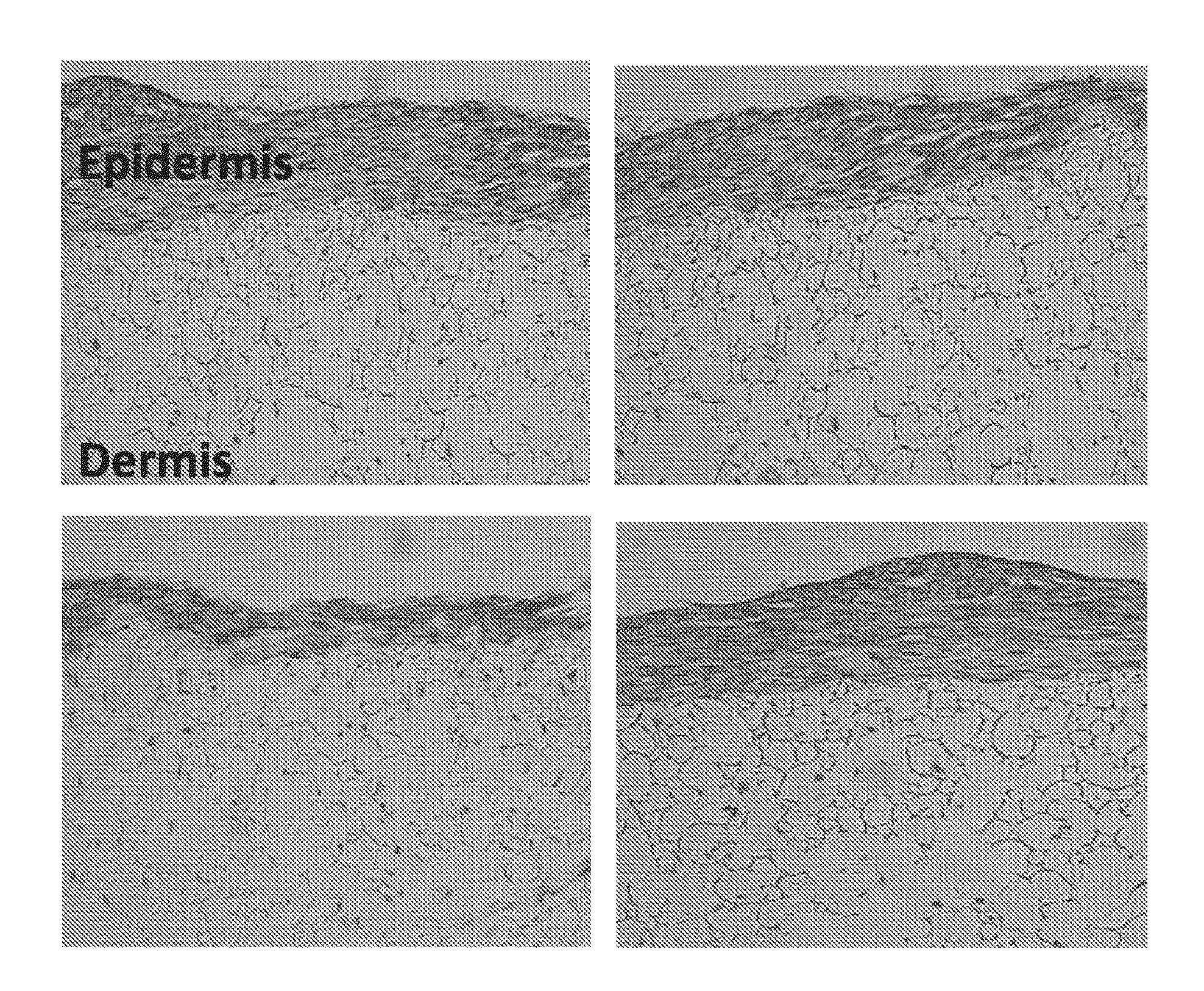
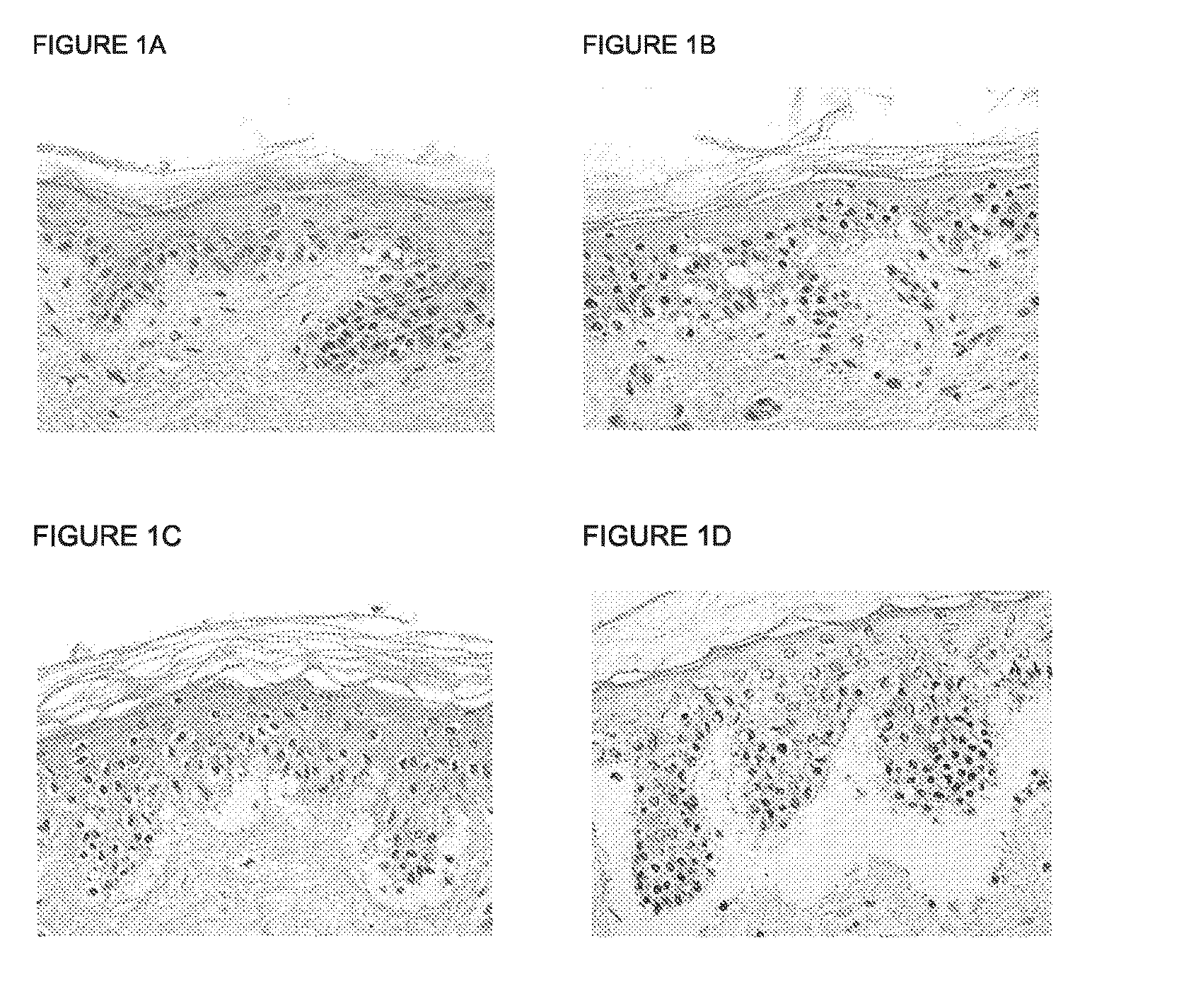
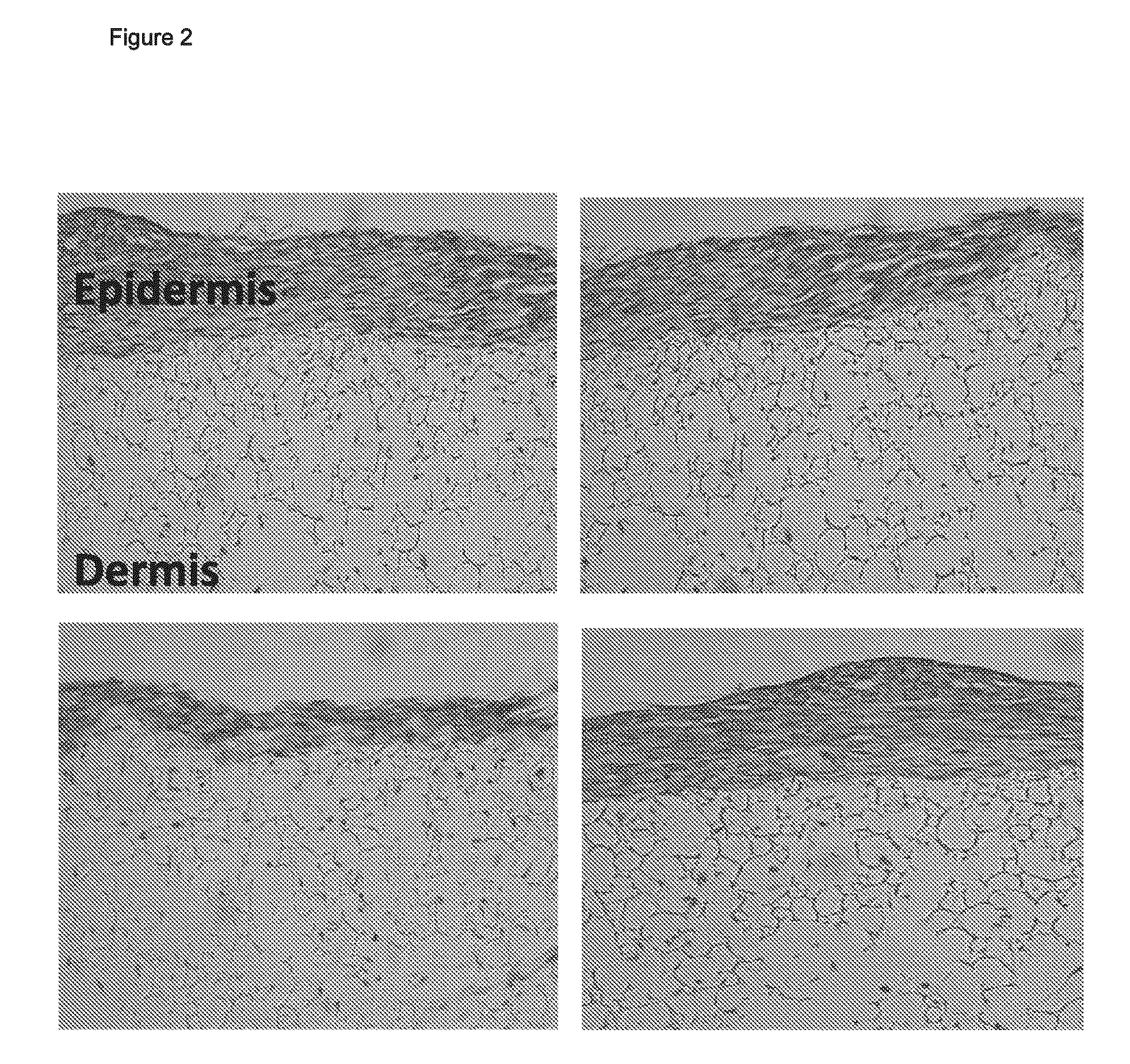
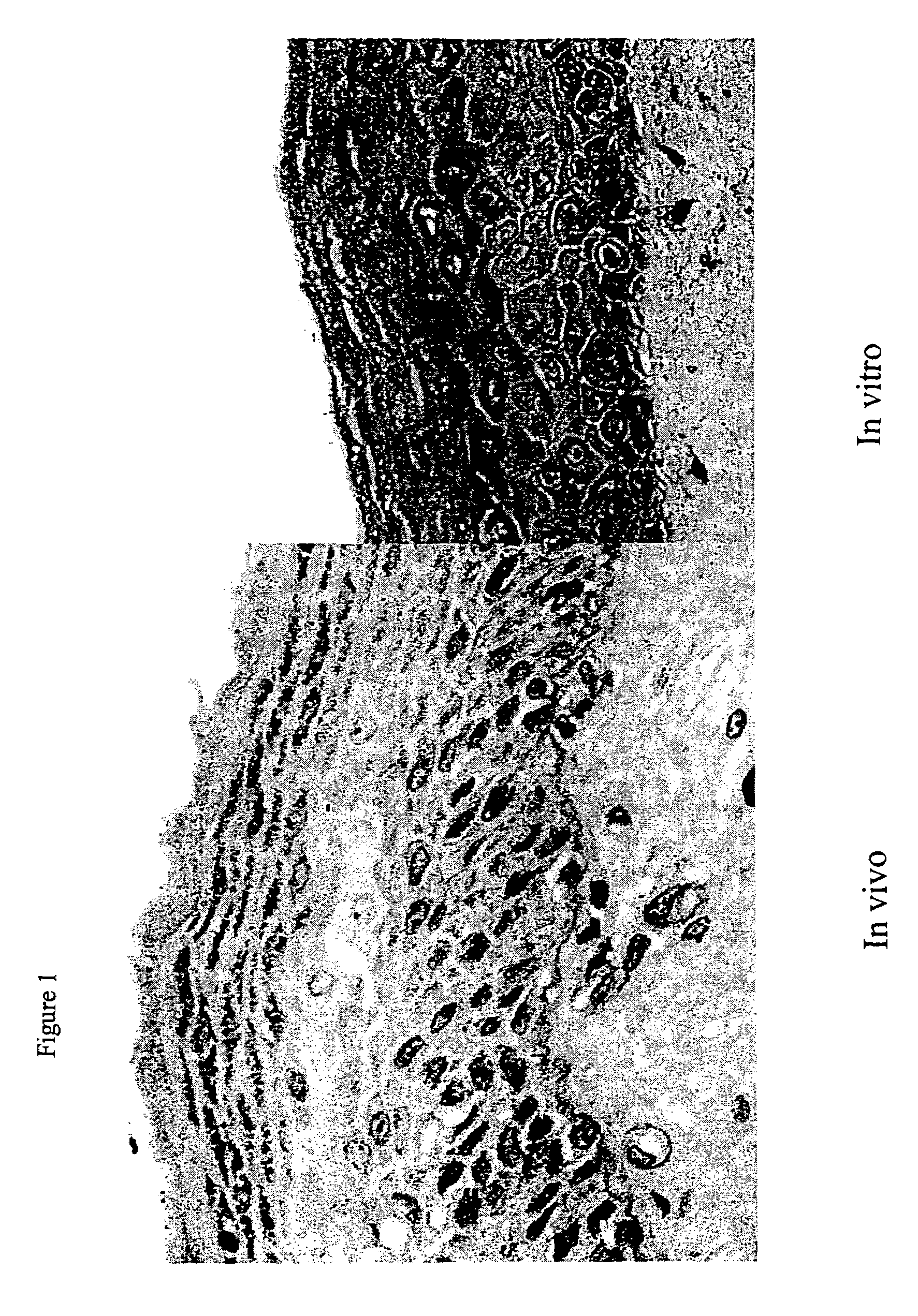
Patents
Literature
36 results about "Skin equivalent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A skin equivalent is an in vitro skin model using to conduct experiments on processes involving the skin, such as wound healing and keratinocyte migration. It is a more complex form of the dermal equivalent.
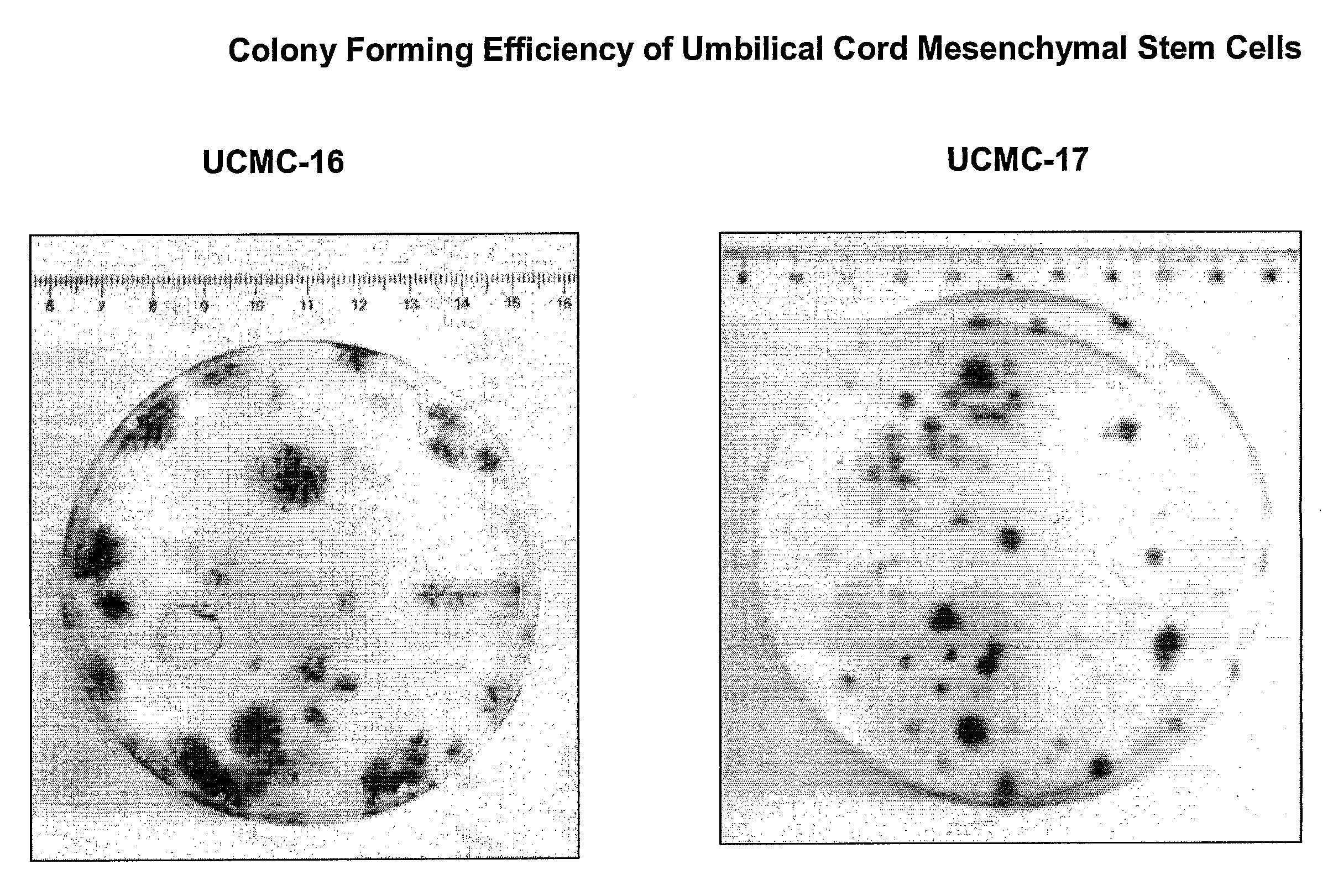
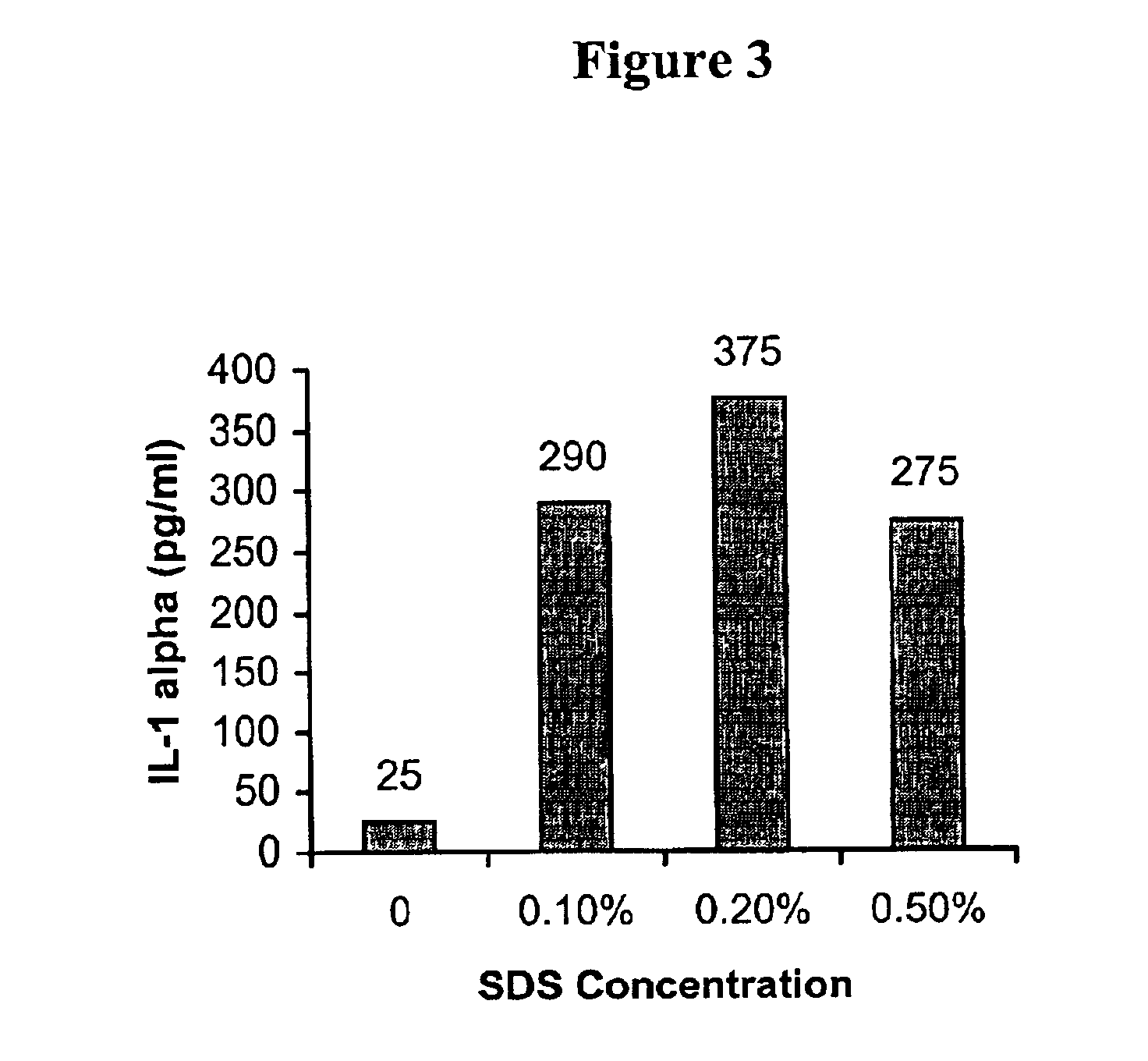
Isolation and Cultivation of Stem/Progenitor Cells From the Amniotic Membrane of Umbilical Cord and Uses of Cells Differentiated Therefrom
The present invention relates to a skin equivalent and a method for producing the same, wherein the skin equivalent comprises a scaffold and