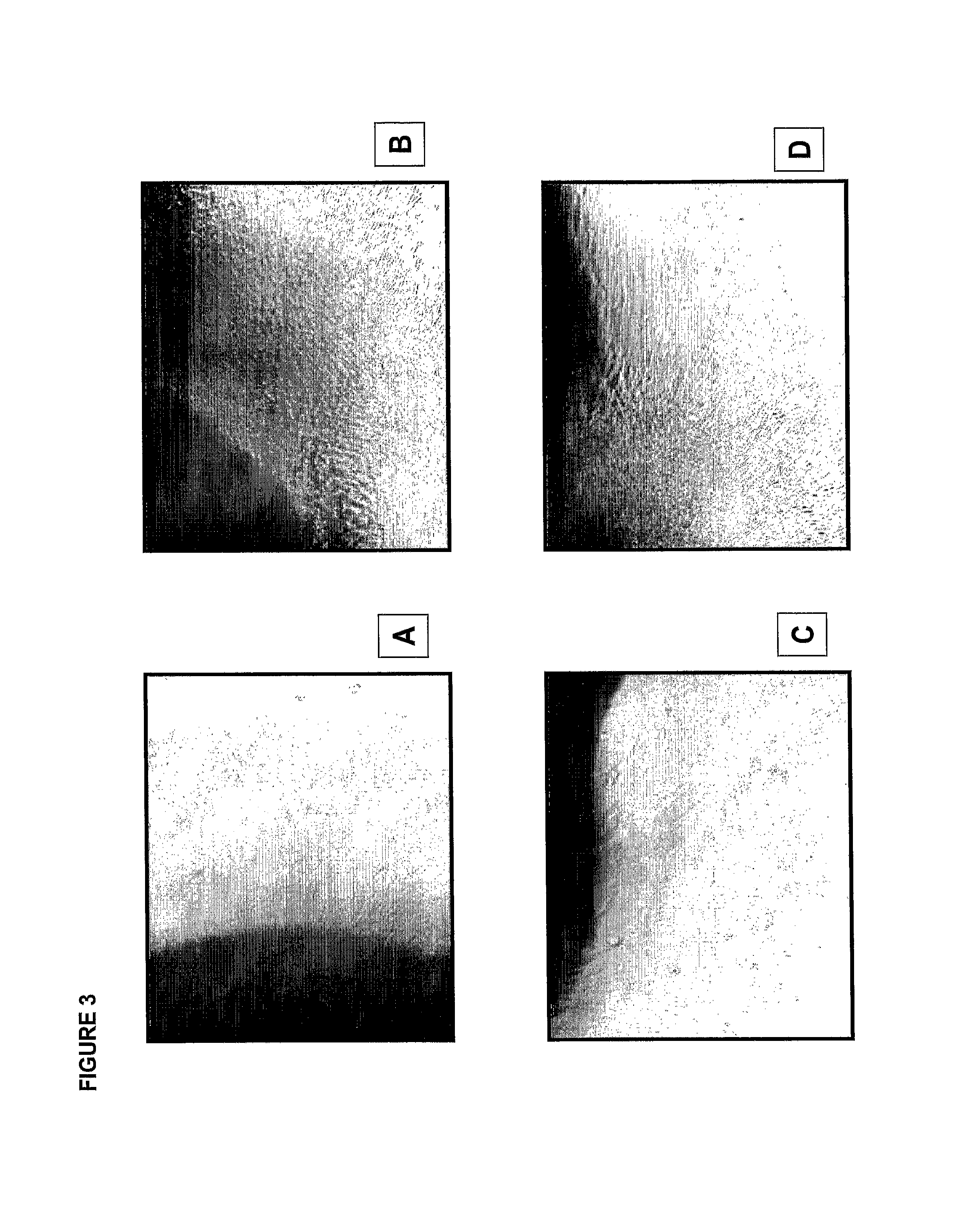
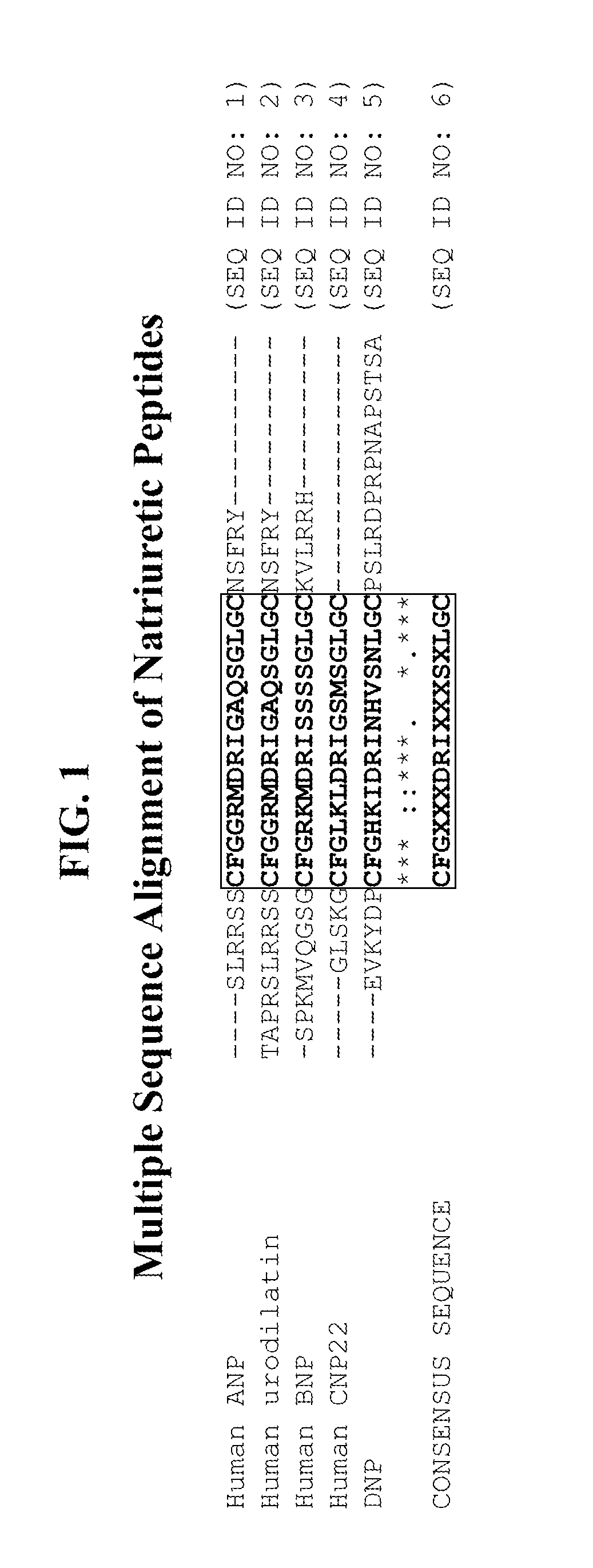
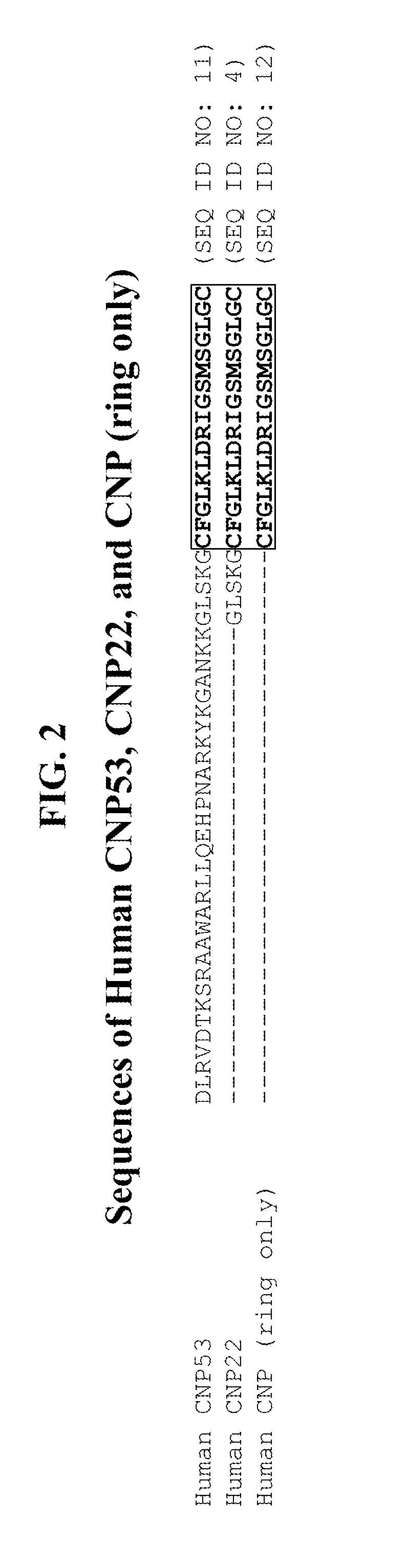
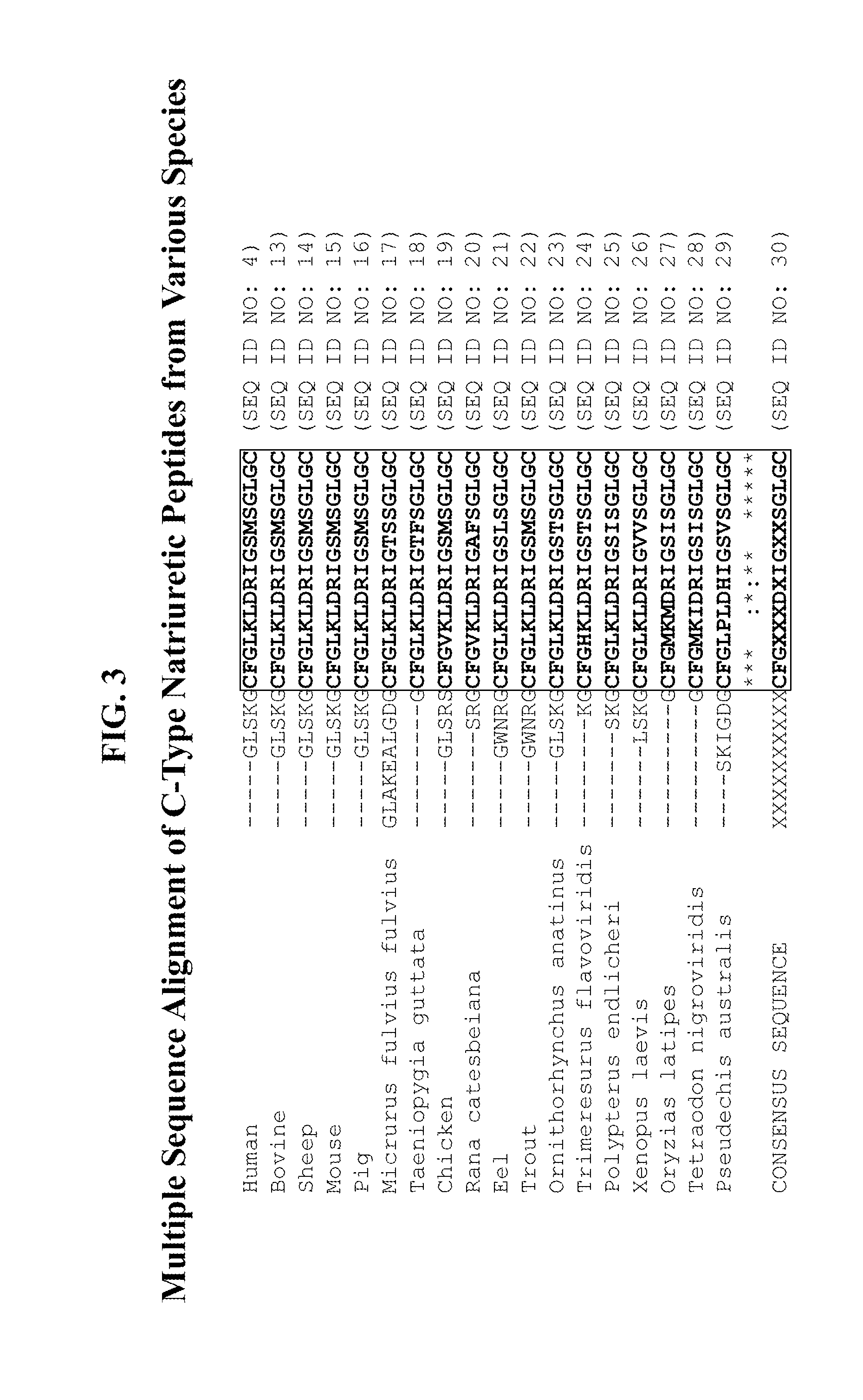
Compositions comprising alkaline phosphatase and/or natriuretic peptide and methods of use thereof
ActiveUS20130323244A1Reduced dose-dependent side effectPeptide/protein ingredientsHydrolasesDiseaseNeurofibromatosis type I
The present invention provides methods, compositions, and kits for the treatment of neurocutaneous syndromes, such as neurofibromatosis type I; disorders associated with overactivation of FGFR3, such as achondroplasia; bone or cartilage disorders; or vascular smooth muscle disorders; or for the elongation of bone. In some embodiments, the present invention provides polypeptides having an alkaline phosphatase peptide fused to an Fc domain of an immunoglobulin or a natriuretic peptide fused to an Fc domain of an immunoglobulin. Such polypeptides can be administered to subjects, e.g., subcutaneously, to treat a neurocutaneous syndrome, a disorder associated with overactivation of FGFR3, a bone or cartilage disorder, or a vascular smooth muscle disorder, or to elongate bone. The invention also features nucleic acid molecules encoding such polypeptides and the use of the nucleic acid molecules for treating neurocutaneous syndromes, disorders associated with overactivation of FGFR3, bone or cartilage disorders, or vascular smooth muscle disorders, or for elongating bone.
Owner:VANDERBILT UNIV +1
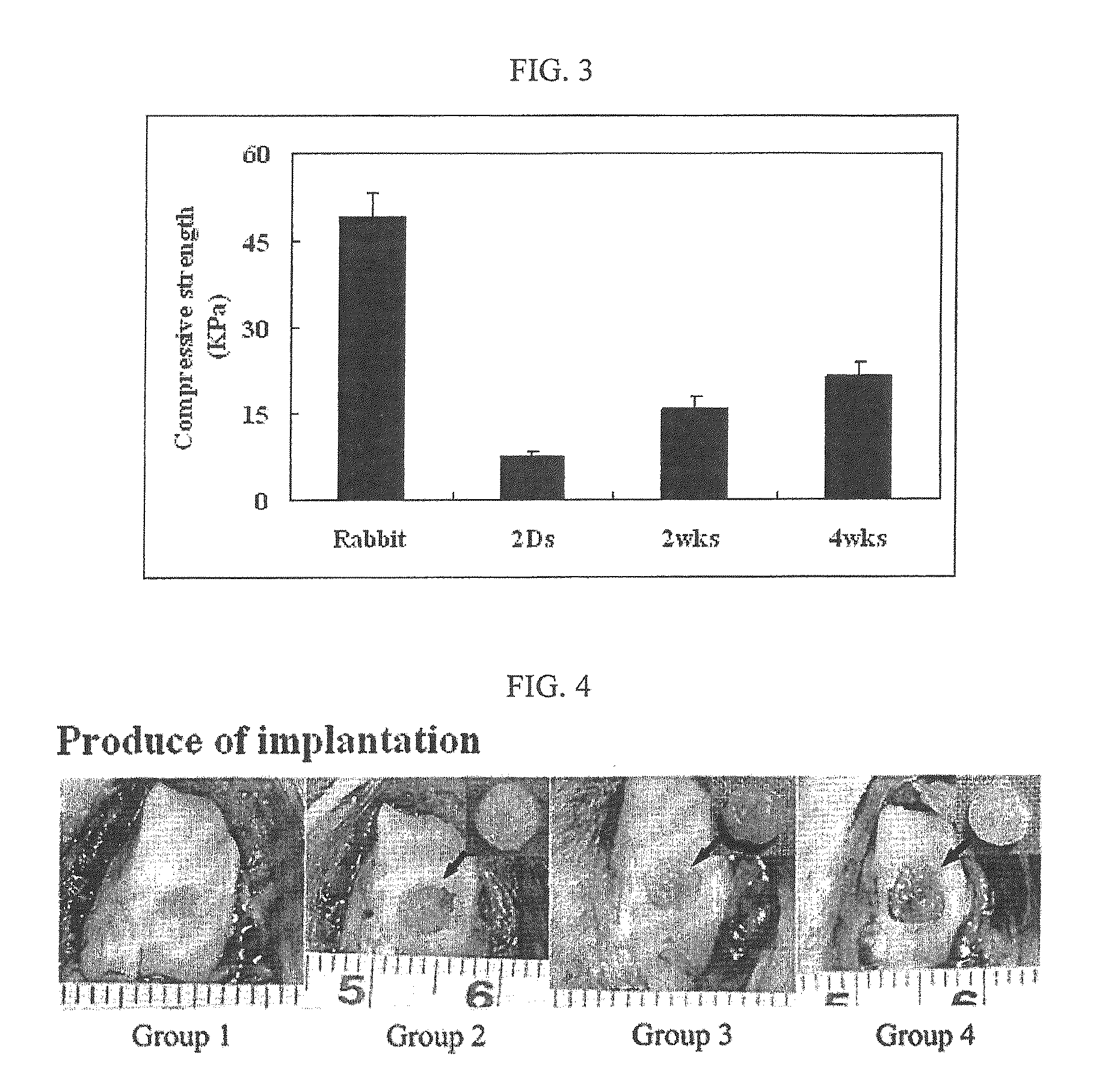
Therapeutic composite for cartilage disorder using extracellular matrix (ECM) scaffold
The present invention relates to a method for preparing a cell-derived ECM scaffold to which chondrocytes or stem cells are attached, a method for cartilage regeneration by tissue engineering, which comprises using the cell-derived ECM scaffold, and a therapeutic composition for treating cartilage disorder, which contains the ECM scaffold as an effective component. More specifically, the present invention relates to a method for cartilage regeneration by tissue engineering, which comprises transplanting ECM scaffold, having chondrocytes or stem cells attached thereto, into cartilage defects, and a therapeutic composition for treating cartilage disorder, which contains the ECM scaffold, having chondrocytes or stem cells attached thereto, as an effective component. According to the present invention, when the inventive ECM scaffold having chondrocytes or stem cells attached thereto is transplanted into a cartilage defect, mature articular cartilage having the same appearance and characteristics as those of natural cartilage tissue, can be regenerated without side effects such as inflammatory responses.
