Compositions and methods for treating articular cartilage disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Use of IGF-I in a Model of Canine Osteoarthritis
[0065] A canine study was conducted to evaluate the efficacy and safety of intra-articular administration of recombinant human insulin-like growth factor I (rhIGF-I) and a sustained-release formulation of rhIGF-I, referred to as Depo IGF-I, in a model of canine osteoarthritis (OA).
[0066] Fifty-six dogs underwent surgical transection of the right anterior cruciate ligament by the method of Pond and Nuki (Ann. Rheum. Dis. 32 (1973):887-888). Such transection induces joint instability, leading to production of erosions in the articular cartilage similar to those seen with human osteoarthritis. The animals were premedicated with atropine (0.02 mg / kg, intramuscular (IM)) and acetylpromazine (0.2 mg / kg, IM) prior to induction of anesthesia. Animals were anesthetized with methohexital (7-12 mg / kg, intravenous (IV)), then intubated and maintained in anesthesia with isoflurane inhalant anesthetic delivered through a volume-regulated respirato...
example 2
IGF-I Stimulation of Proteoglycan in Cell Culture
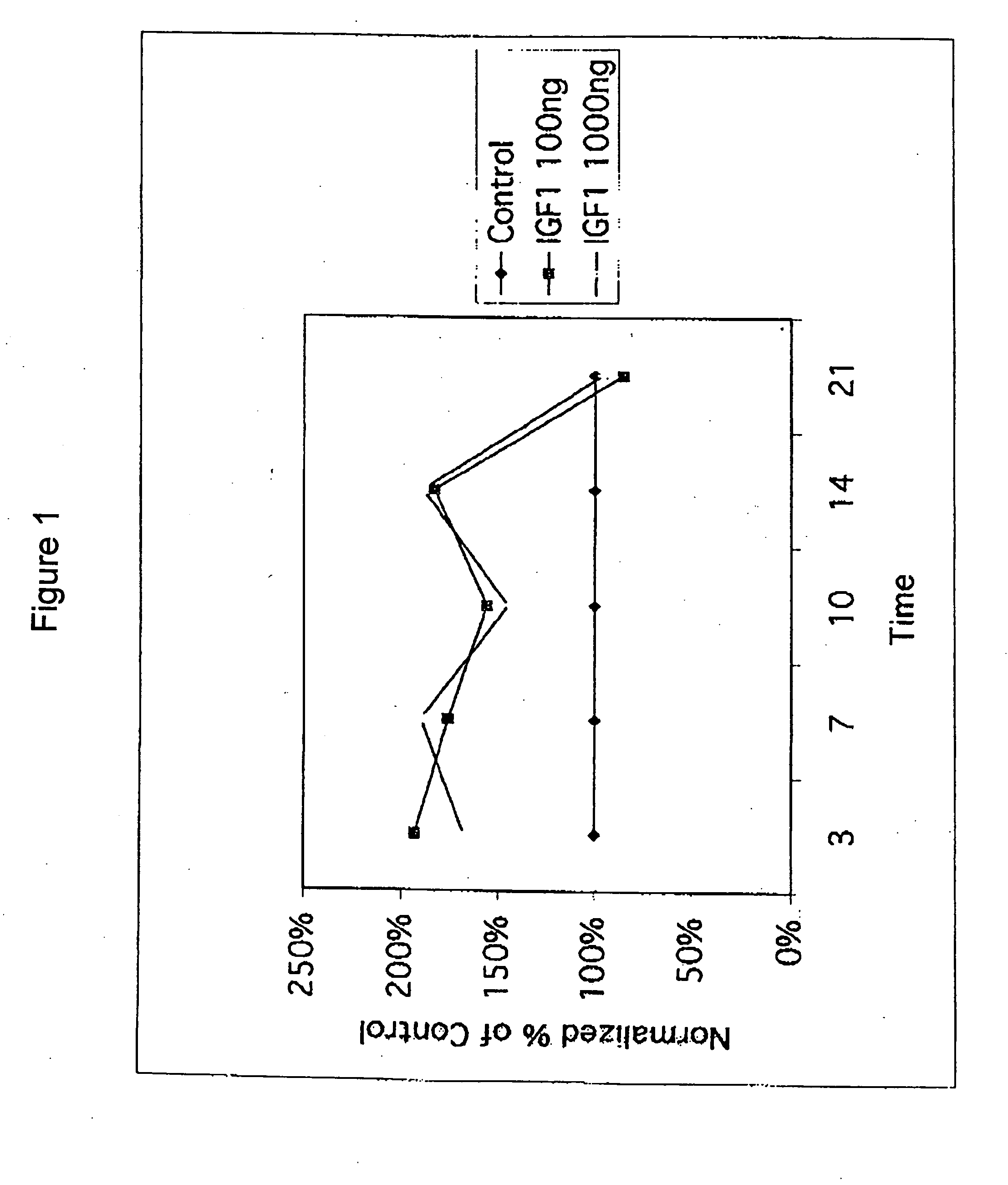
[0108] Chondrocytes were obtained from a human with osteoarthritis. Cells in suspension (alginate beads) were exposed to 100 ng / ml or 1,000 ng / ml rhIGF-I for 10 days. The IGF-I response (35S incorporation into proteoglycan) was assessed on days 3, 7, and 10. rhIGF-I was then removed from the media, and the IGF-I response was assessed again on days 14 and 21. Proteoglycan was measured in the media, the cell pellet, and in the alginate.
[0109] The subject's cells showed IGF-I stimulation of proteoglycan synthesis during the first 10 days as compared to the control cells, which were not exposed to rhIGF-I (FIG. 1). Further, chondrocytes continued to demonstrate enhanced proteoglycan synthesis from day 10 to day 14, four days after removal of IGF-I. These data provide additional evidence for the benefit of intermittent dosing in the treatment of osteoarthritis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com