stem / progenitor cells isolated from the amniotic membrane of umbilical cord. These stem / progenitor cells may be mesenchymal (UCMC) and / or epithelial (UCEC) stem cells, which may then be further differentiated to fibroblast and keratinocytes. Further described is a method for isolating stem / progenitor cells from the amniotic membrane of umbilical cord, wherein the method comprises separating the amniotic membrane from the other components of the umbilical cord in vitro, culturing the amniotic membrane tissue under conditions allowing cell proliferation, and isolating the stem / progenitor cells from the tissue cultures. The invention also refers to therapeutic uses of these skin equivalents. Another aspect of the invention relates to the generation of a mucin-producing cell using stem / progenitor cells obtained from the amniotic membrane of umbilical cord and therapeutic uses of such mucin-producing cells. In yet another aspect, the invention relates to a method for generating an insulin-producing cell using stem / progenitor cells isolated from the amniotic membrane of umbilical cord and therapeutic uses thereof. The invention further refers to a method of treating a bone or cartilage disorder using UCMC. Furthermore, the invention refers to a method of generating a dopamin and tyrosin hydroxylase as well as a HLA-G and hepatocytes using UCMC and / or UCEC. The present invention also refers to a method of inducing proliferation of aged keratinocytes using UCMC.
Owner:CELLRESEARCH CORP PTE LTD
Skin substitutes and uses thereof