Owner:MIN BYOUNG HYUN
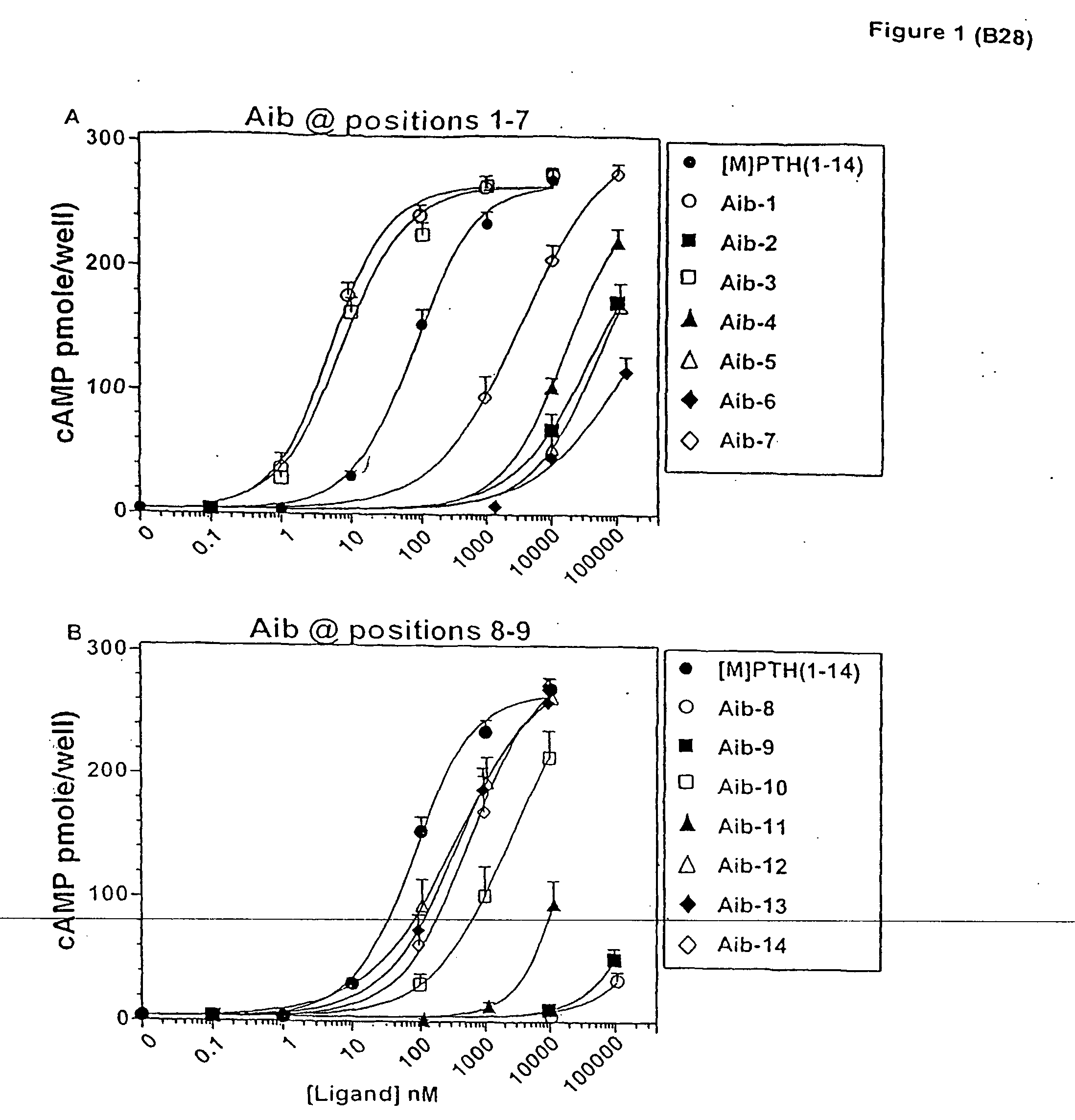
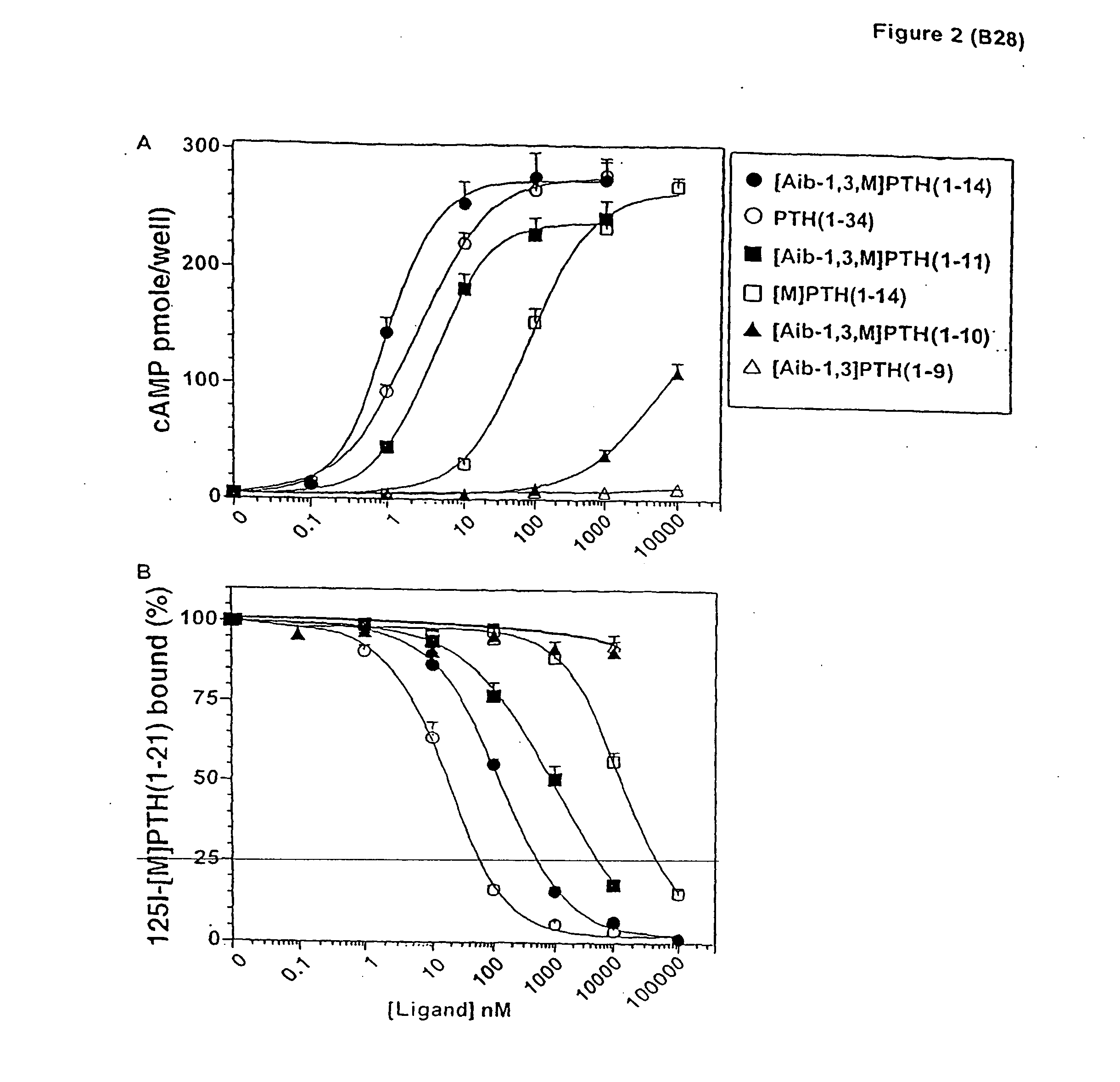
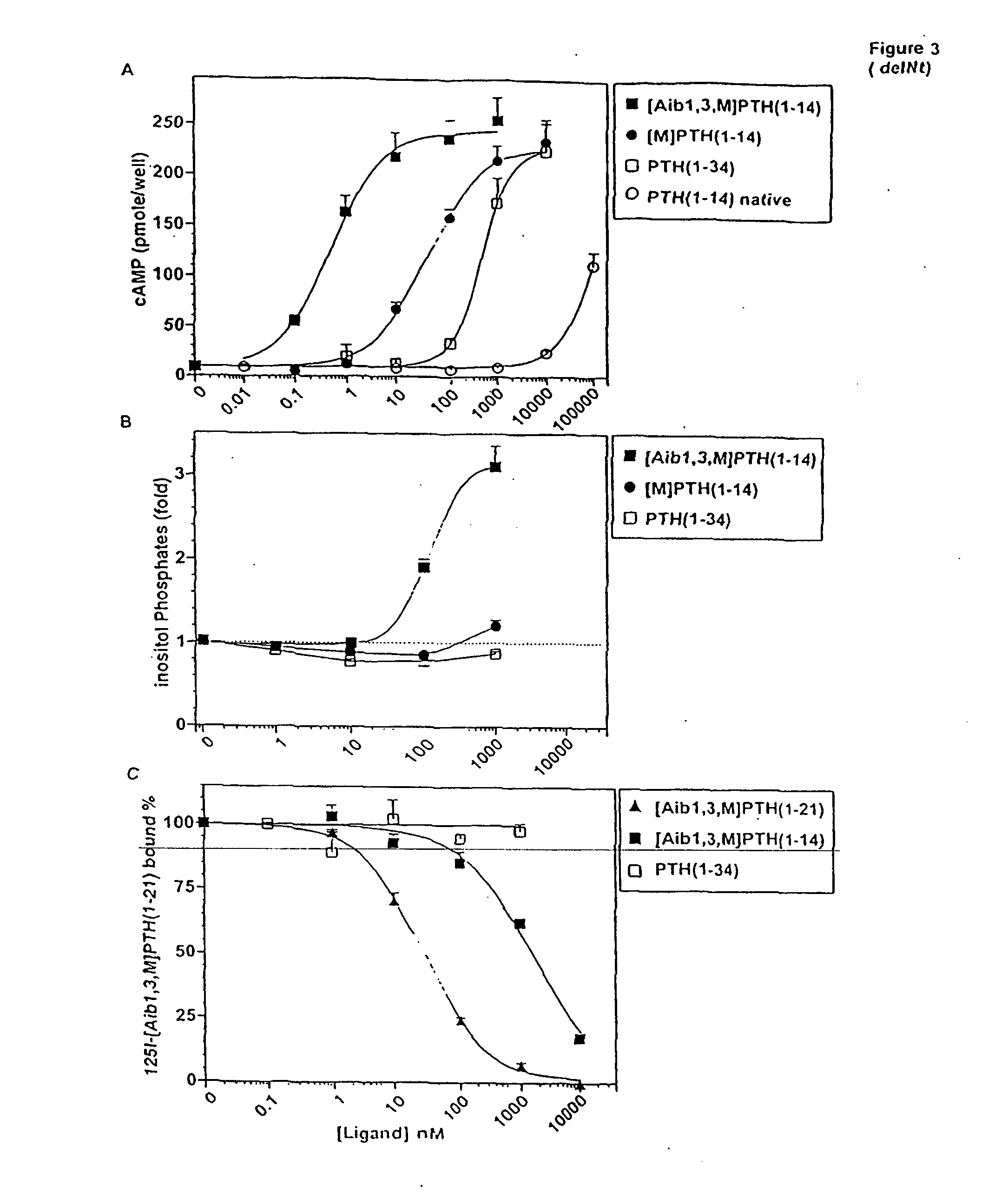
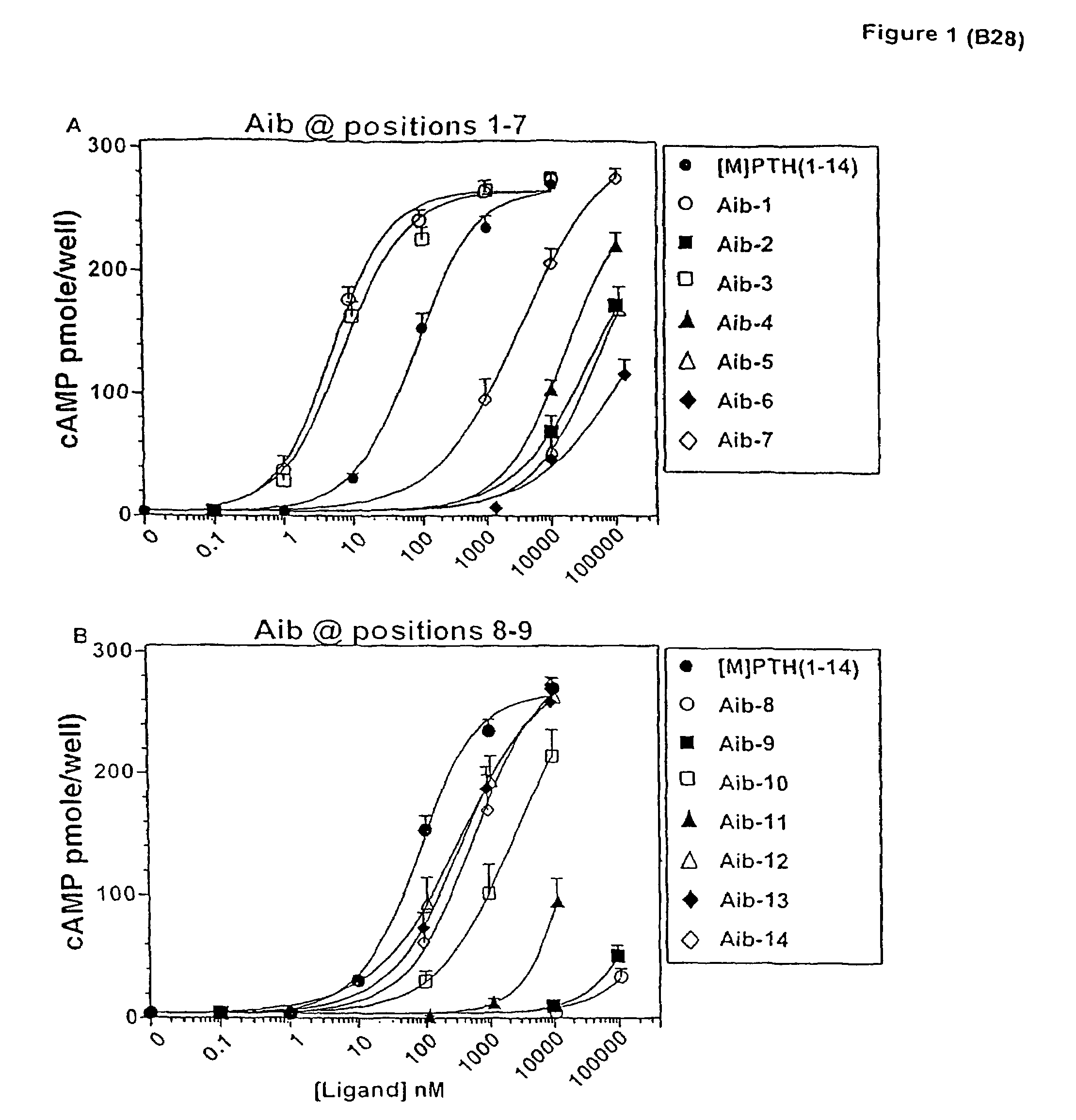
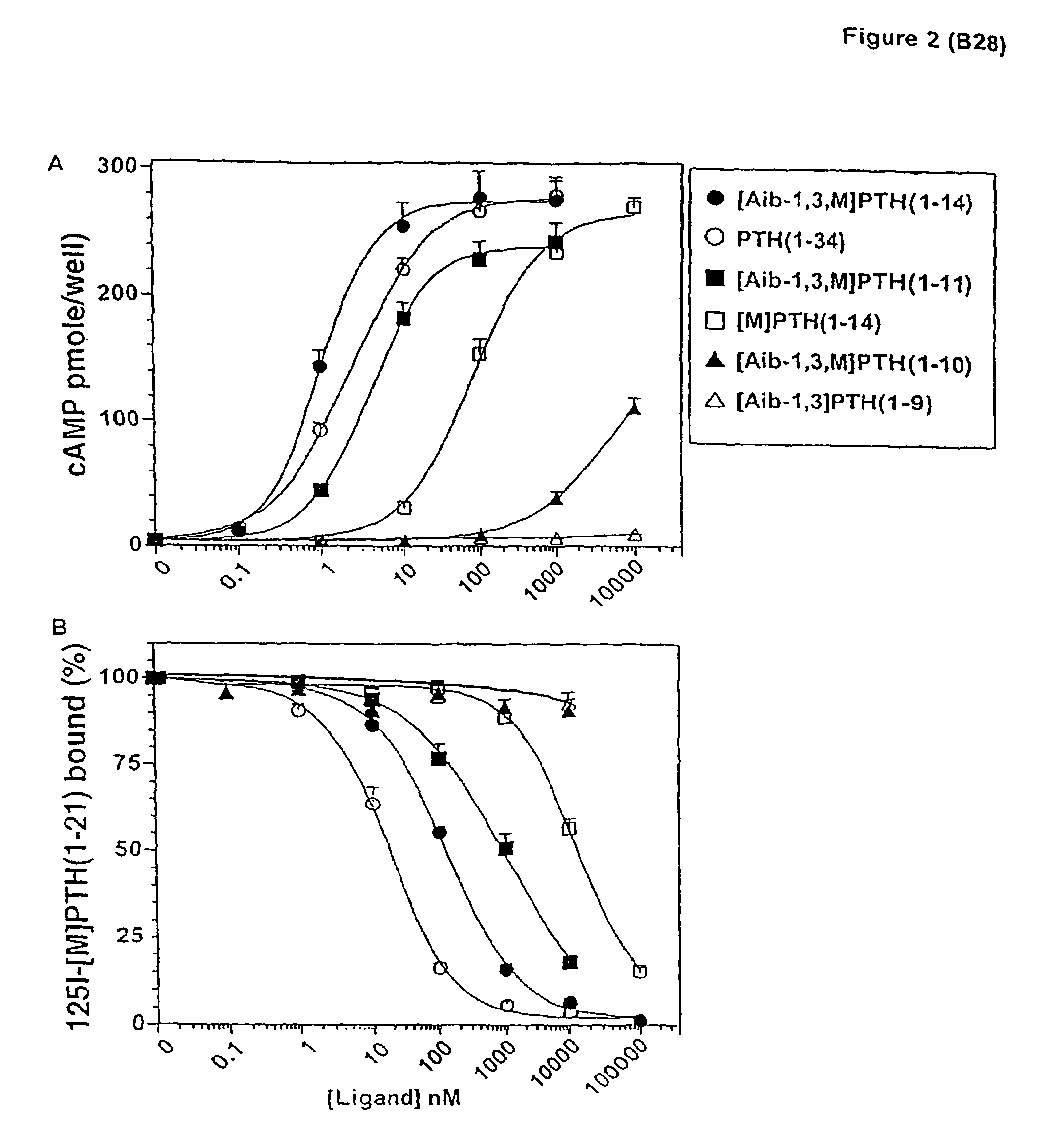
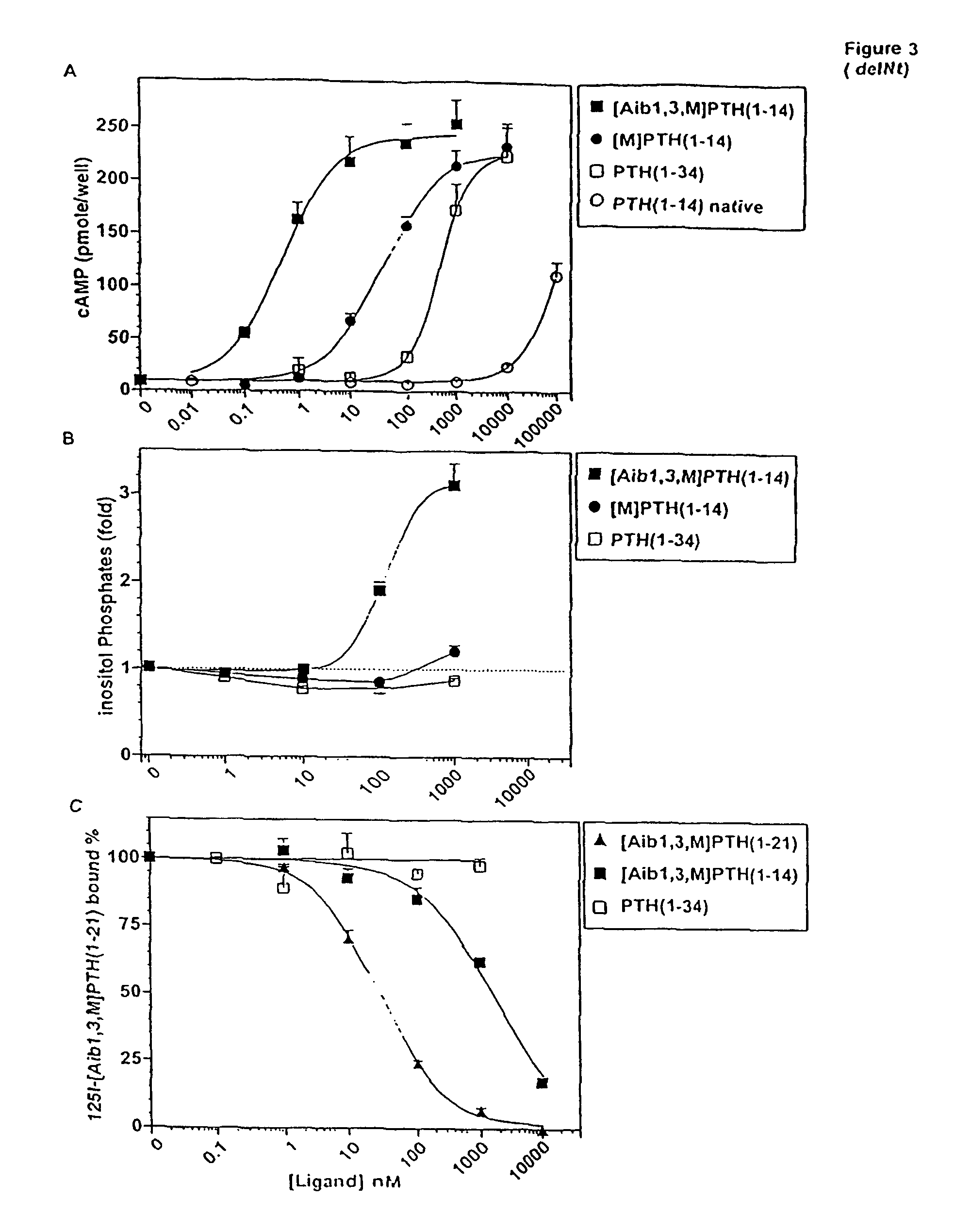
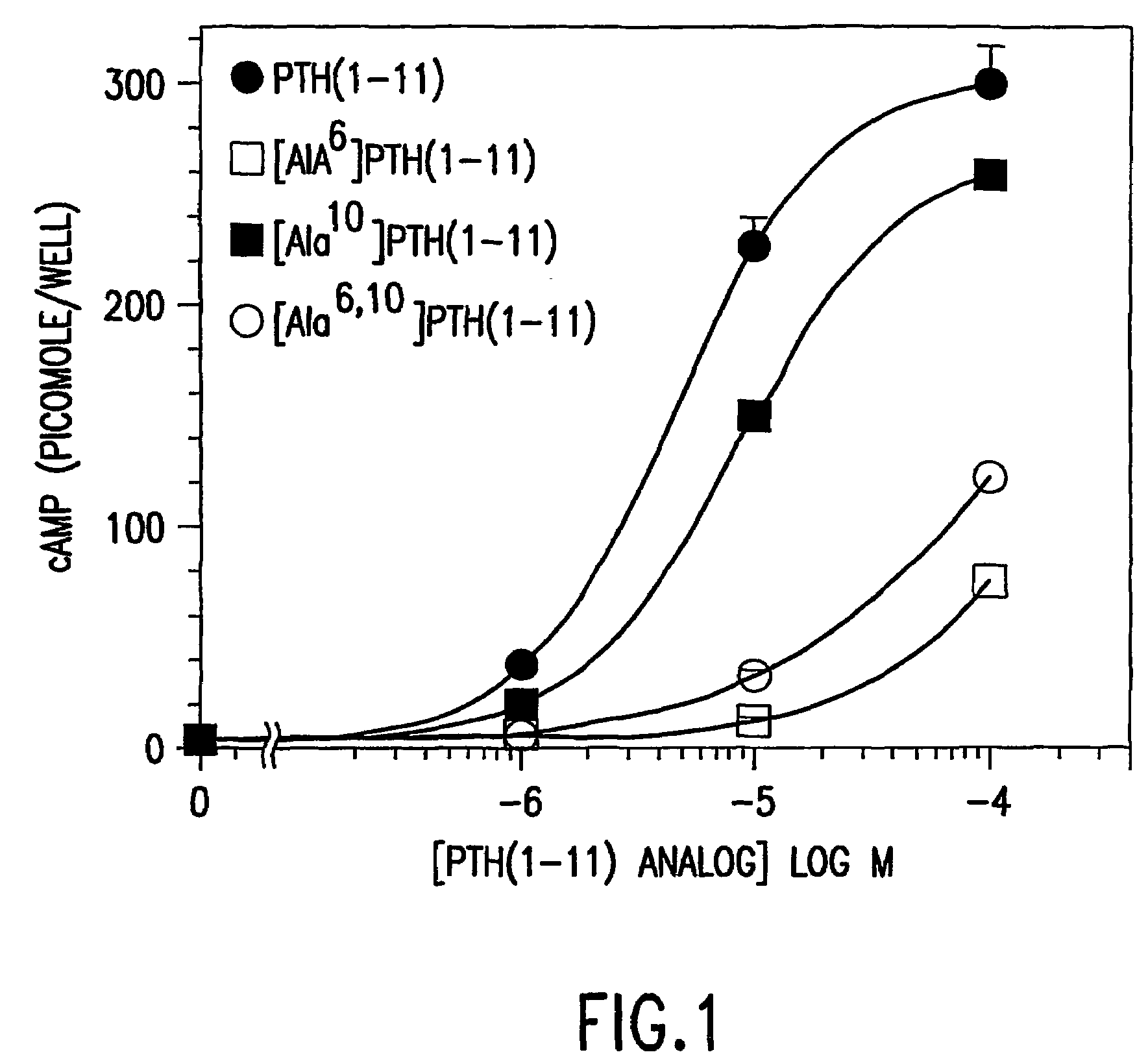
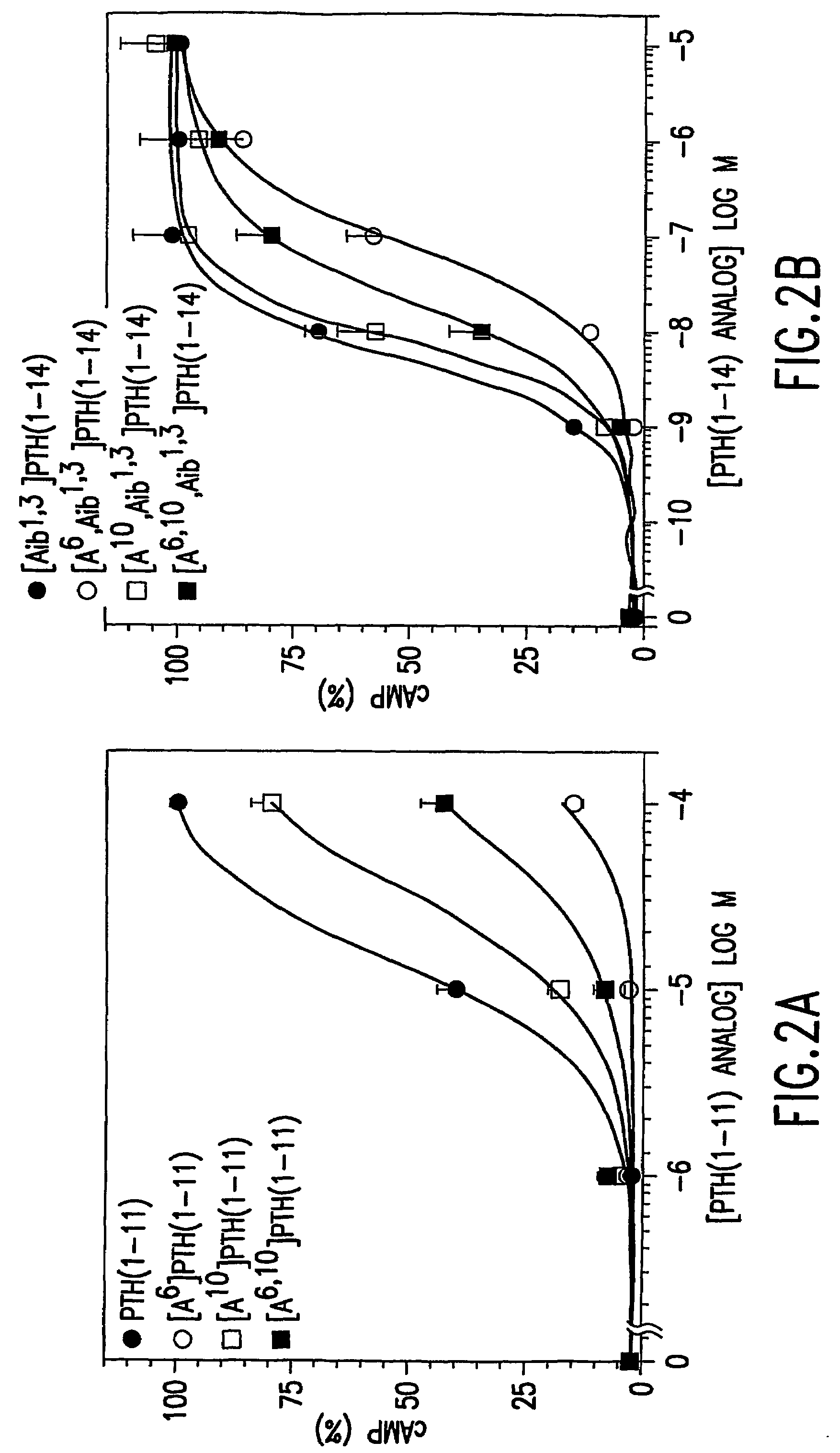
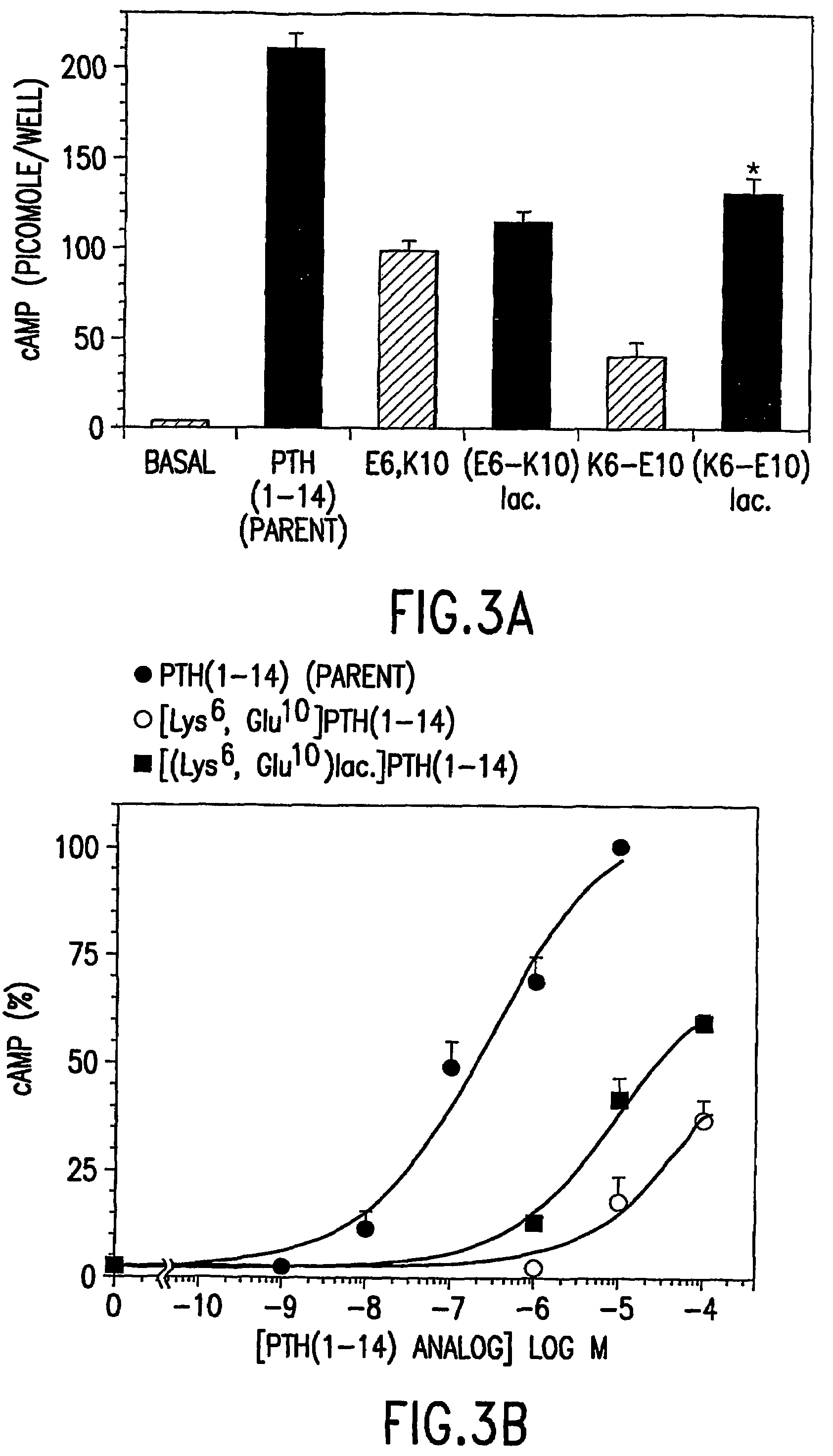
Conformationally constrained parathyroid hormone (pth) analogs
ActiveUS20050026839A1Decrease in bone massQuality improvementIn-vivo radioactive preparationsPeptide/protein ingredientsDiseaseAmino acid substitution
The present invention relates to conformationally constrained parathyroid hormone (PTH) analogs, and methods of preparing and using the PTH analogs. The invention provides novel PTH polypeptide derivatives containing amino acid substitutions at selected positions in the polypeptides. The invention provides derivatives of PTH (1-34), PTH(1-21), PTH(1-20), PTH(1-19), PTH(1-18), PTH(1-17), PTH(1-16), PTH(1-15), PTH(1-14), PTH(1-13), PTH(1-12), PTH(1-11) and PTH(1-1 0) polypeptides, wherein at least one residue in each polypeptide is a helix, preferably an a-helix, stabilizing residue. The invention also provides methods of making such peptides. Further, the invention encompasses compositions and methods for use in limiting undesired bone loss in a vertebrate at risk of such bone loss, in treating conditions that are characterized by undesired bone loss or by the need for bone growth, e.g. in treating fractures or cartilage disorders and for raising cAMP levels in cells where deemed necessary.
Owner:THE GENERAL HOSPITAL CORP
Implant composite material
InactiveUS20090157194A1Rapid hydrolysisImprove biological activityJoint implantsLigamentsKnee JointLigament structure
An implant composite material is provided which is for use in the treatment of articular cartilage disorders such as hip joint femur head necrosis and knee joint bone head necrosis, the reconstruction / fixing of a bio-derived or artificial ligament or tendon, the uniting / fixing of a bone, etc. Part of the implant composite material is replaced by bone tissues in an early stage to enable the material to stably bond with a living bone, while the other part retains a necessary strength over a necessary time period. Finally, the implant composite material is wholly replaced by the living bone and disappears.It is an implant composite material having a constitution which comprises a compact composite of a biodegradable and bioabsorbable polymer containing bioabsorbable and bioactive bioceramic particles and a porous composite of a biodegradable and bioabsorbable polymer containing bioabsorbable and bioactive bioceramic particles, the porous composite being united with the compact composite. The porous composite is replaced by bone tissues in an early stage to enable the material to stably bond with a living bone, while the compact composite retains a necessary strength over a necessary time period. Finally, the material is wholly replaced by the living bone and disappears. Consequently, this implant composite material can sufficiently meet desires in this medical field.
Owner:TAKIRON CO LTD
Skin equivalents derived from umbilical cord mesenchymal stem/progenitor cells and umbilical cord epithelial stem/progenitor cells
The present invention relates to a skin equivalent and a method for producing the same, wherein the skin equivalent comprises a scaffold and stem / progenitor cells isolated from the amniotic membrane of umbilical cord. These stem / progenitor cells may be mesenchymal (UCMC) and / or epithelial (UCEC) stem cells, which may then be further differentiated to fibroblast and keratinocytes. Further described is a method for isolating stem / progenitor cells from the amniotic membrane of umbilical cord, wherein the method comprises separating the amniotic membrane from the other components of the umbilical cord in vitro, culturing the amniotic membrane tissue under conditions allowing cell proliferation, and isolating the stem / progenitor cells from the tissue cultures. The invention also refers to therapeutic uses of these skin equivalents. Another aspect of the invention relates to the generation of a mucin-producing cell using stem / progenitor cells obtained from the amniotic membrane of umbilical cord and therapeutic uses of such mucin-producing cells. In yet another aspect, the invention relates to a method for generating an insulin-producing cell using stem / progenitor cells isolated from the amniotic membrane of umbilical cord and therapeutic uses thereof. The invention further refers to a method of treating a bone or cartilage disorder using UCMC. Furthermore, the invention refers to a method of generating a dopamin and tyrosin hydroxylase as well as a HLA-G and hepatocytes using UCMC and / or UCEC. The present invention also refers to a method of inducing proliferation of aged keratinocytes using UCMC.
Owner:CELLRESEARCH CORP PTE LTD
Compositions comprising natriuretic peptides and methods of use thereof
Owner:ALEXION PHARMA INC
Conformationally constrained parathyroid hormone (PTH) analogs
ActiveUS7572765B2In-vivo radioactive preparationsPeptide/protein ingredientsAmino acid substitutionCvd risk
The present invention relates to conformationally constrained parathyroid hormone (PTH) analogs, and methods of preparing and using the PTH analogs. The invention provides novel PTH polypeptide derivatives containing amino acid substitutions at selected positions in the polypeptides. The invention provides derivatives of PTH (1-34), PTH(1-21), PTH(1-20), PTH(1-19), PTH(1-18), PTH(1-17), PTH(1-16), PTH(1-15), PTH(1-14), PTH(1-13), PTH(1-12), PTH(1-11) and PTH(1-1 0) polypeptides, wherein at least one residue in each polypeptide is a helix, preferably an a-helix, stabilizing residue. The invention also provides methods of making such peptides. Further, the invention encompasses compositions and methods for use in limiting undesired bone loss in a vertebrate at risk of such bone loss, in treating conditions that are characterized by undesired bone loss or by the need for bone growth, e.g. in treating fractures or cartilage disorders and for raising cAMP levels in cells where deemed necessary.
Owner:THE GENERAL HOSPITAL CORP
Conformationally constrained parathyroid hormone (PTH) analogs with lactam bridges
Owner:THE GENERAL HOSPITAL CORP
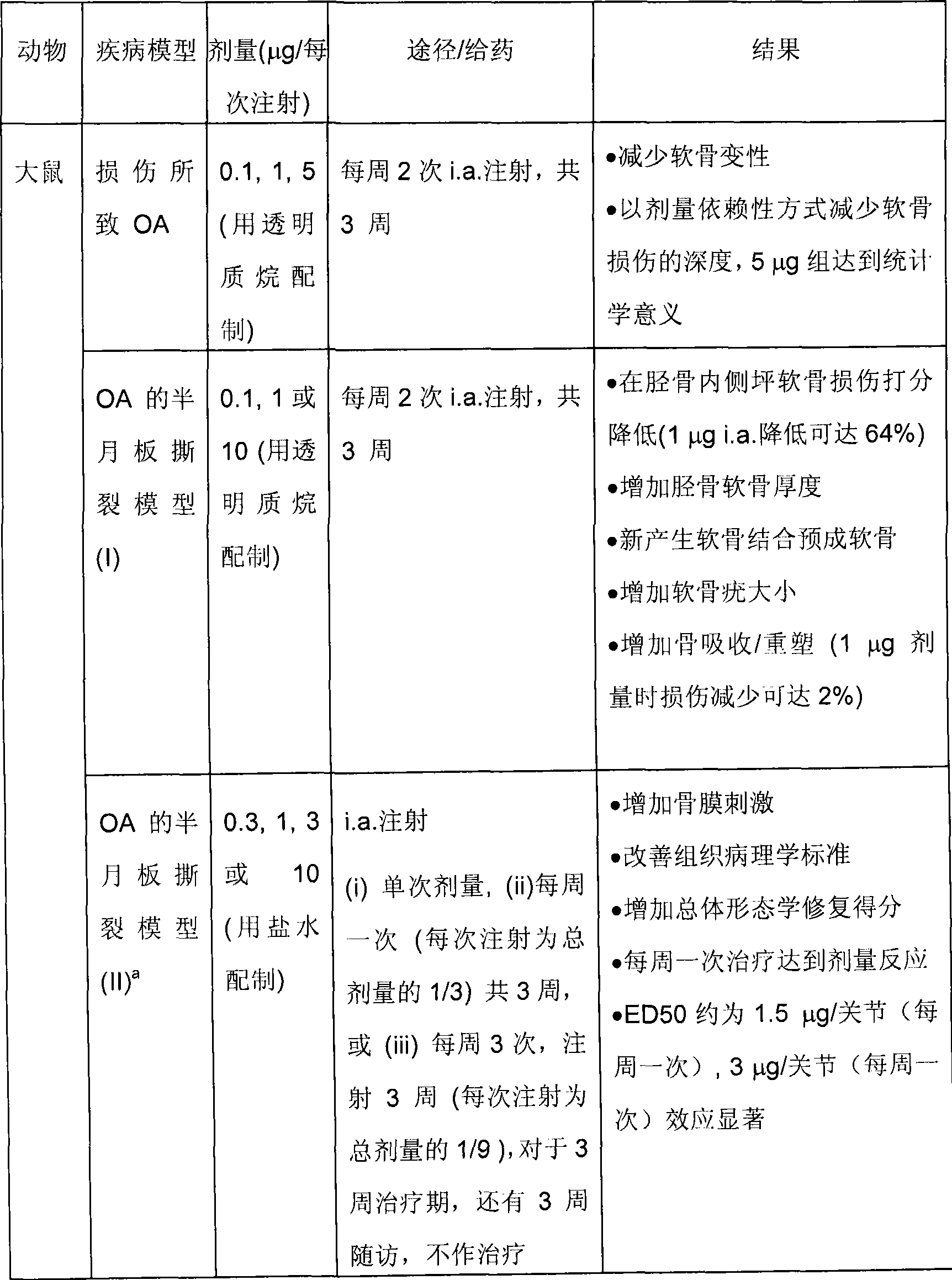
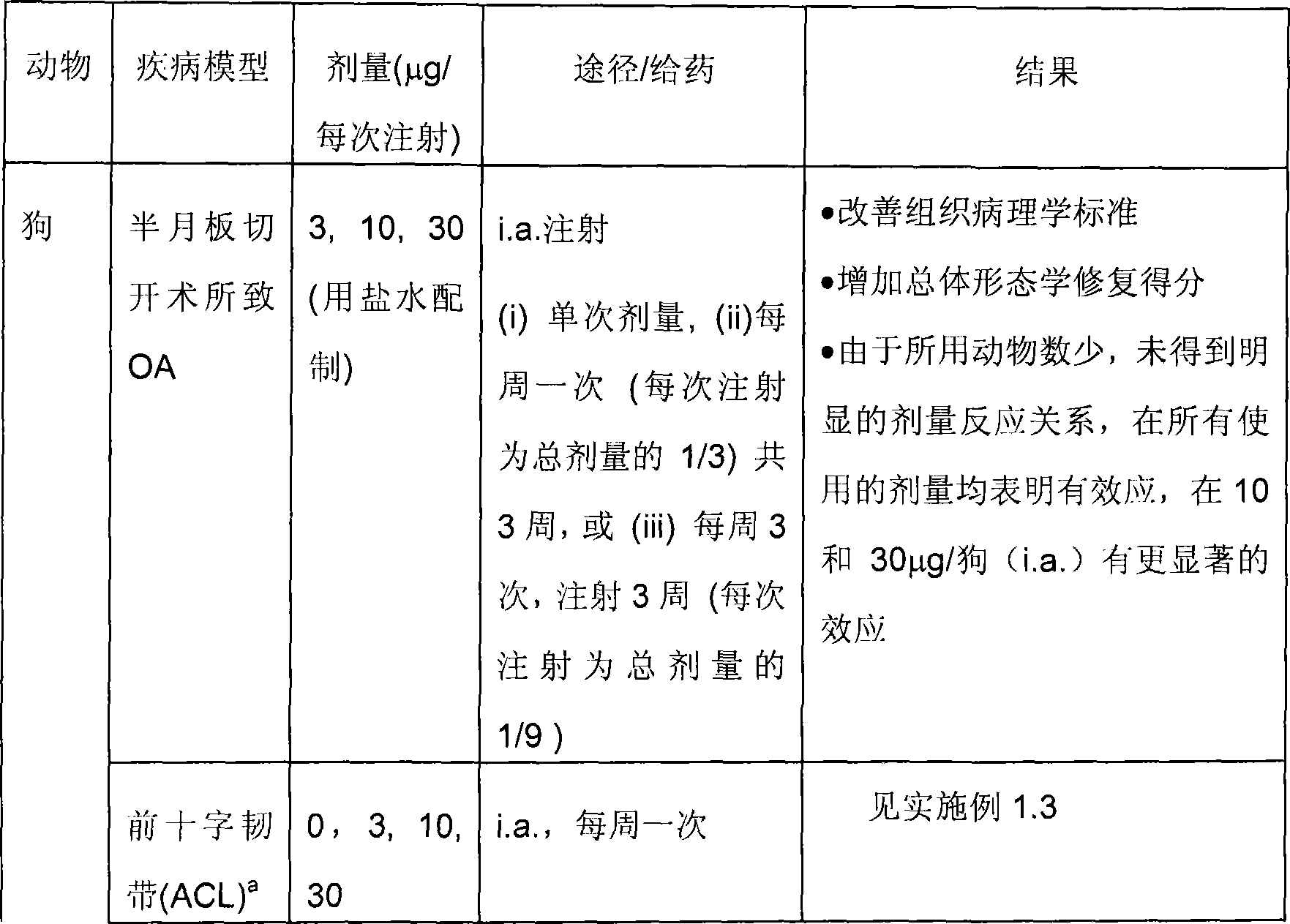
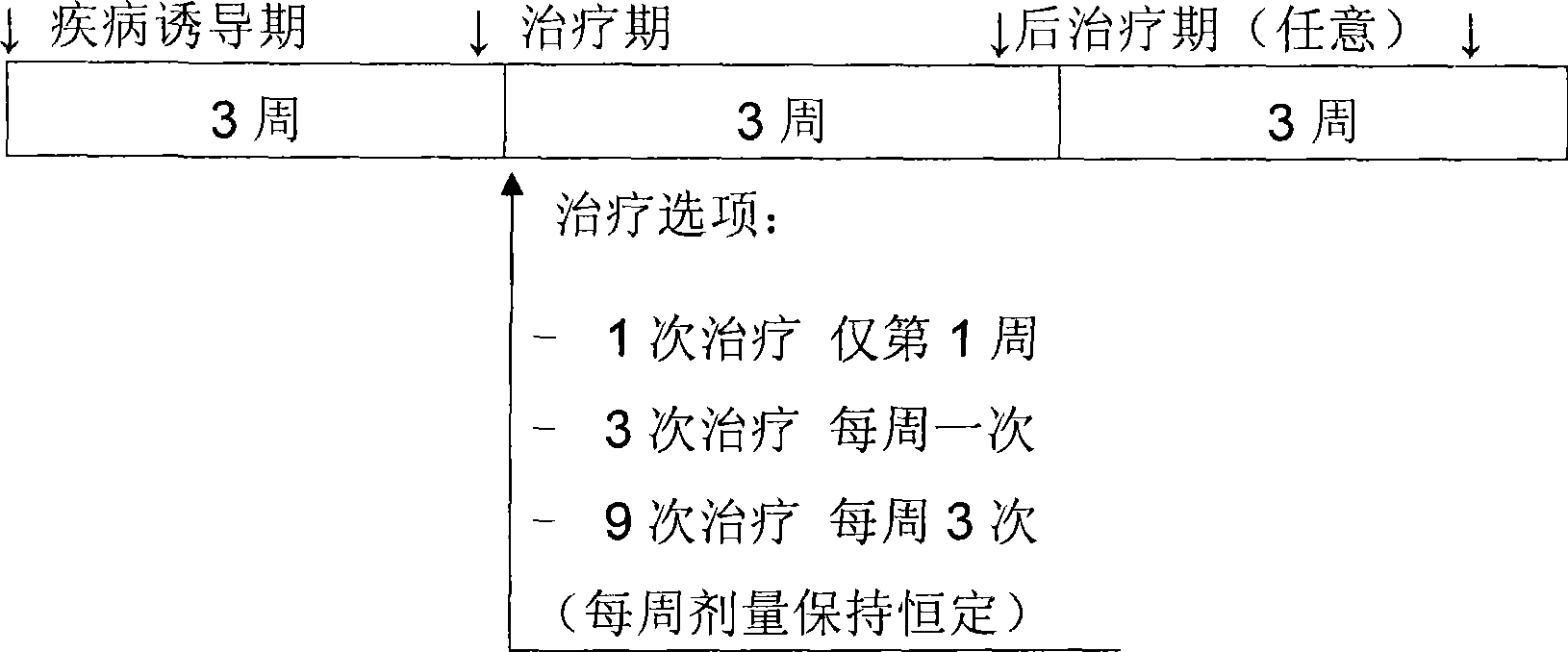
Treatment of cartilage disorders with fgf-18
This invention concerns the treatment of cartilage disorder and osteoarthritis in particular. More specifically, it relates to the use of FGF-18 in treatment regimens and for the manufacture of a medicament for the treatment of patients having a cartilage disorder such as osteoarthritis, such as for example knee osteoarthritis or secondary hip osteoarthritis. Specifically provided is a preferred treatment scheme comprising once weekly administration of an FGF-18 compound per treatment cycle.