The present invention relates to in vitro cultured skin substitutes, and in particular to improved methods for organotypic culture of skin substitutes. In some embodiments, the dermal equivalent of the skin substitute is lifted to air interface of the culture prior to seeding with keratinocytes. In other embodiments, increased concentrations of collagen are used to form the dermal equivalent. In still other embodiments, optimized media are utilized to maintain the skin equivalents.
Owner:STRATATECH
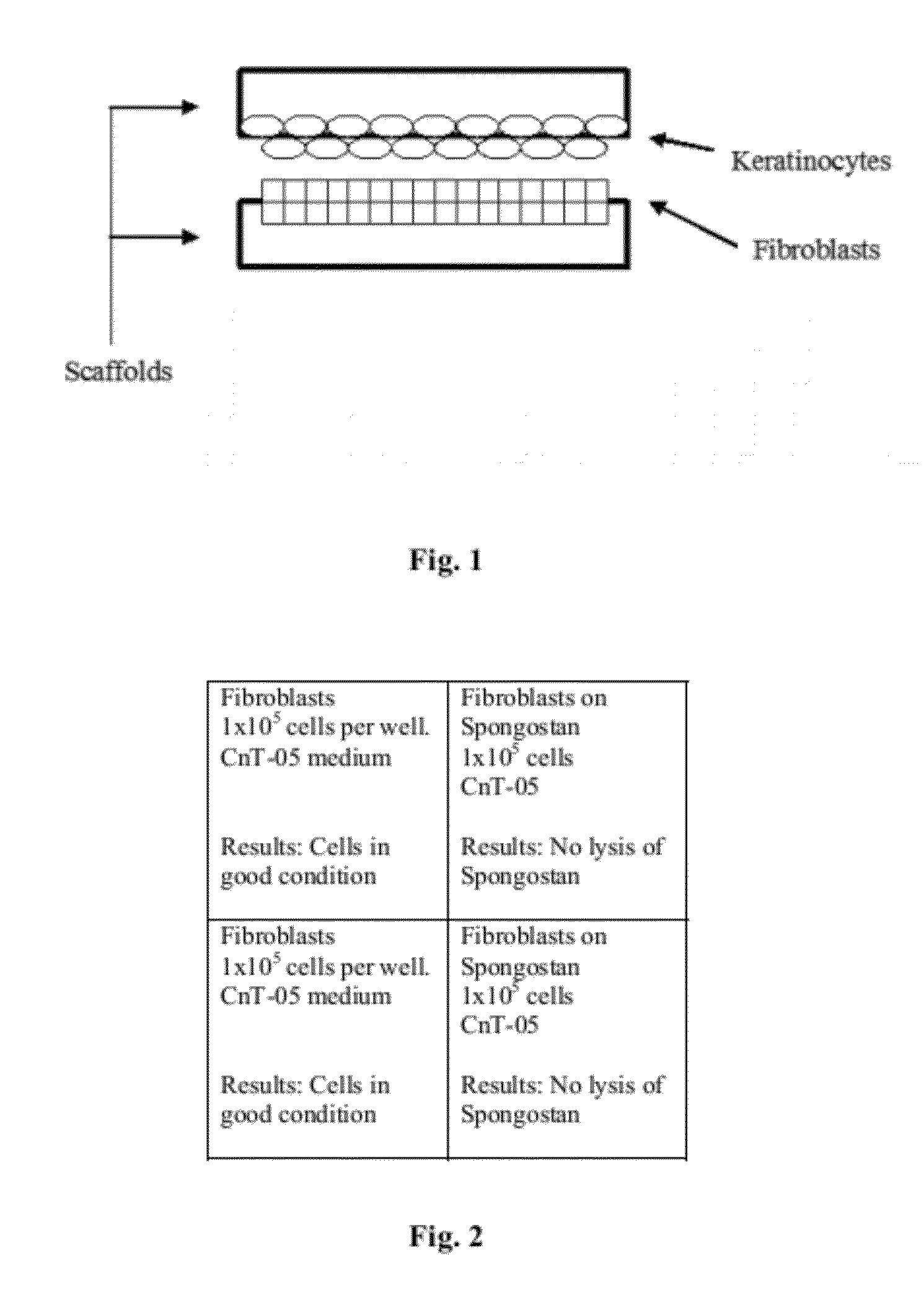
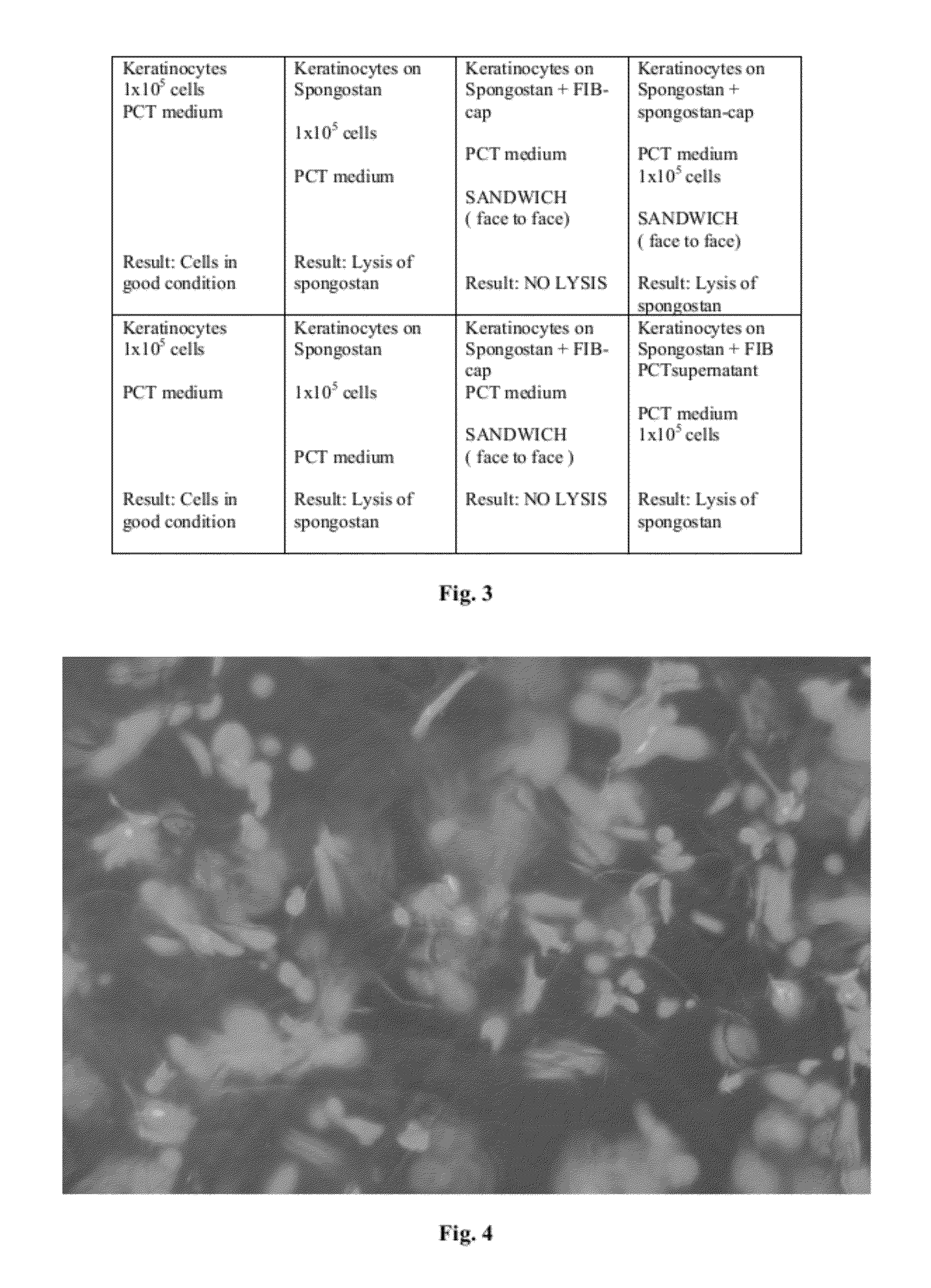
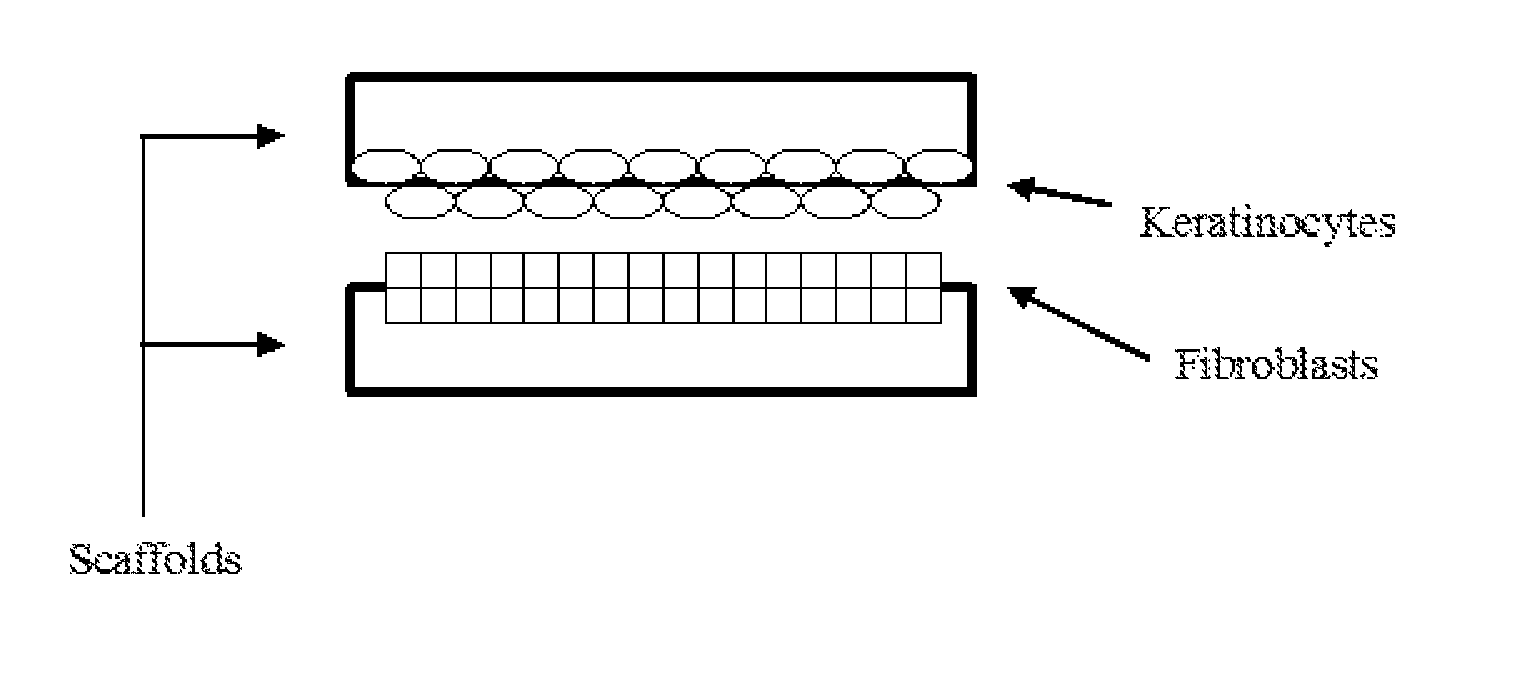
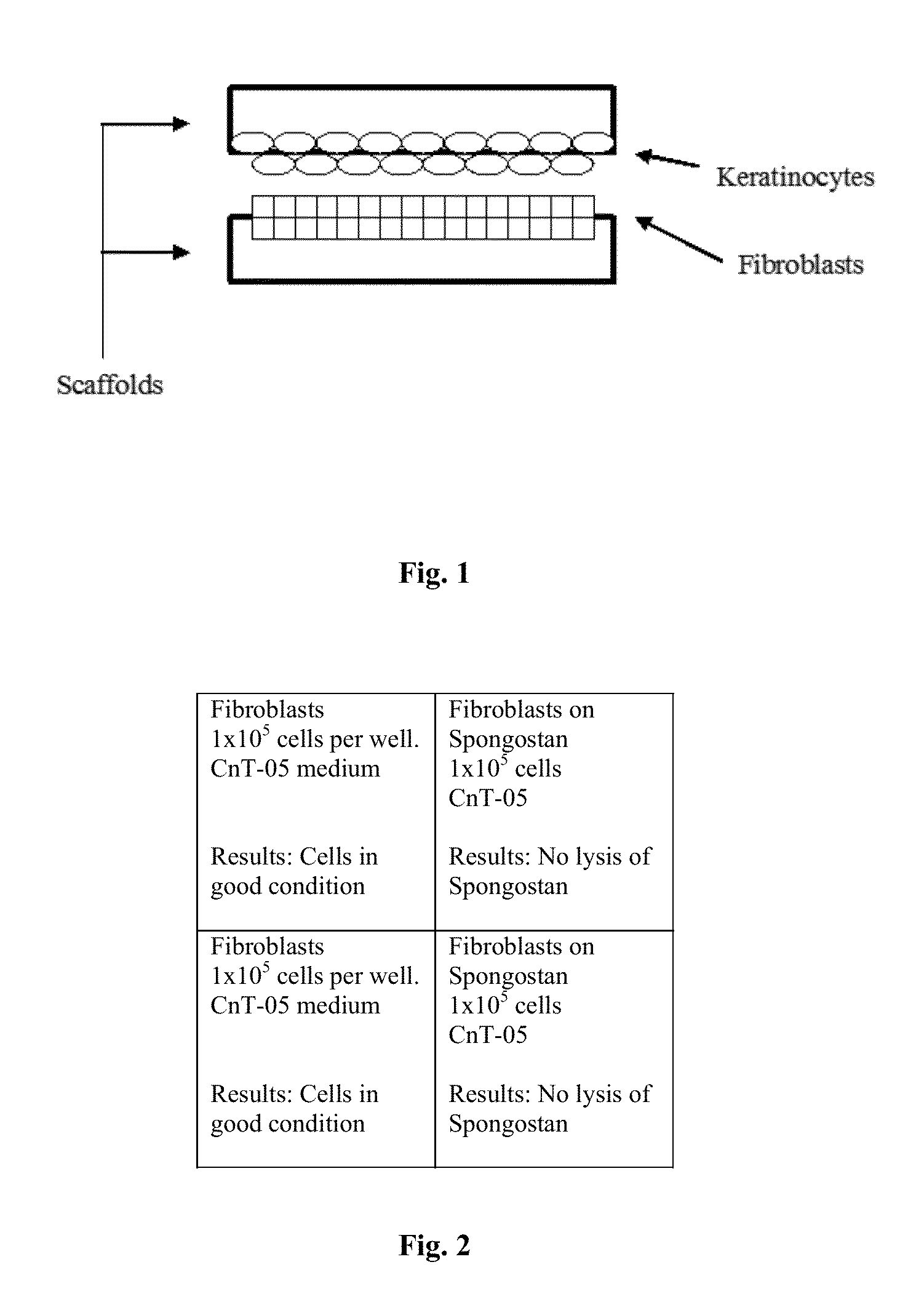
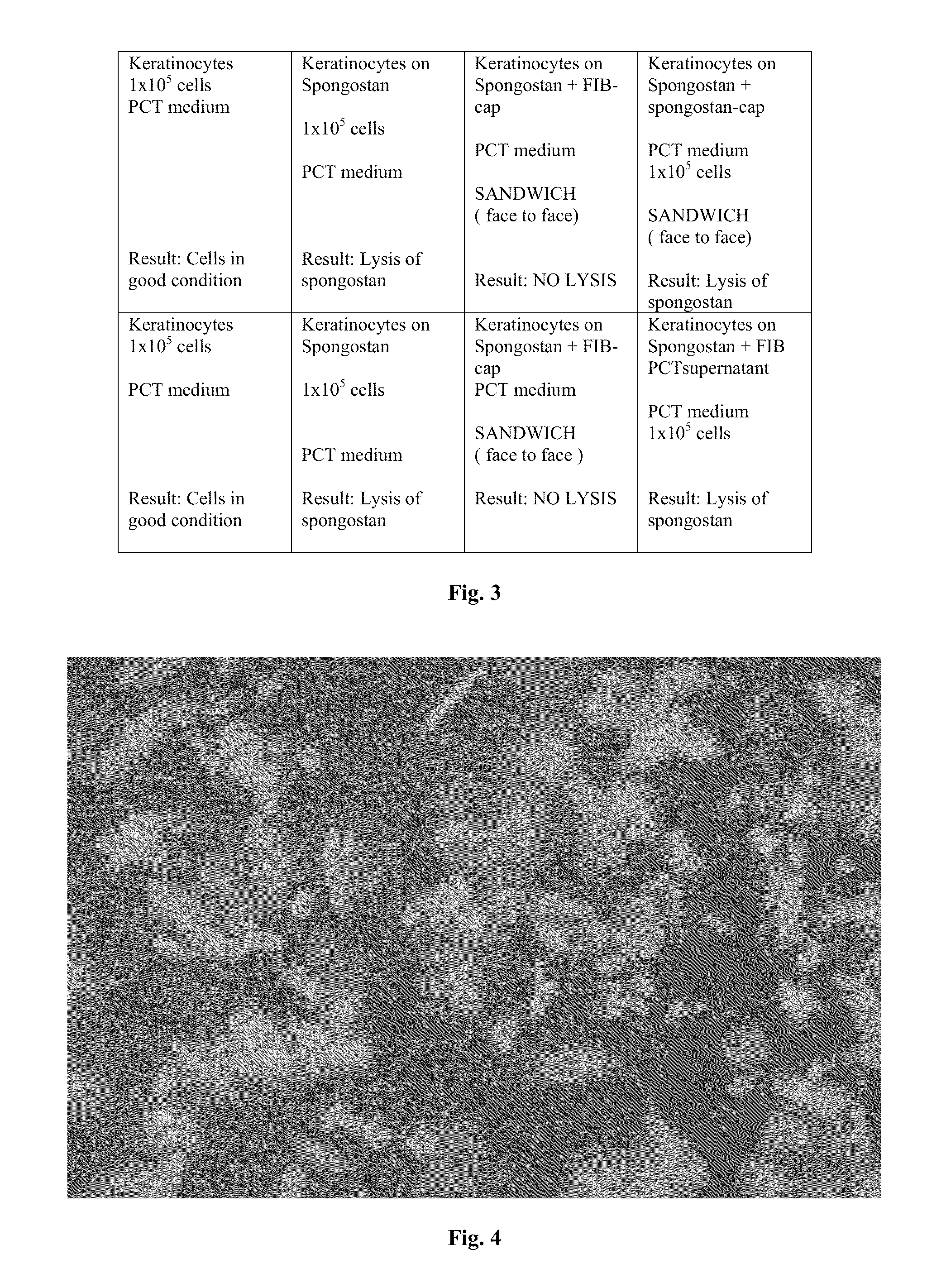
Cellular scaffold
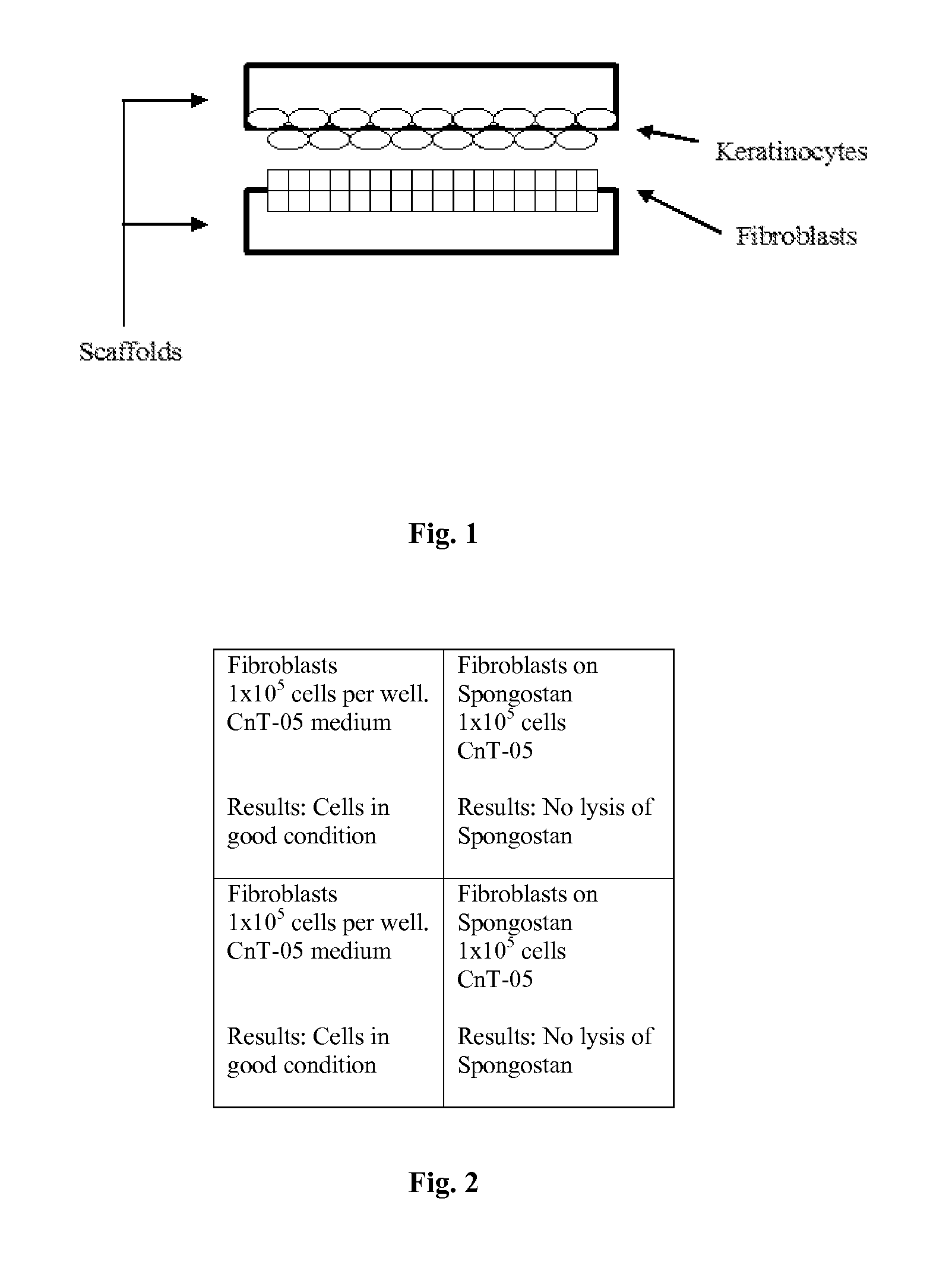
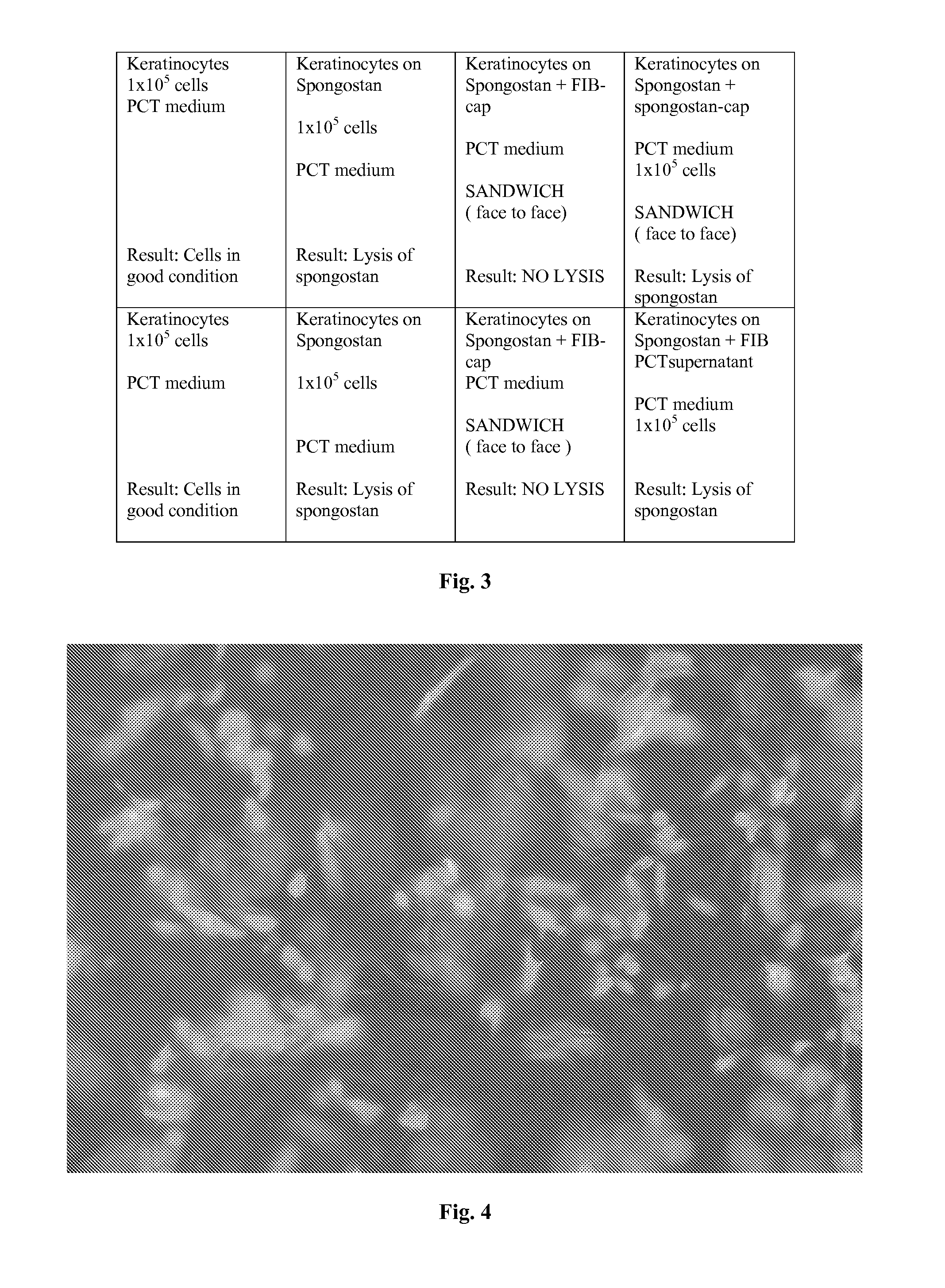
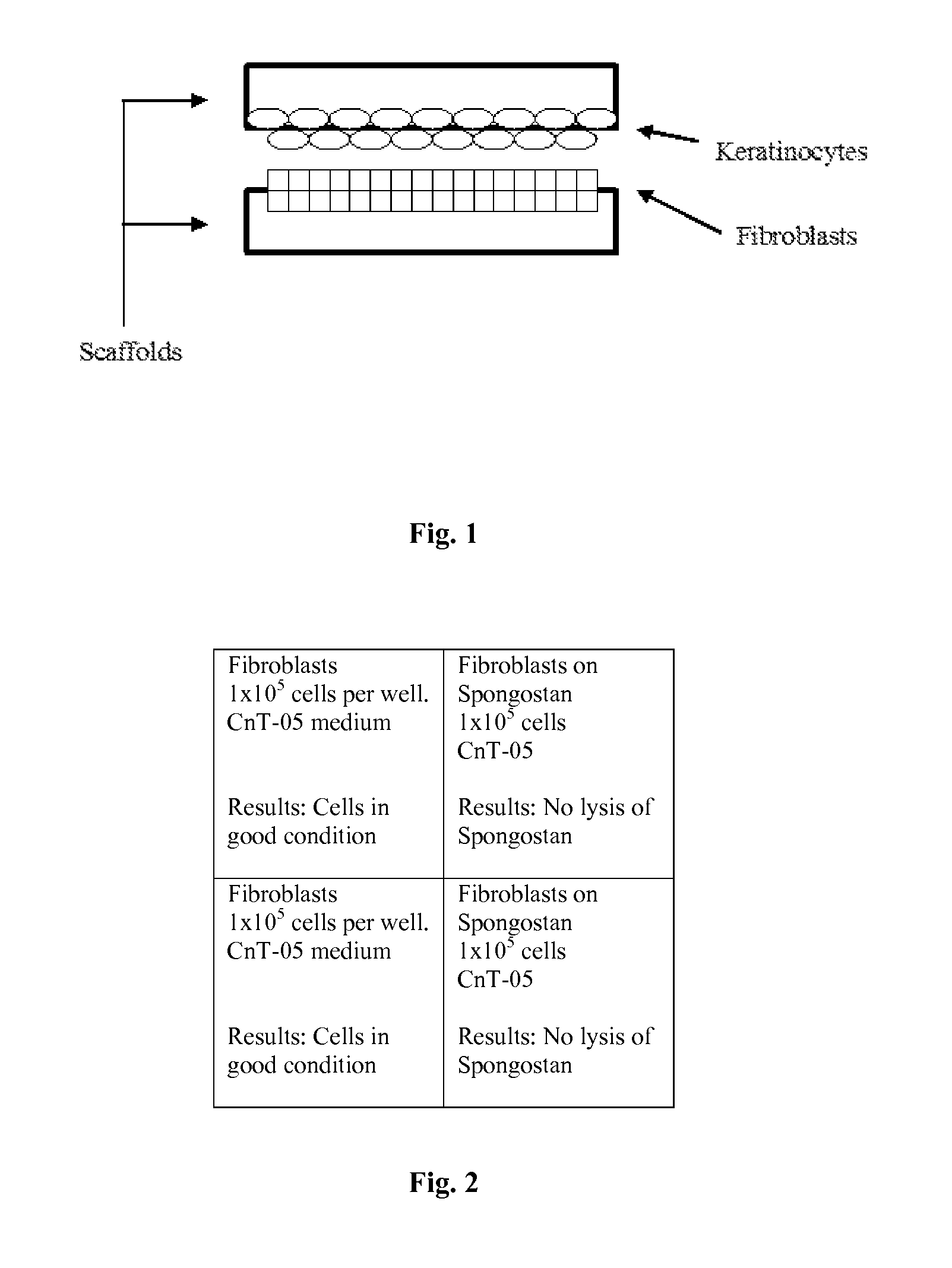
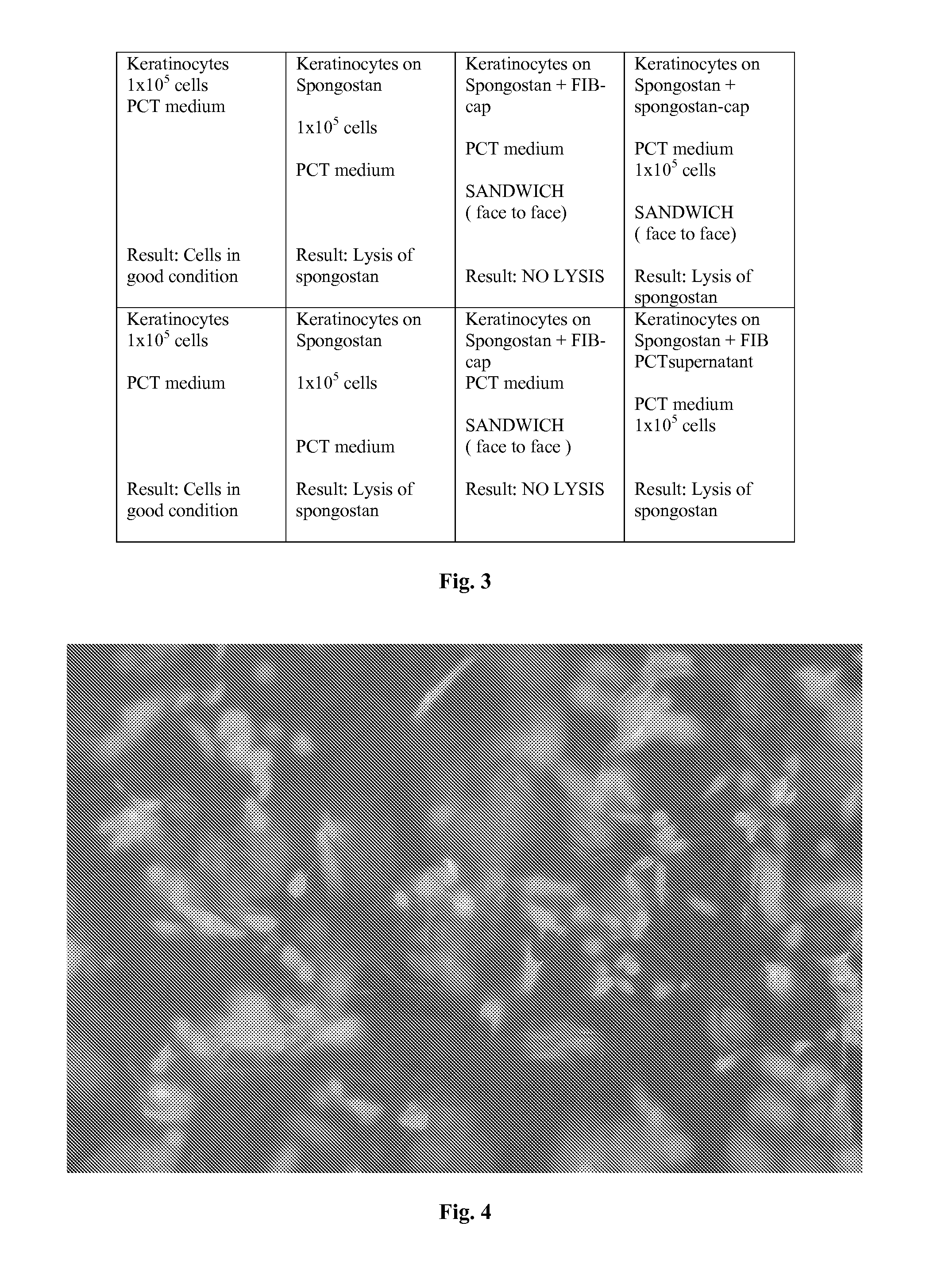