Owner:ARES TRADING SA
Compositions and methods for treating articular cartilage disorders
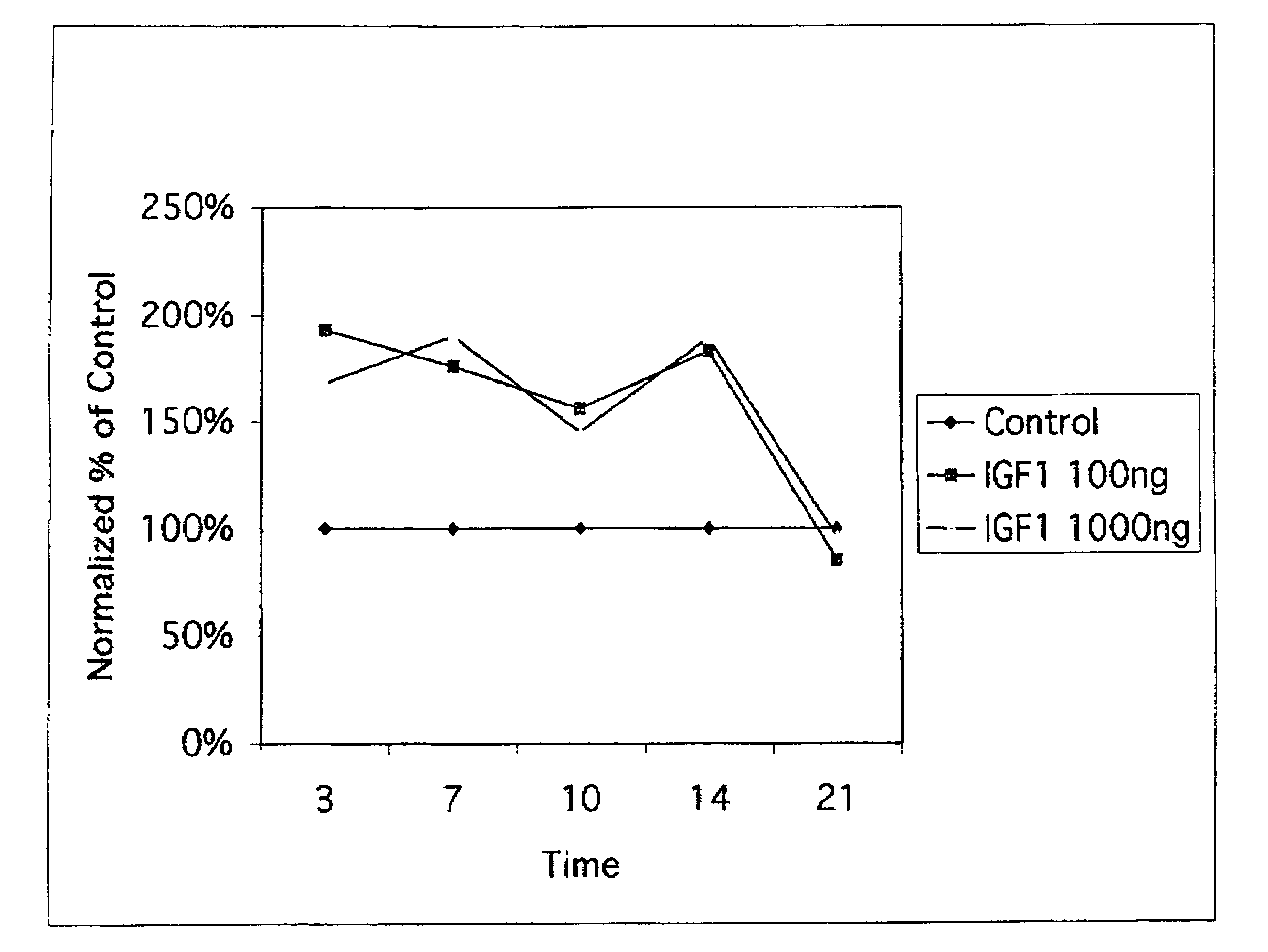
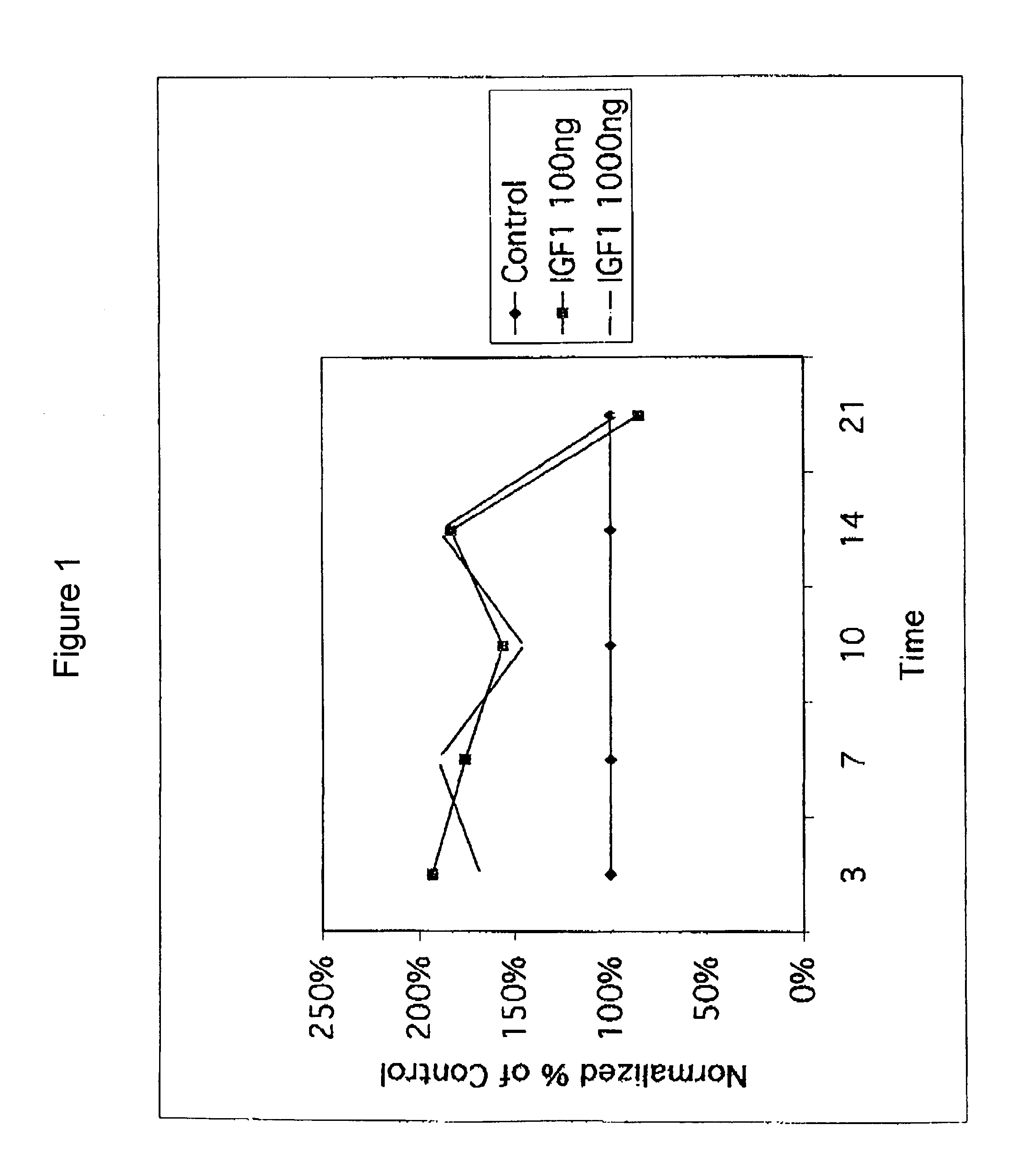
InactiveUS7141545B2Increase volumeEasy maintenancePeptide/protein ingredientsSkeletal disorderInsulin-like growth factorDisease
A method for treating mammalian articular cartilage disorders, more particularly osteoarthritis, and trauma-related cartilage injuries using insulin-like growth factor I (IGF-I) is provided. The method comprises increasing the amount of IGF-I at the diseased or injured articular site to a therapeutically effective level that is capable of maintenance and / or regeneration of cartilage, which is beneficial to the long-term treatment of osteoarthritis and trauma-related injuries to cartilage tissues. In one embodiment of the invention, single doses of at least 0.01 mg of pharmaceutically effective IGF-I are administered intermittently such that the duration of time off of therapy is greater than the time on therapy, more preferably with a frequency of administration of about once per week or less.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
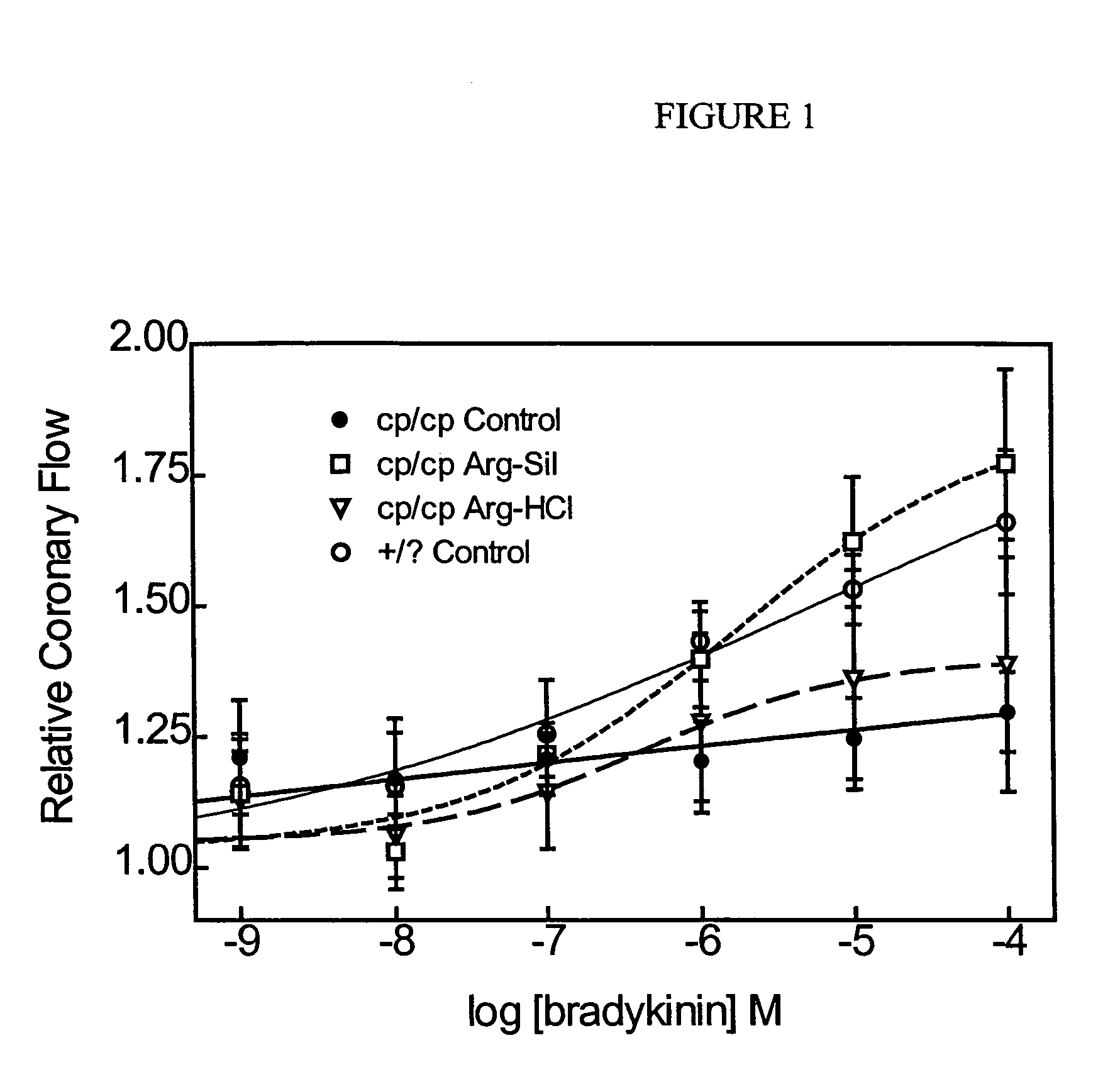
Arginine silicate inositol complex and use thereof
ActiveUS7576132B2Improving and reducing infectionDecreases the inflammatory markersBiocideNervous disorderArginineHigh doses
A method for preventing and treating a variety of disease states and disorders is provided, comprising the administration of an arginine silicate inositol complex to an individual in the presence of medical therapy or absence of medical therapy. Examples of said disease states and disorders include bone and cartilage disorders and cardiovascular disease and its associated micro and macro vascular complications including infections and inflammation of all these diseases in combination or without. Advantageously, the amount of arginine silicate inositol complex administered per day is between about 2 mg / Kg body weight to 2,500 mg / Kg body weight or from a low dose to a higher dose to observe normal metabolic functions and healthy and the delivery is parenteral, oral or intravenous or topical by solid or liquid or both.
Owner:NUTRITION 21 INC
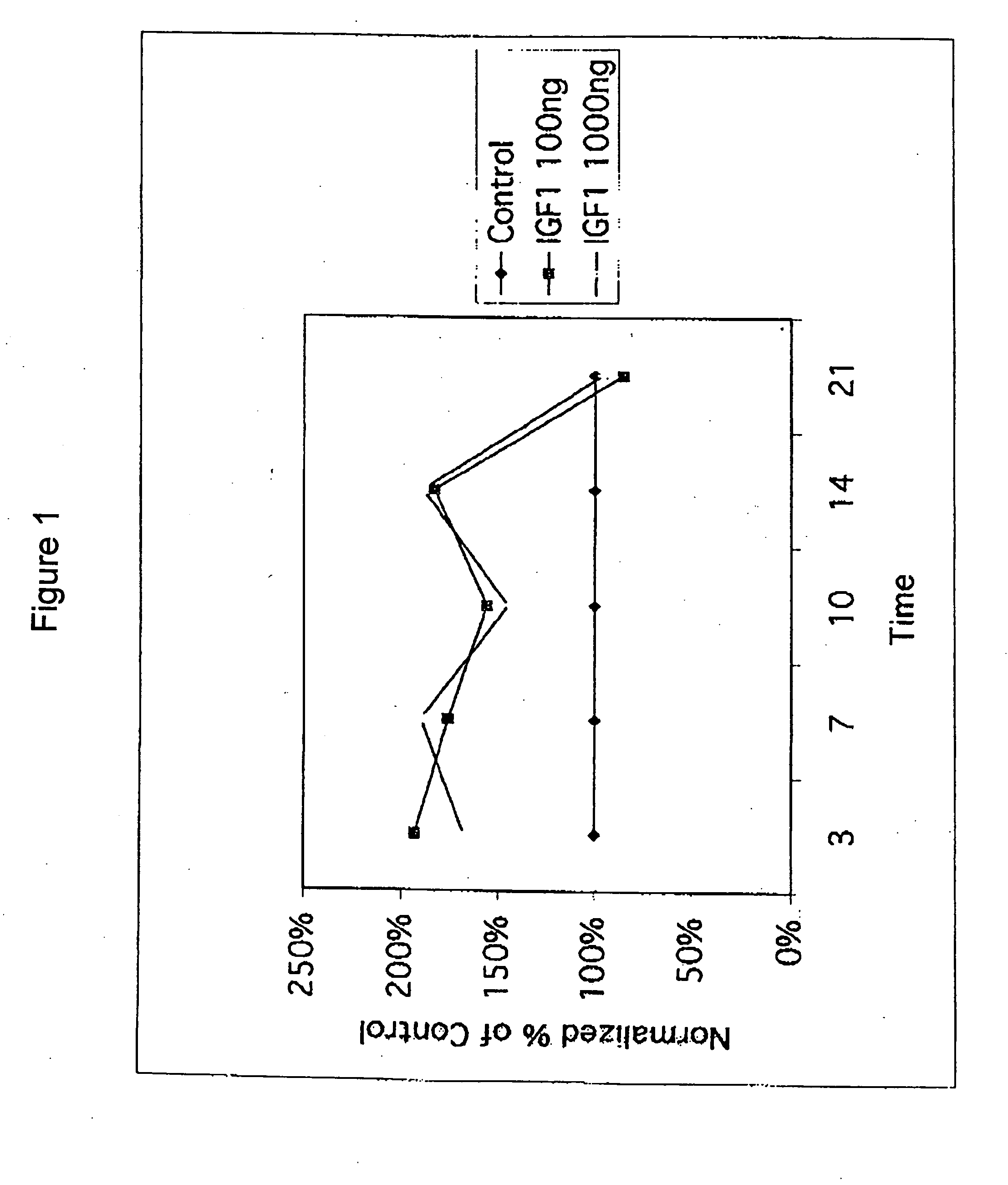
Compositions and methods for treating articular cartilage disorders
InactiveUS20060258588A1Enables maintenanceIncrease volumePeptide/protein ingredientsSkeletal disorderDiseaseInsulin-like growth factor
A method for treating mammalian articular cartilage disorders, more particularly osteoarthritis, and trauma-related cartilage injuries using insulin-like growth factor I (IGF-I) is provided. The method comprises increasing the amount of IGF-I at the diseased or injured articular site to a therapeutically effective level that is capable of maintenance and / or regeneration of cartilage, which is beneficial to the long-term treatment of osteoarthritis and trauma-related injuries to cartilage tissues. In one embodiment of the invention, single doses of at least 0.01 mg of pharmaceutically effective IGF-I are administered intermittently such that the duration of time off of therapy is greater than the time on therapy, more preferably with a frequency of administration of about once per week or less.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Treatment of cartilage disorders
This invention concerns the treatment of cartilage disorder and osteoarthritis in particular. More specifically, it relates to the use of FGF-18 in treatment regimens and for the manufacture of a medicament for the treatment of patients having a cartilage disorder such as osteoarthritis, such as for example knee osteoarthritis or secondary hip osteoarthritis. Specifically provided is a preferred treatment scheme comprising once weekly administration of an FGF-18 compound per treatment cycle.
Owner:ARES TRADING SA
Homing in mesenchymal stem cells
InactiveUS20110165128A1Improve abilitiesImprove adhesionBiocideMammal material medical ingredientsCXCR4Liver disorder
The present invention relates to expression of CXCR4 in mesenchymal stem cells (MSCs) and homing of MSCs to sites of injury. In particular, the invention provides expanded cultures of MSCs which maintain cell surface expression of CXCR4. The MSCs are capable of homing to sites of injury and are suitable for treatment of ischemic disorders, including cardiac disorders, bone and cartilage disorders, liver disorders, inflammatory disorders, and stroke.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Method for treatment of cartilage disorders with centella extract
The invention is a method for the treatment of mammalian articular cartilage disorders, inflammatory joint disease, trauma-related cartilage injuries, and degenerative disc disease. The method involves treating the affected area with a composition containing a therapeutically effective dose of Centella extract. The composition is delivered locally by parenteral administration to the affected site.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Treatment of cartilage disorders with FGF-18
This invention concerns the treatment of cartilage disorder and osteoarthritis in particular. More specifically, it relates to the use of FGF-18 in treatment regimens of patients having a cartilage disorder such as osteoarthritis, such as for example knee osteoarthritis or secondary hip osteoarthritis. Specifically provided is a preferred treatment scheme comprising once weekly administration of an FGF-18 compound per treatment cycle.
Owner:ARES TRADING SA
Compositions comprising bone marrow cells together with demineralized and/or mineralized bone matrix and uses thereof in the induction of bone and cartilage formation
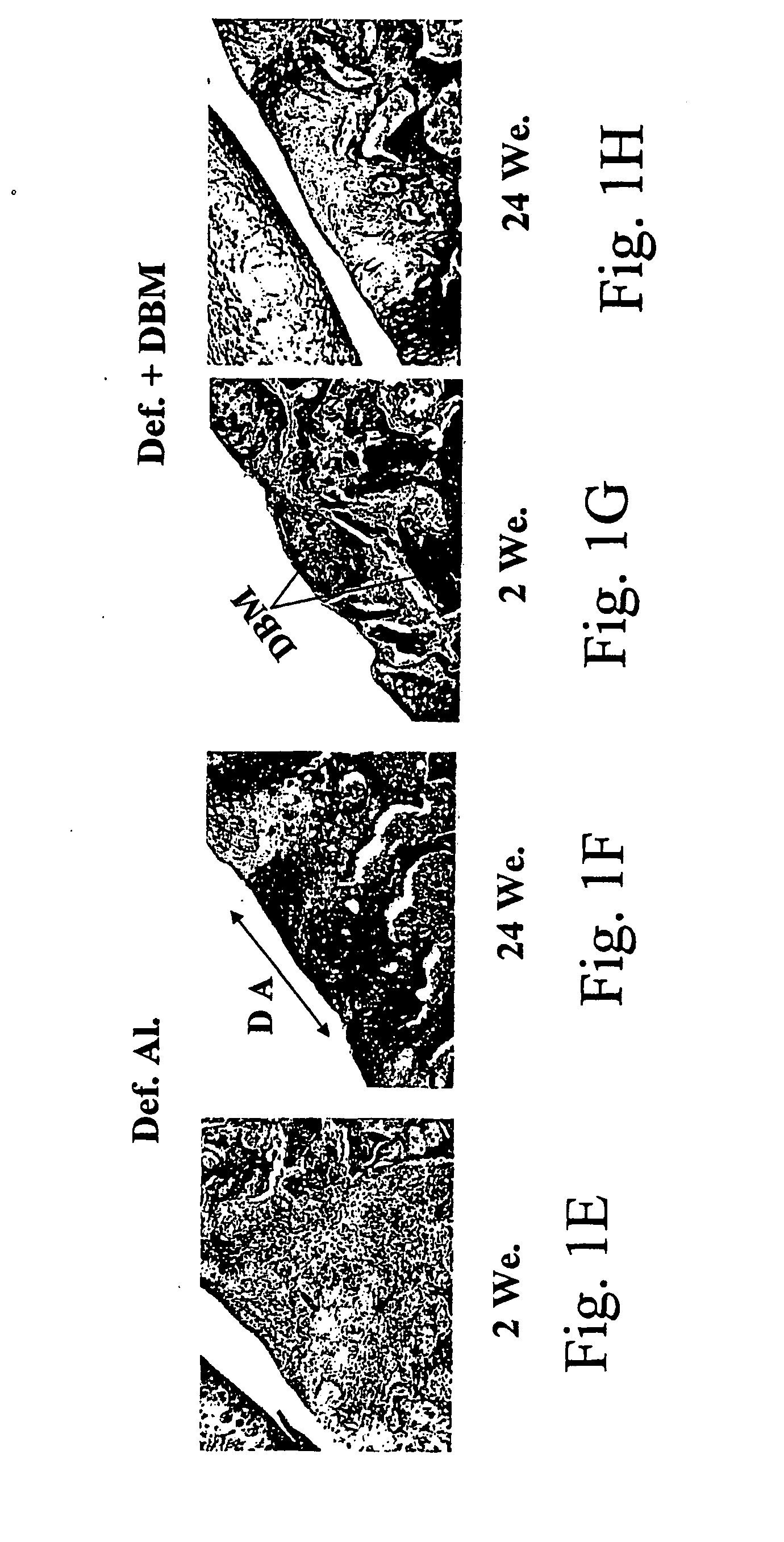
A composition comprising bone marrow cells (BMC) and demineralized bone matrix (DBM) and / or mineralized bone matrix (MBM) and optionally comprising bone morphogenetic protein / s (BMP) and / or other active agents, particularly for use in the transplantation of mesenchymal progenitor cells into a joint and / or a cranio-facial maxillary bone, for restoring and / or enhancing the formation of a new hyaline cartilage and subchondral bone structure. The composition of the invention and method of treatment employing the same may be used for the treatment of hereditary or acquired bone disorders, hereditary or acquired cartilage disorders, malignant bone or cartilage disorders, metabolic bone diseases, bone infections, conditions involving bone or cartilage deformities and Paget's disease. The composition and method may further be used for the correction of complex fractures, bone replacement and formation of new bone in plastic or sexual surgery, for support of implants of joints, cranio-facial-maxillary bones, or other musculoskeletal implants, including artificial implants. The method of the invention may further be used for treating damaged joints or degenerative arthropathy associated with malformation and / or dysfunction of cartilage and / or subchondral bone. A kit is provided for performing transplantation into a joint or a cranio-facial-maxillary bone of a mammal of the composition of the invention.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
Fgf-18 formulation in alginate/collagen hydrogels
ActiveUS20160303291A1Prevent possible protein lossesOintment deliverySkeletal disorderCartilage injuryPharmaceutical formulation
The invention relates to the field of pharmaceutical formulations. More particularly it is directed to homogeneous hydrogels comprising Fibroblast Growth Factor 18 (FGF-18) compound and to methods of producing such hydrogels. The hydrogels of the invention can be used, once formed in situ, for the treatment of cartilage disorders such as osteoarthritis or cartilage injury.
Owner:ARES TRADING SA
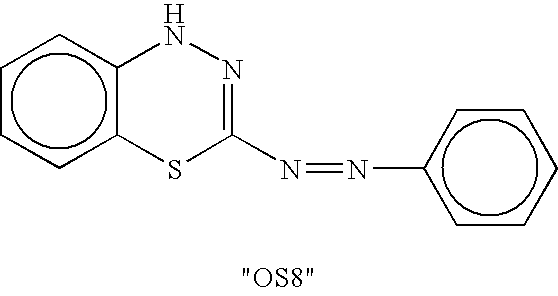
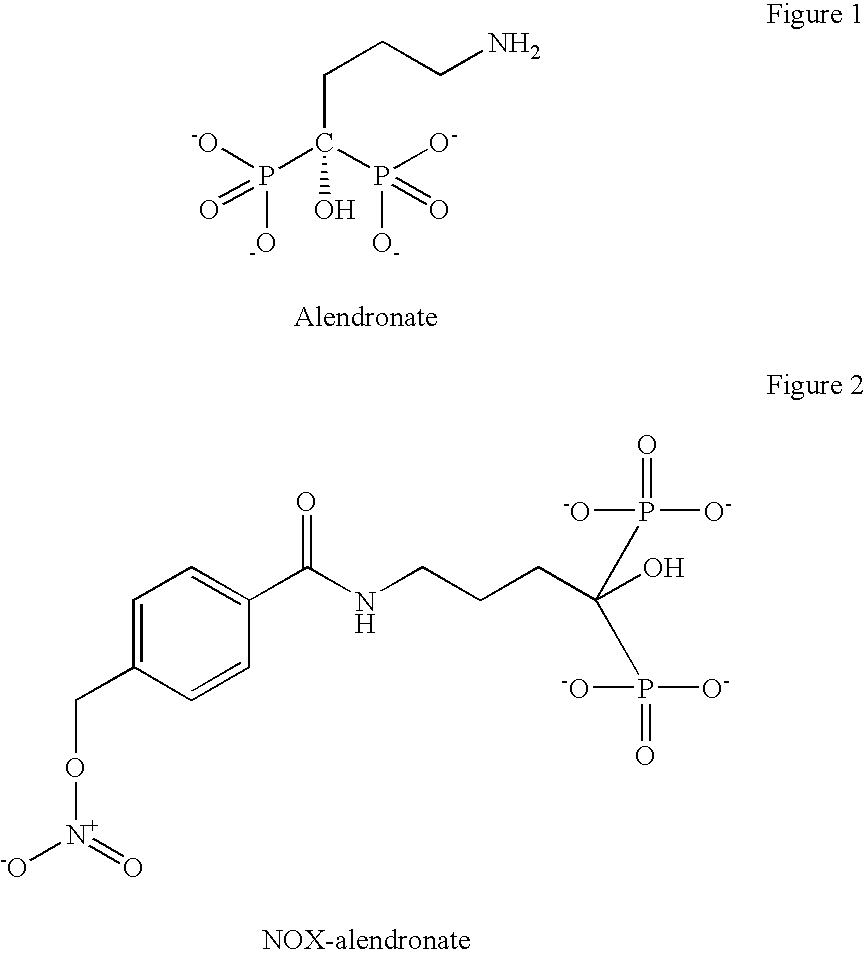
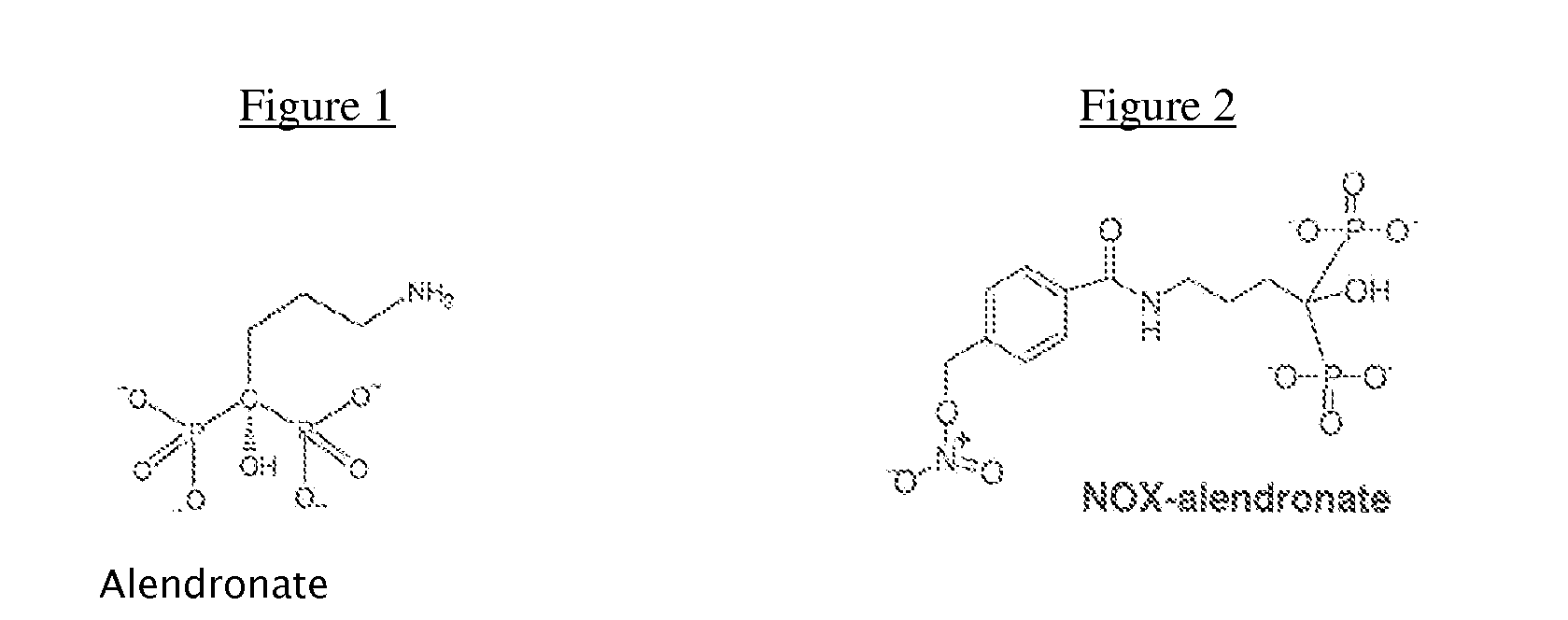
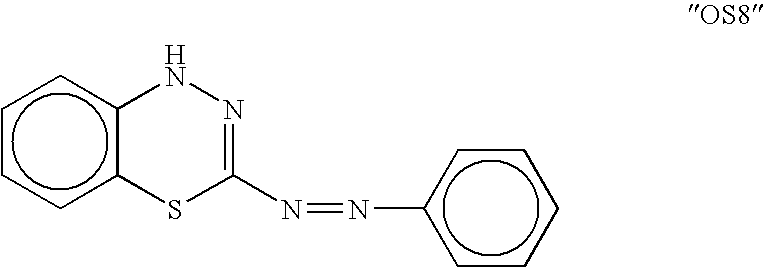
Methods and compositions for stimulating bone growth using nitric oxide releasing bisphosphonate conjugates
InactiveUS7288535B2Improve bone formationEnhance bone bone mineral densityBiocidePhosphorous compound active ingredientsDiseaseMedicine
The invention relates to compositions and methods for use in treating skeletal system disorders in a vertebrate at risk for bone loss, and in treating conditions that are characterized by the need for bone growth, in treating fractures, and in treating cartilage disorders. More specifically, the invention concerns the use of NO-bisphosphonate assembly for enhancing bone growth.
Owner:OSTEOSCREEN IP
Methods and compositions for stimulating bone growth using nitric oxide releasing bisphosphonate conjugates (no-bisphosphonate)
The invention relates to compositions and methods for use in treating skeletal system disorders in a vertebrate at risk for bone loss, and in treating conditions that are characterized by the need for bone growth, in treating fractures, and in treating cartilage disorders. More specifically, the invention concerns the use of NO-bisphosphonate assembly for enhancing bone growth.