A cellular scaffold that is suitable for tissue regeneration, cell culture and in vitro assays. The invention relates to a layered cell scaffold that is seeded with mesenchymal and ectodermal cells. The layered cellular scaffold comprises an inoculum of mesenchymal cells and ectodermal cells positioned between two opposing scaffolds in a sandwich configuration. The layered cell scaffold provides a functional skin equivalent that is suitable for transplantation and in vitro cell-based assays.
Owner:STEMEDICA CELL TECH
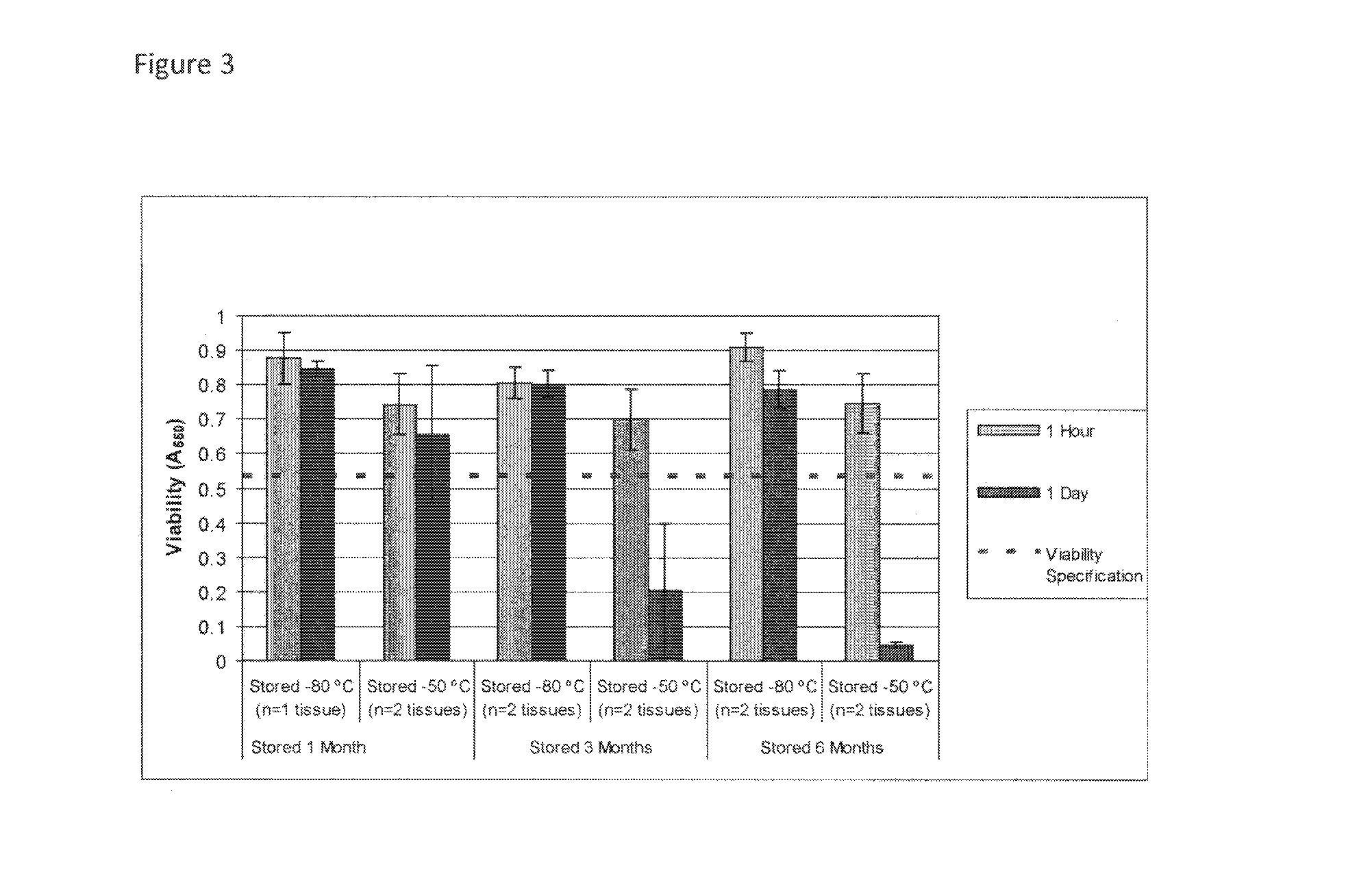
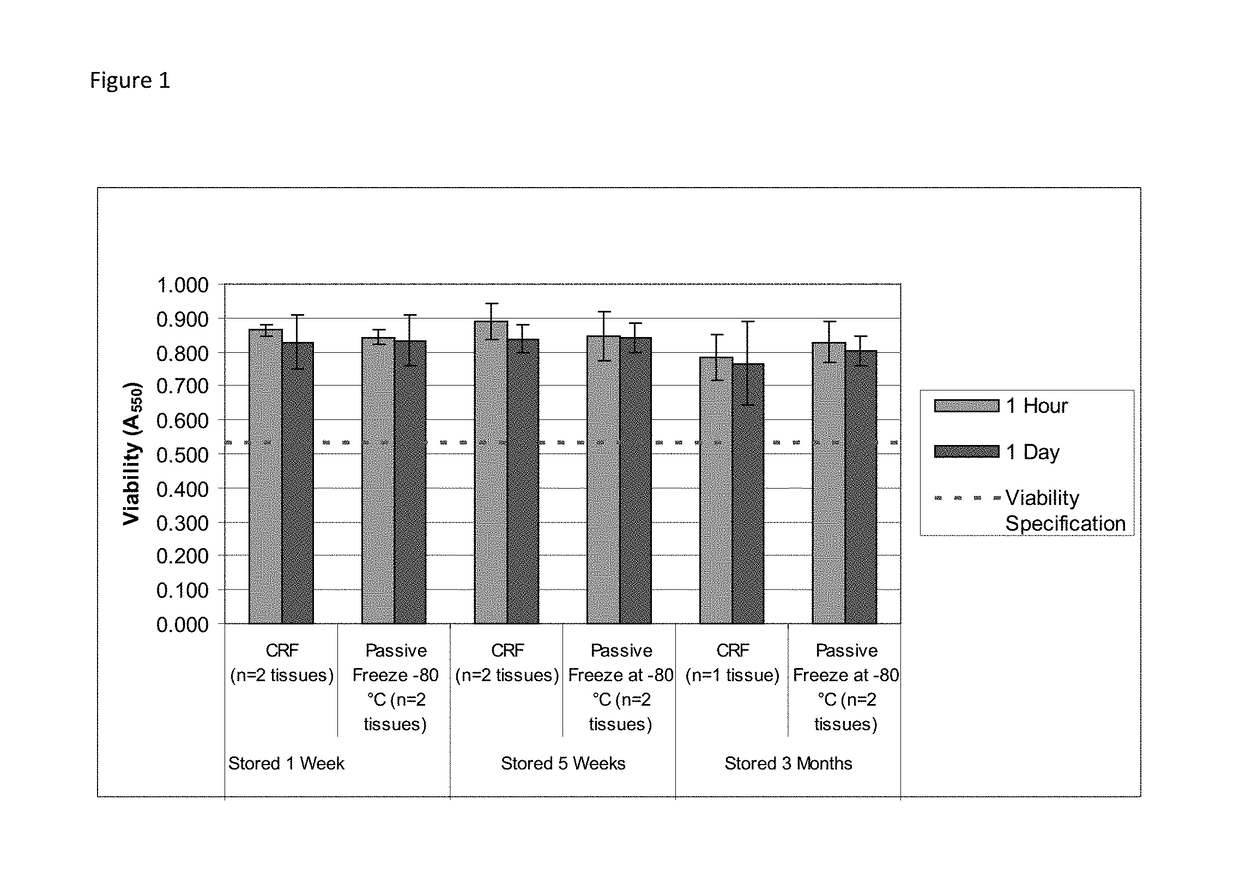
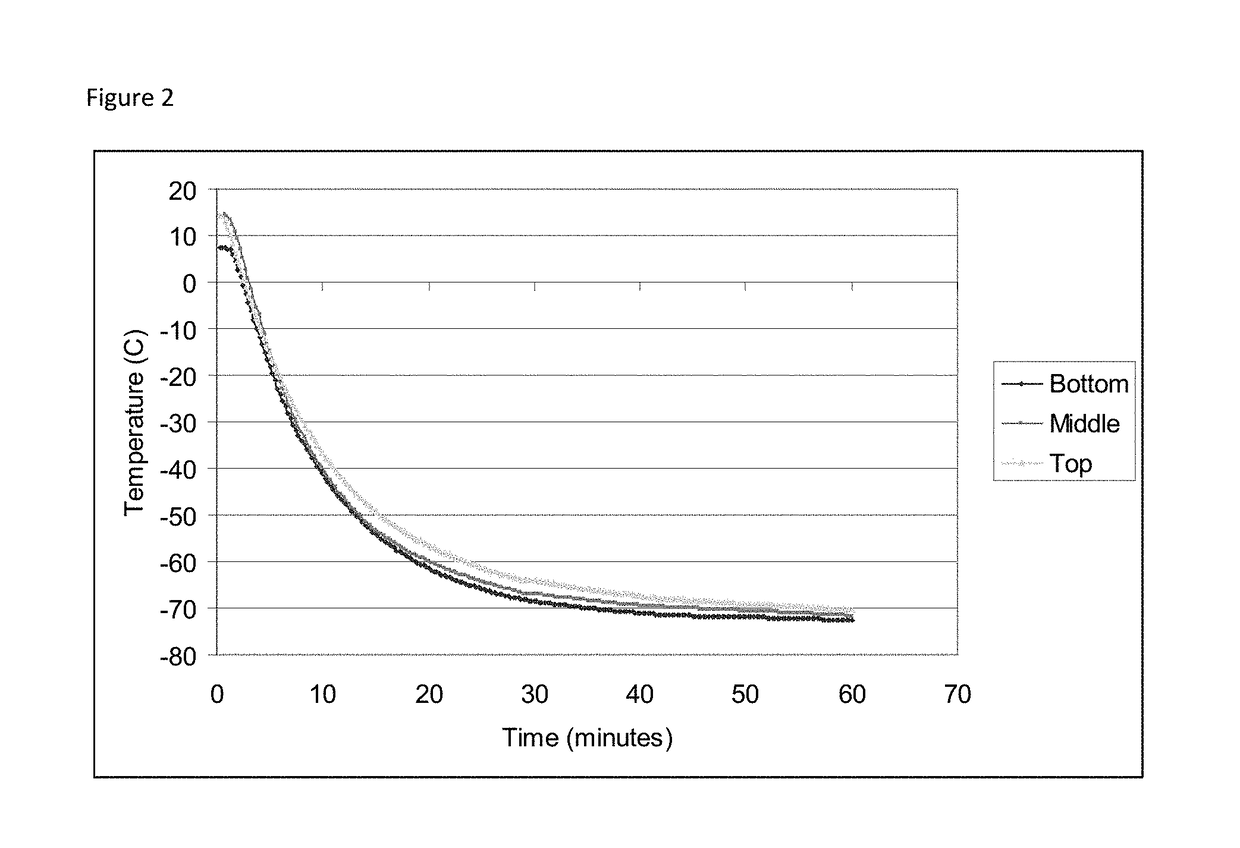
Cryopreservation of viable human skin substitutes
ActiveUS20140271583A1Simple methodBiocideGenetic material ingredientsSkin equivalentCryopreservation
Owner:STRATATECH
Skin equivalents derived from umbilical cord mesenchymal stem/progenitor cells and umbilical cord epithelial stem/progenitor cells