Owner:GARRETT I ROSS
Chondrocyte proliferation promoting agent
ActiveUS20150164973A1Improve securityFacilitated DiffusionHydrolysed protein ingredientsSkeletal disorderDiseaseJoint pain
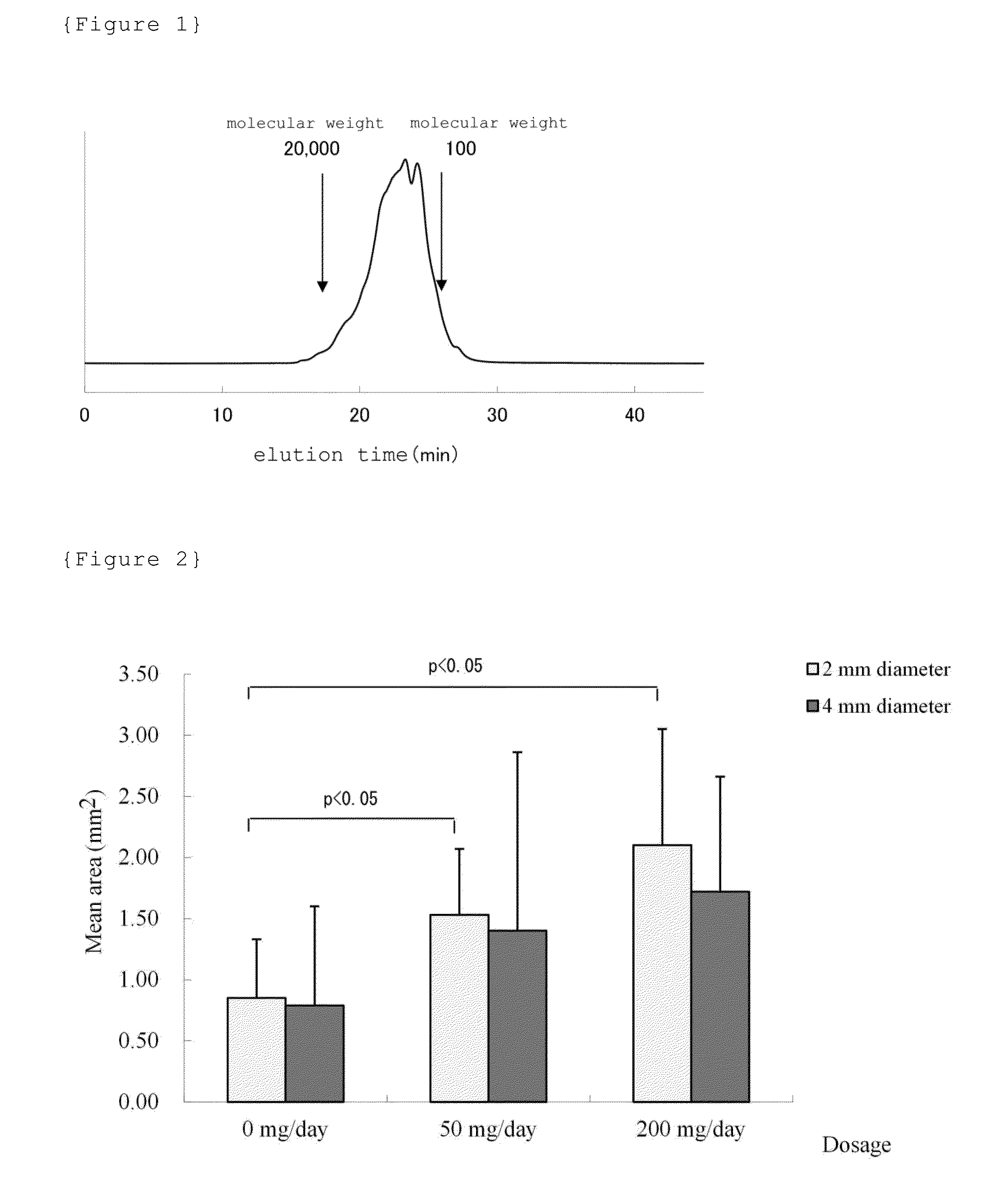
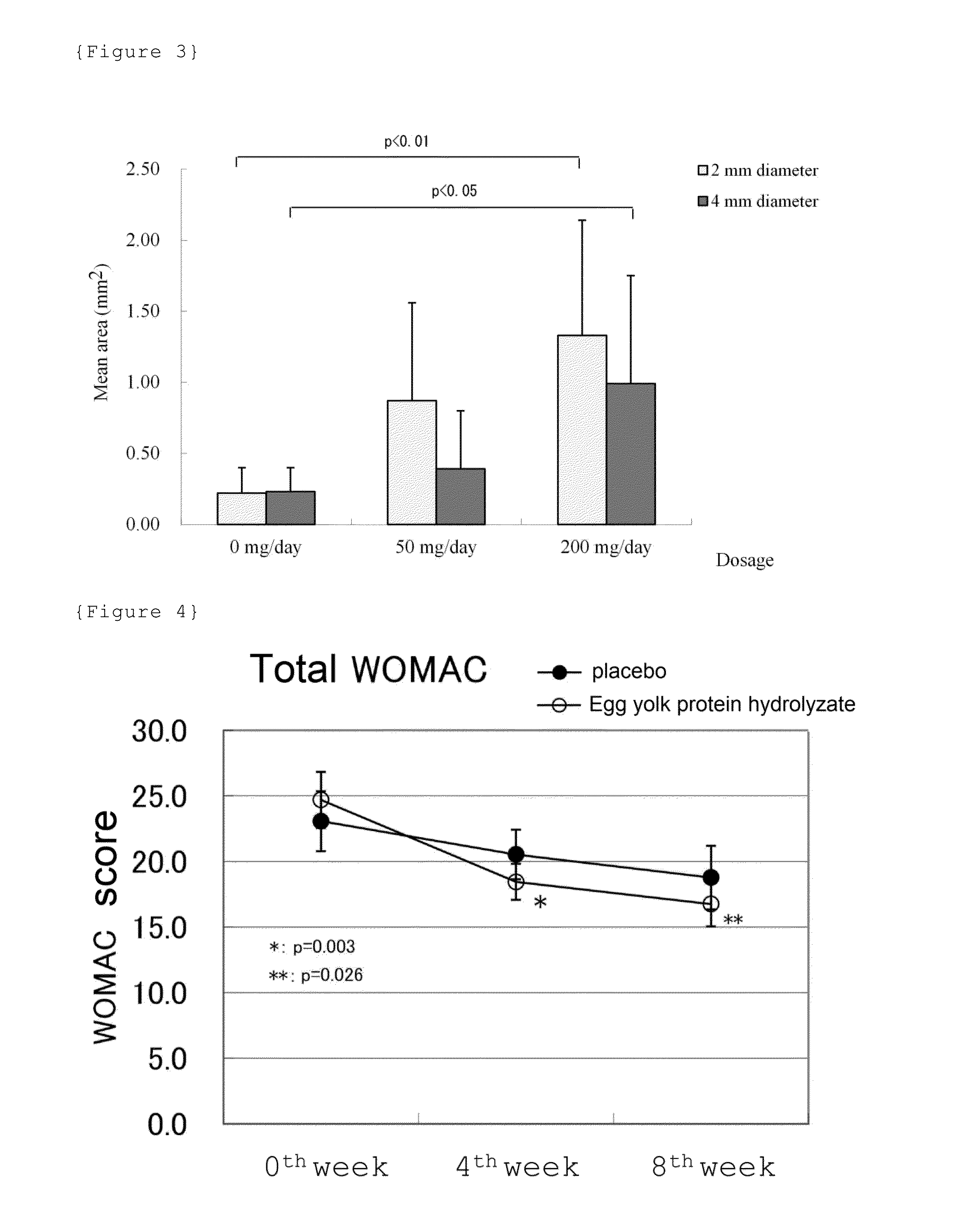
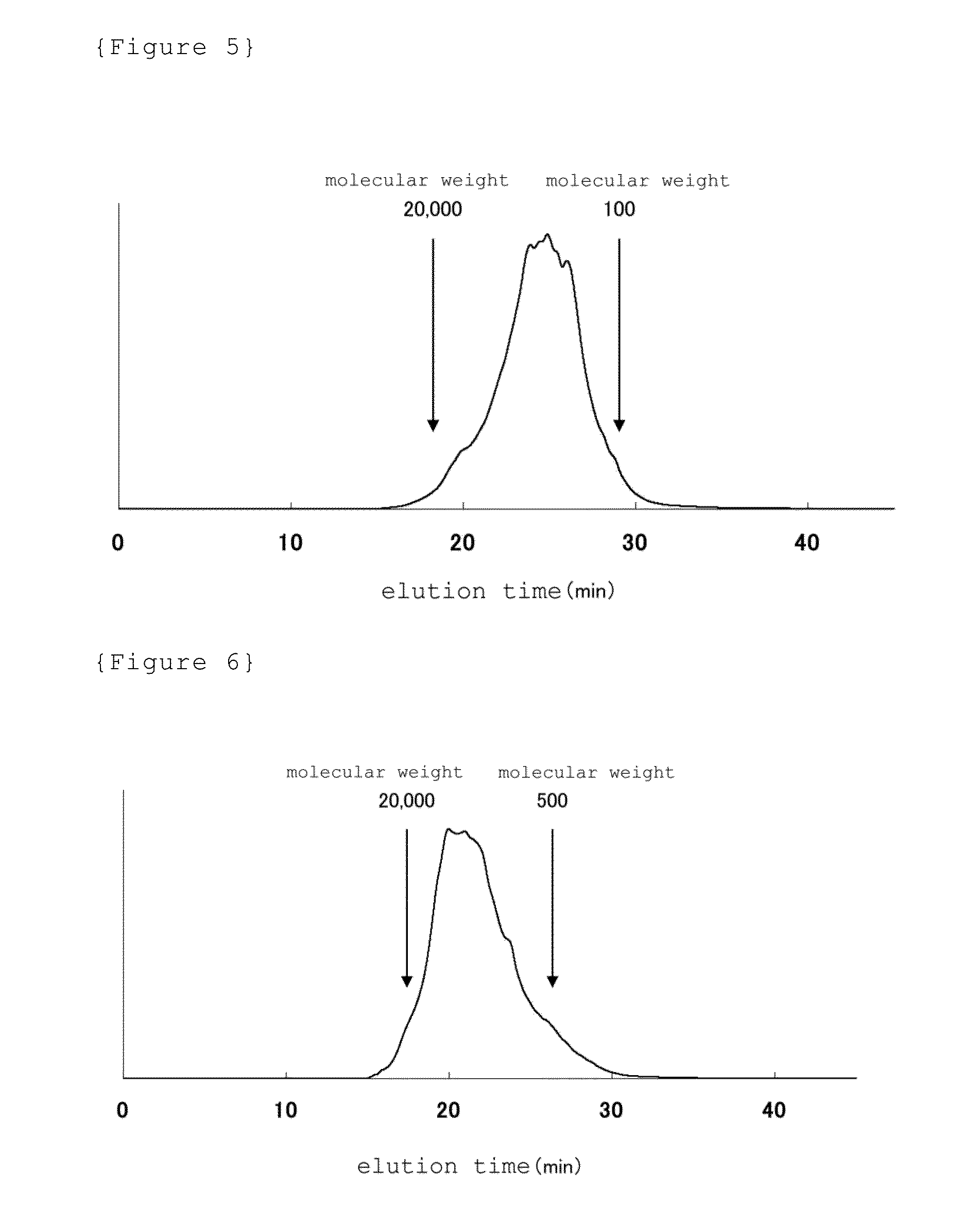
The present invention provides a novel active ingredient which can be safely used in preventing, ameliorating or treating diseases related to the cartilage such as cartilage damages and cartilage disorders. Since the egg yolk protein hydrolyzate has an effect on chondrocyte proliferation promotion, it is useful as an active ingredient for preventing, ameliorating or treating cartilage disorders and for preventing, ameliorating or treating joint pain. The egg yolk protein hydrolyzate is a natural-origin material with high safety and, therefore, can be widely used in foods and drinks, medicines, feeds and the like which can be daily ingested.
Owner:PHARMA FOODS INT CO LTD
Compositions for treatment of osteochondral disorders
ActiveUS20180153940A1Improve performanceRobust long-term outcomeCulture processCell culture mediaDiseaseMedicine
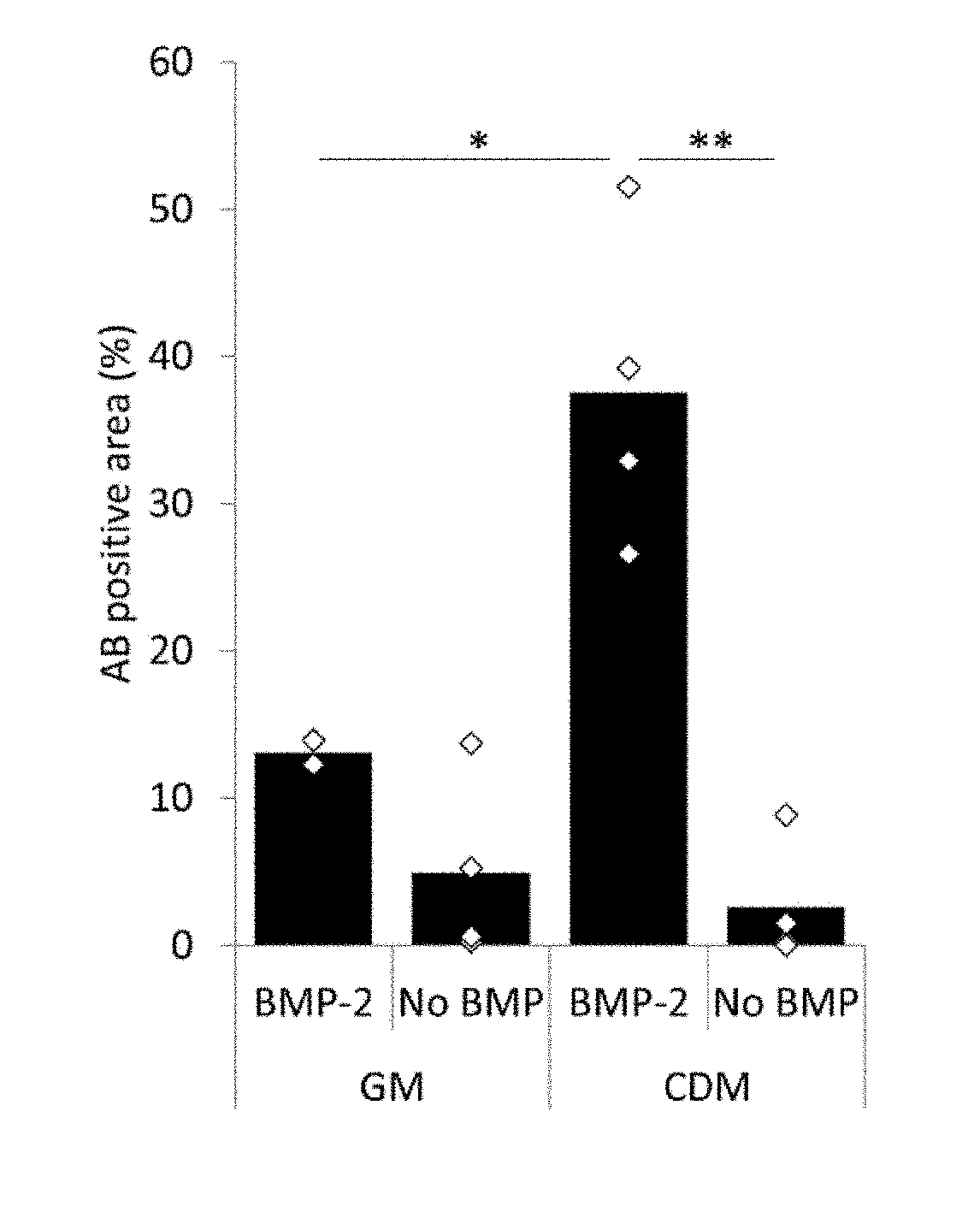
The application provides biocompatible carriers comprising bone forming and / or cartilage forming cells and methods for making them. The application further provides pharmaceutical compositions comprising said ATMPs and method of treatments using said ATMPs. The application further relates to said ATMPS for use in the treatment of bone disorders, cartilage disorders and joint disorders. The current invention further relates to method of treatments of bone disorders, cartilage disorders and joint disorders.
Owner:KATHOLIEKE UNIV LEUVEN
Compositions and methods for managing or improving bone disorders, cartilage disorders, or both
The present disclosure provides mixtures of prenylated flavonoids, stilbenes, or both with flavans or curcuminoids or both capable of useful for promoting, managing or improving bone health, cartilage health or both, or for preventing or treating a bone disorder, cartilage disorder or both. Such a mixture of prenylated flavonoids, stilbenes, or both with flavans or curcuminoids or both can optionally be used in combination with other bone and cartilage management agents, such as calcium, magnesium, zinc, boron, vitamin D, vitamin K, glucosamine and / or chondroitin compounds, non-steroidal anti-inflammatory agents / analgesics, COX / LOX inhibiting agents, neuropathic pain relief agents, or the like.