The present invention relates to a skin equivalent and a method for producing the same, wherein the skin equivalent comprises a scaffold and stem / progenitor cells isolated from the amniotic membrane of umbilical cord. These stem / progenitor cells may be mesenchymal (UCMC) and / or epithelial (UCEC) stem cells, which may then be further differentiated to fibroblast and keratinocytes. Further described is a method for isolating stem / progenitor cells from the amniotic membrane of umbilical cord, wherein the method comprises separating the amniotic membrane from the other components of the umbilical cord in vitro, culturing the amniotic membrane tissue under conditions allowing cell proliferation, and isolating the stem / progenitor cells from the tissue cultures. The invention also refers to therapeutic uses of these skin equivalents. Another aspect of the invention relates to the generation of a mucin-producing cell using stem / progenitor cells obtained from the amniotic membrane of umbilical cord and therapeutic uses of such mucin-producing cells. In yet another aspect, the invention relates to a method for generating an insulin-producing cell using stem / progenitor cells isolated from the amniotic membrane of umbilical cord and therapeutic uses thereof. The invention further refers to a method of treating a bone or cartilage disorder using UCMC. Furthermore, the invention refers to a method of generating a dopamin and tyrosin hydroxylase as well as a HLA-G and hepatocytes using UCMC and / or UCEC. The present invention also refers to a method of inducing proliferation of aged keratinocytes using UCMC.