Owner:UNIGEN
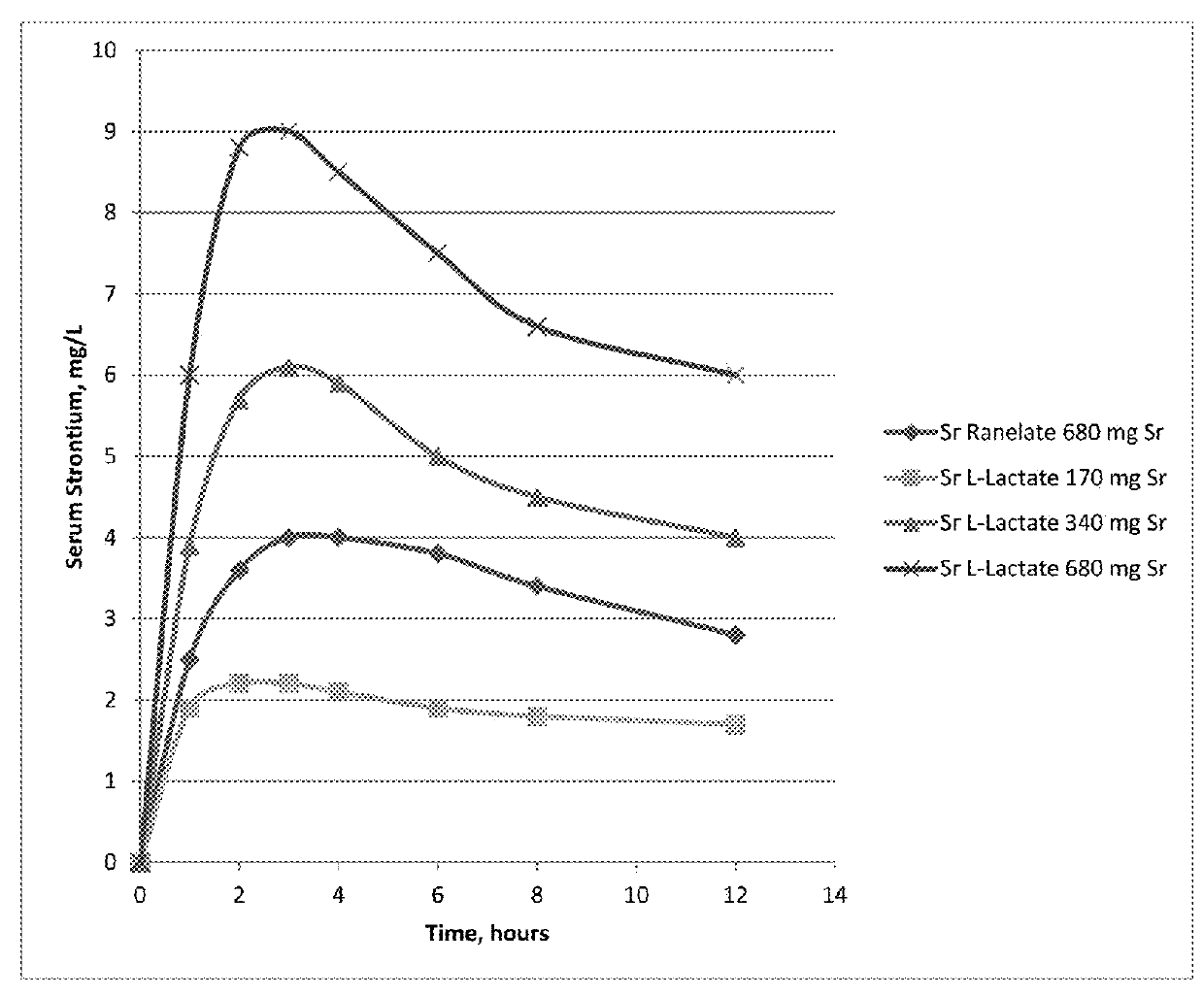
Pharmaceutical quality strontium l-lactate
ActiveUS20180092869A1Improve bioavailabilityGood for bone healthHeavy metal active ingredientsSkeletal disorderL lactateMedicine
Owner:NELSON DEANNA J
Therapeutic method
InactiveUS7208469B2Increasing active concentrationFacilitated DiffusionBiocidePeptide/protein ingredientsDiseaseMedicine
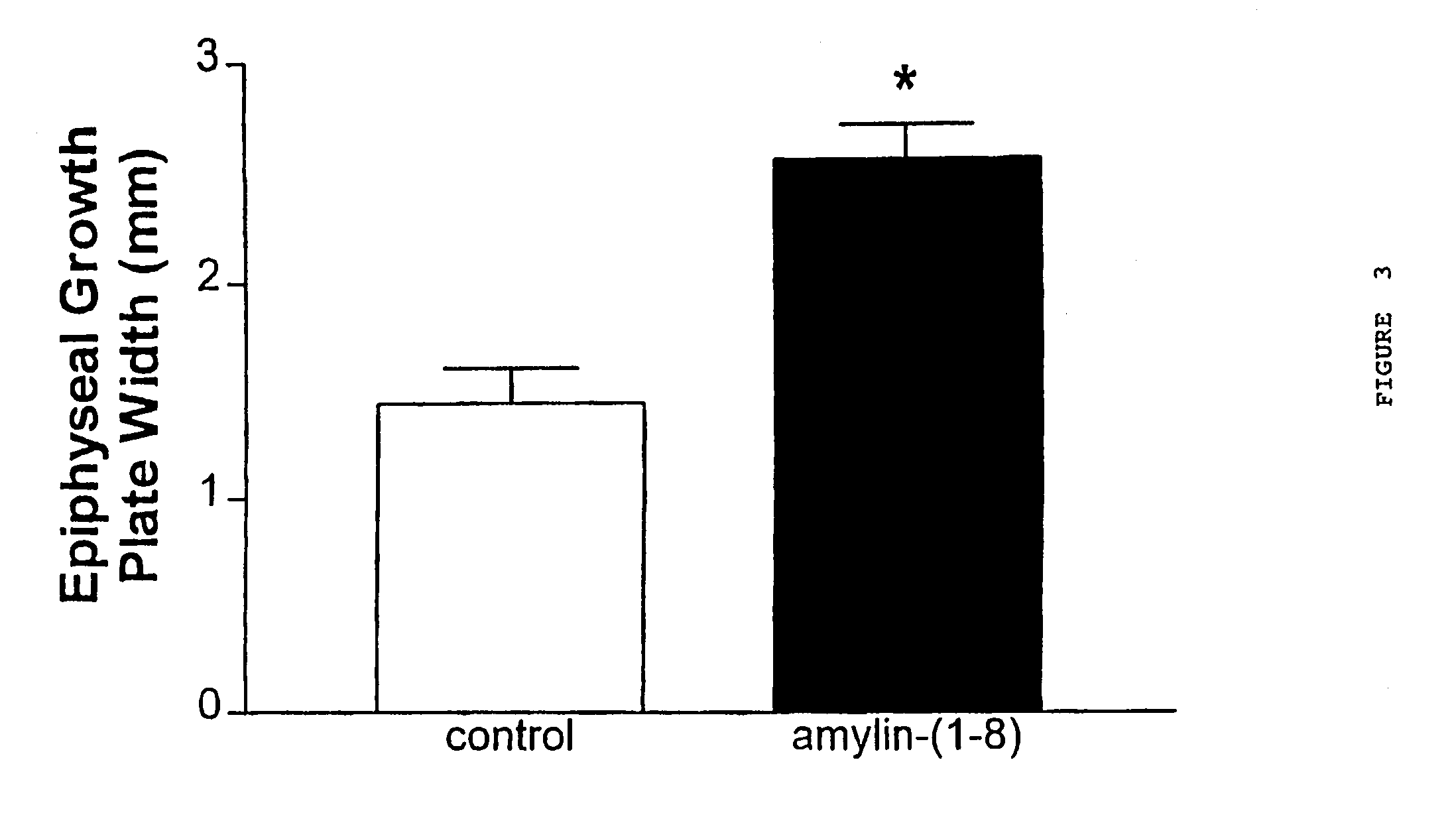
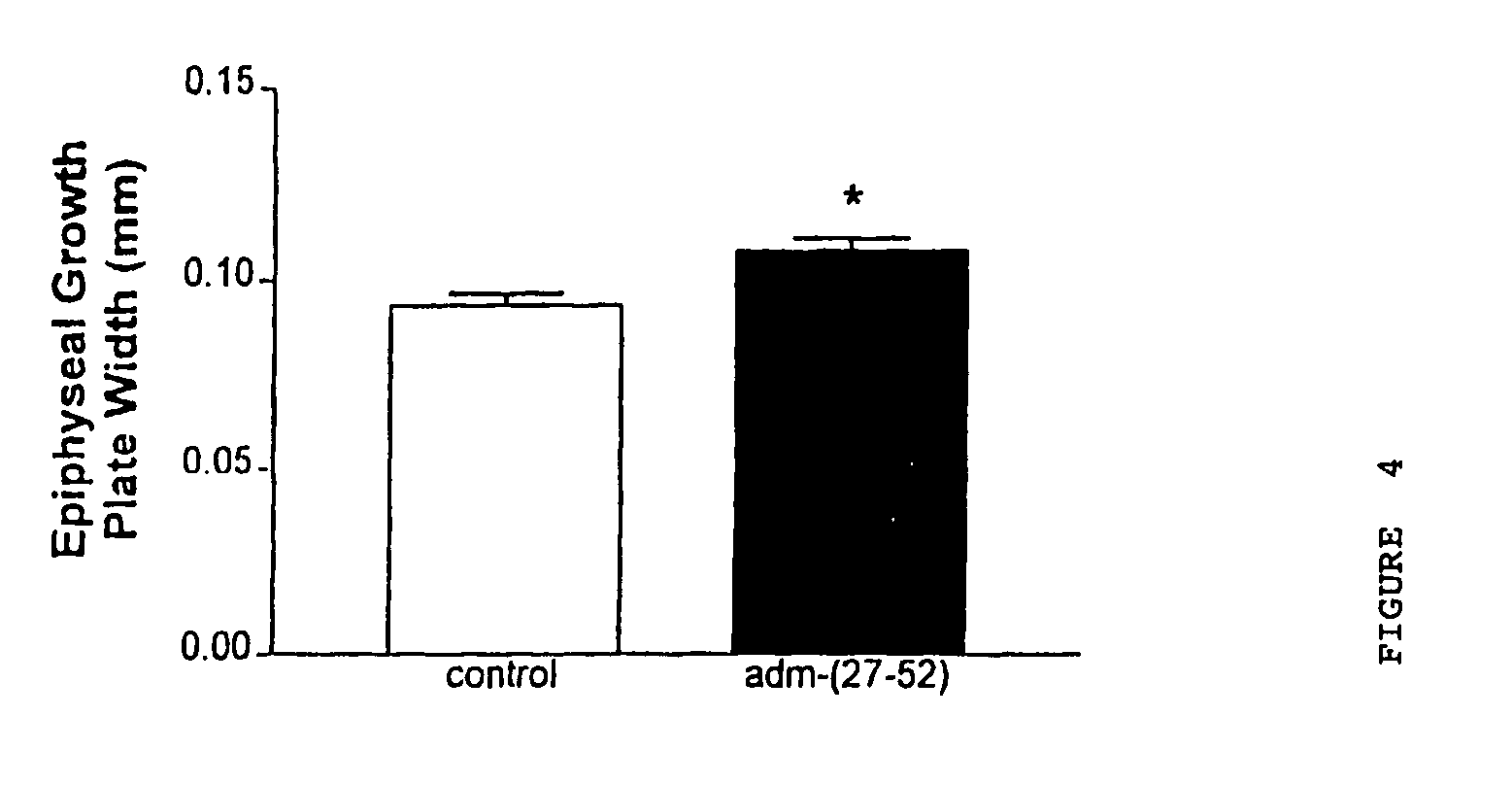
We claim a method of treating a patient to stimulate chondrocyte proliferation comprising administering to said patient an adrenomedullin analog that is adrenomedullin-(27–52) wherein said patient is suffering from cartilage disorders, and a method of stimulating chondrocyte proliferation comprising administering to chondrocyte cells adrenomedullin-(27–52).
Owner:AUCKLAND UNISERVICES LTD
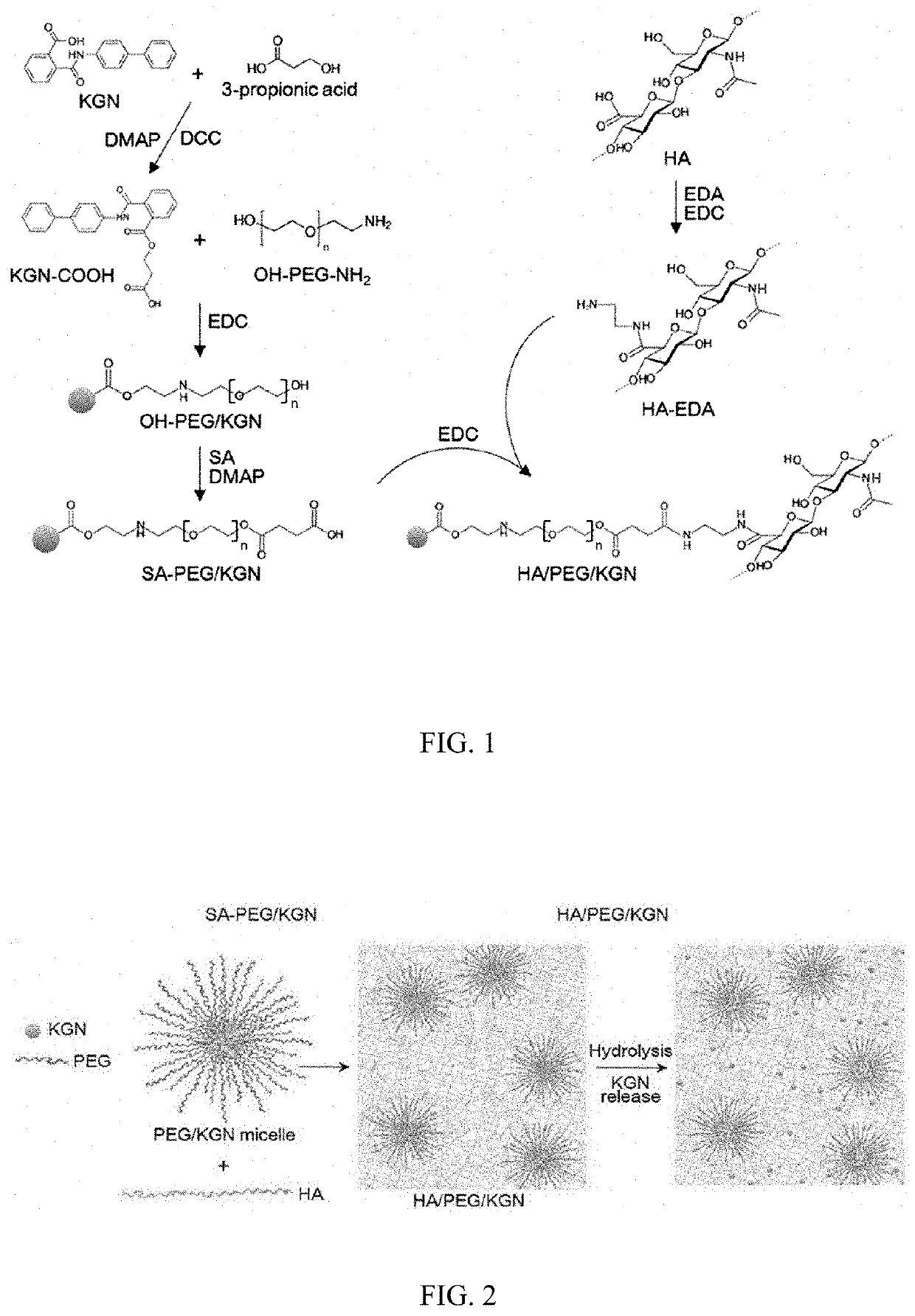
Kartogenin derivative-containing polymeric micelle, hyaluronic acid hydrogel, method for producing the same, and use thereof
InactiveUS20190388558A1Reduce failureFacilitate covalent bondingOrganic active ingredientsOintment deliveryDiseaseDegenerative arthritis
The present invention relates to a polymeric micelle including a kartogenin derivative, a hyaluronic acid hydrogel including the same, a method for producing the same, and a use thereof, and the polymeric micelle and the hyaluronic acid derivative hydrogel including the polymeric micelle slowly release kartogenin, and thus may be usefully used for the purpose of preventing or treating various cartilage disorder-related diseases such as degenerative arthritis because an effect of regenerating chondrocytes while protecting chondrocytes is excellent.
Owner:DONGGUK UNIV IND ACADEMIC COOPERATION FOUND
Fgf-18 formulation in xyloglucan gels
ActiveCN106029085APeptide/protein ingredientsInorganic non-active ingredientsCartilage injuryPharmaceutical formulation
The invention relates to the field of pharmaceutical formulations. More particularly it is directed to xyloglucan hydrogels comprising Fibroblast Growth Factor 18 (FGF-18) compound and to methods of producing such hydrogels. The hydrogels of the invention can be used, once formed in situ, for the treatment of cartilage disorders such as osteoarthritis or cartilage injury.
Owner:ARES TRADING SA
Injectable biocompatible composition
ActiveUS20100322993A1Ensures bioavailabilityAvoid tensionOrganic active ingredientsBiocideSerum protein albuminDrug release
The invention relates to an injectable biocompatible composition based on a polymeric support as well as to a method for producing it, which composition which comprises at least one hydrophilic polymer, wherein the polymer is polymerizable in situ to form a gel, and wherein the hydrophilic polymer is crosslinkable serum albumin or crosslinkable serum protein. The composition can be used in the restoration, the reconstruction, and / or the replacement of tissues and / or organs, or as a drug release implant in mammals. The composition is particularly suitable for treating cartilage disorders of a diseased or injured articular site in a mammal.
Owner:NMI NATURWISSENSCHAFTLICHES & MEDIZINISCHES INST AN DER UNIV TUBINGEN
Genetic markers for predicting responsiveness to FPG-18 compound
ActiveUS10221456B2Dose of FGF-1Reduce doseMicrobiological testing/measurementSkeletal disorderCartilage injuryMedicine
This application is directed to the use of biomarkers for predicting the sensitivity to treatment with an FGF-18 compound of a patient having a cartilage disorder, such as osteoarthritis, cartilage injury, fractures affecting joint cartilage or surgical procedures with impact on joint cartilage (e.g., microfracture), in order to reduce the risk of adverse events and increase the overall benefit after therapy.
Owner:MERCK PATENT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com