Owner:CELLRESEARCH CORP PTE LTD
Vascularized human skin equivalent
InactiveUS20070207125A1Accelerated rate of vascularizationImprove clinical utilityBiocideEpidermal cells/skin cellsSkin equivalentIn vivo
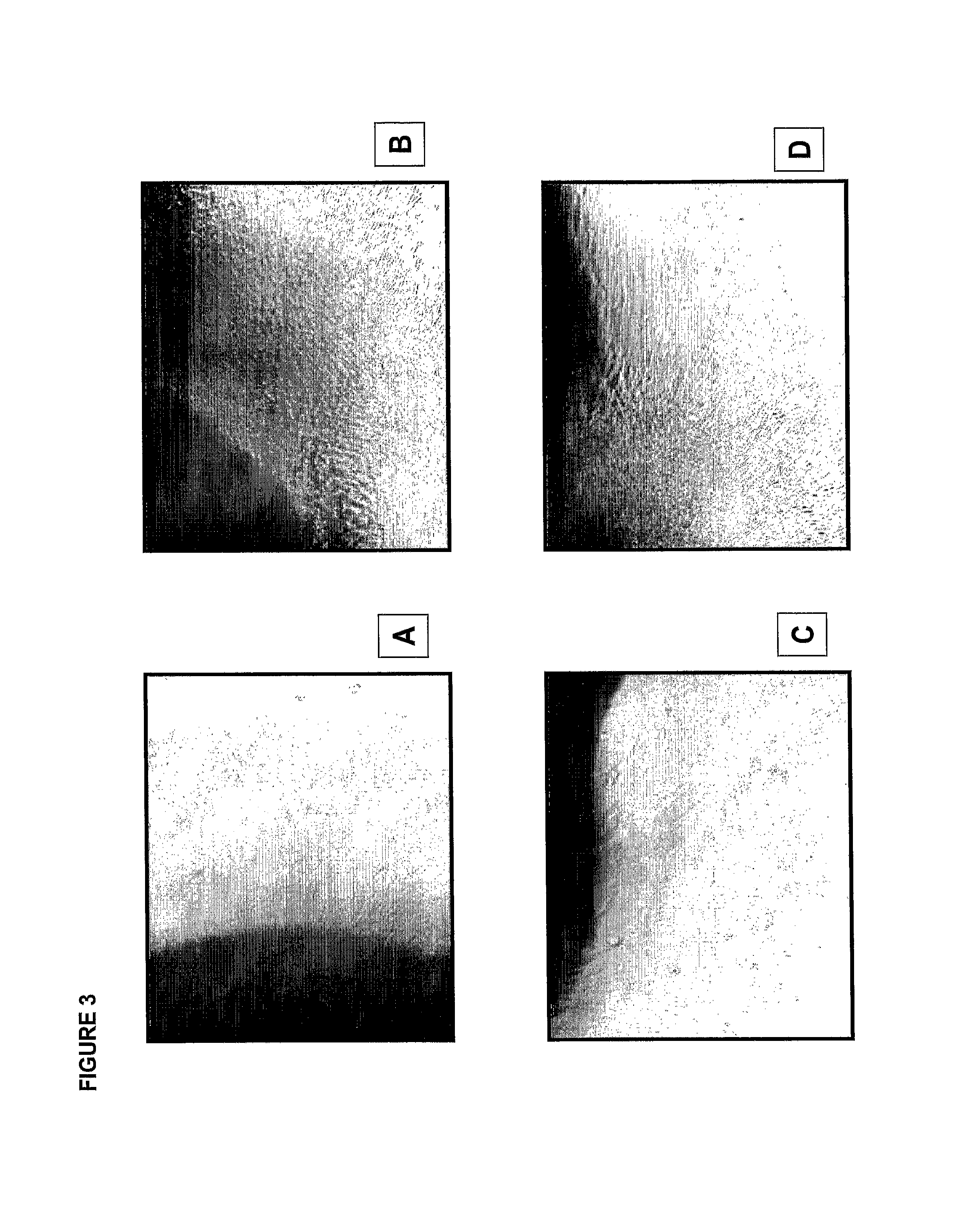
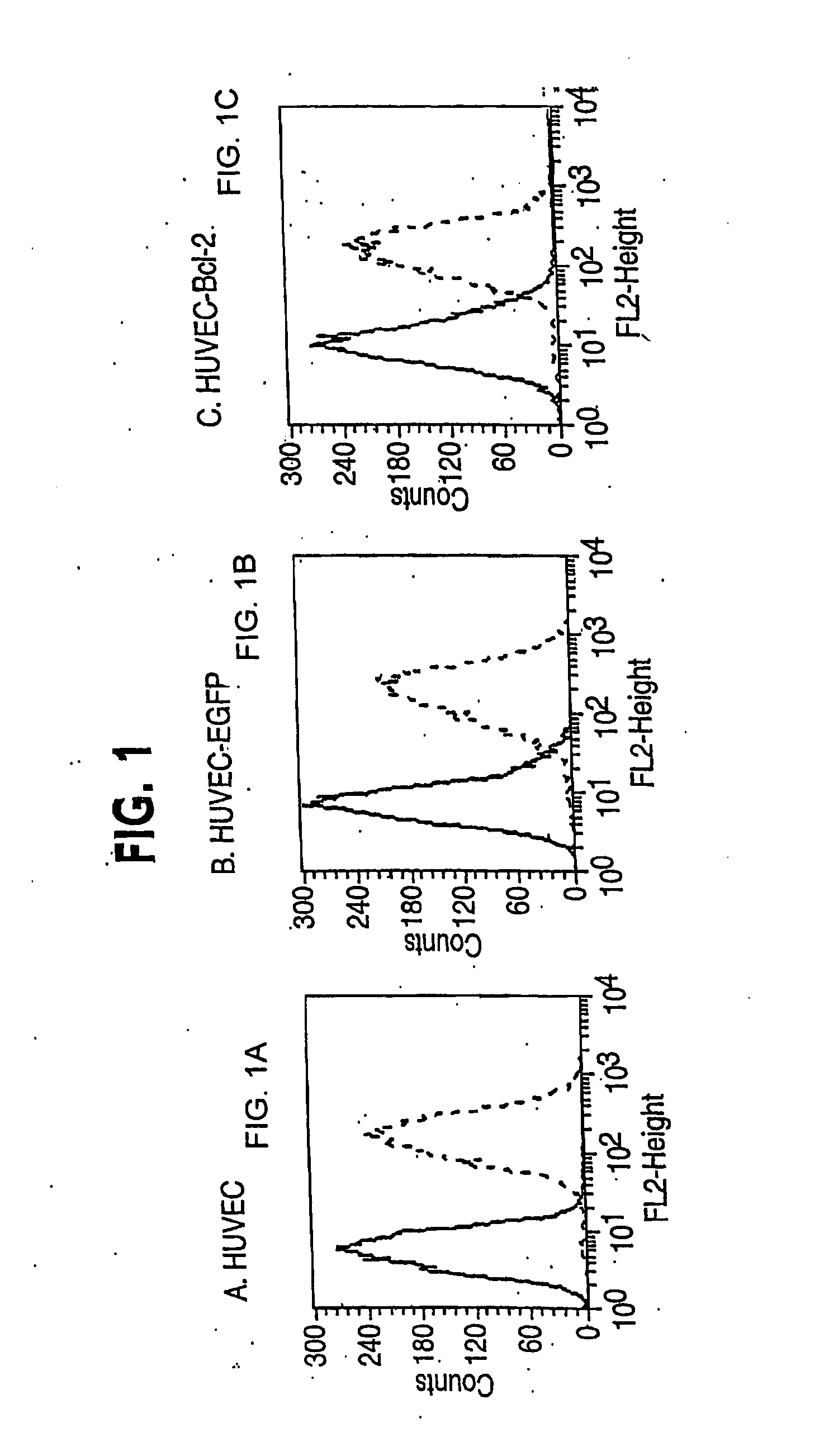
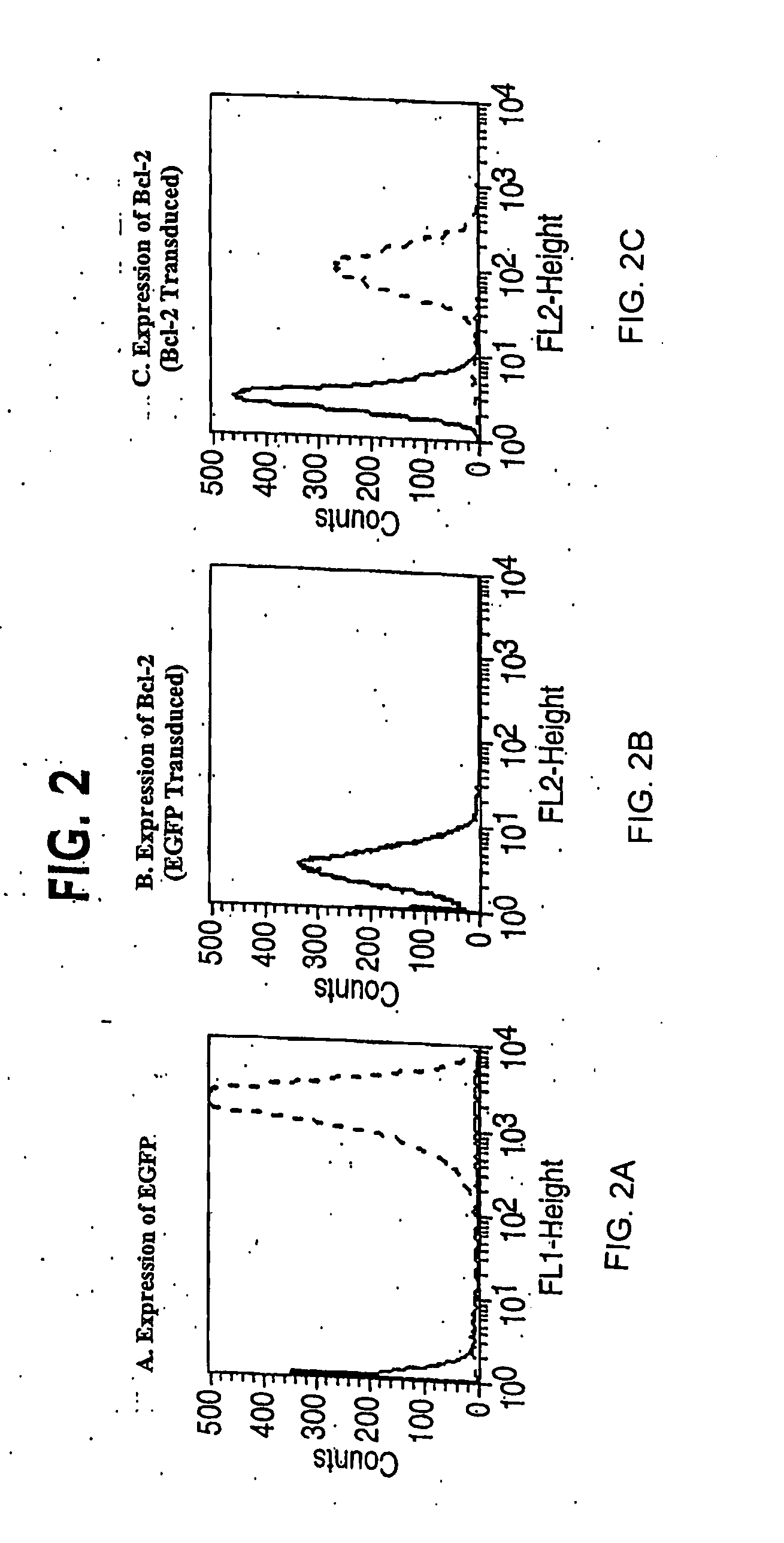
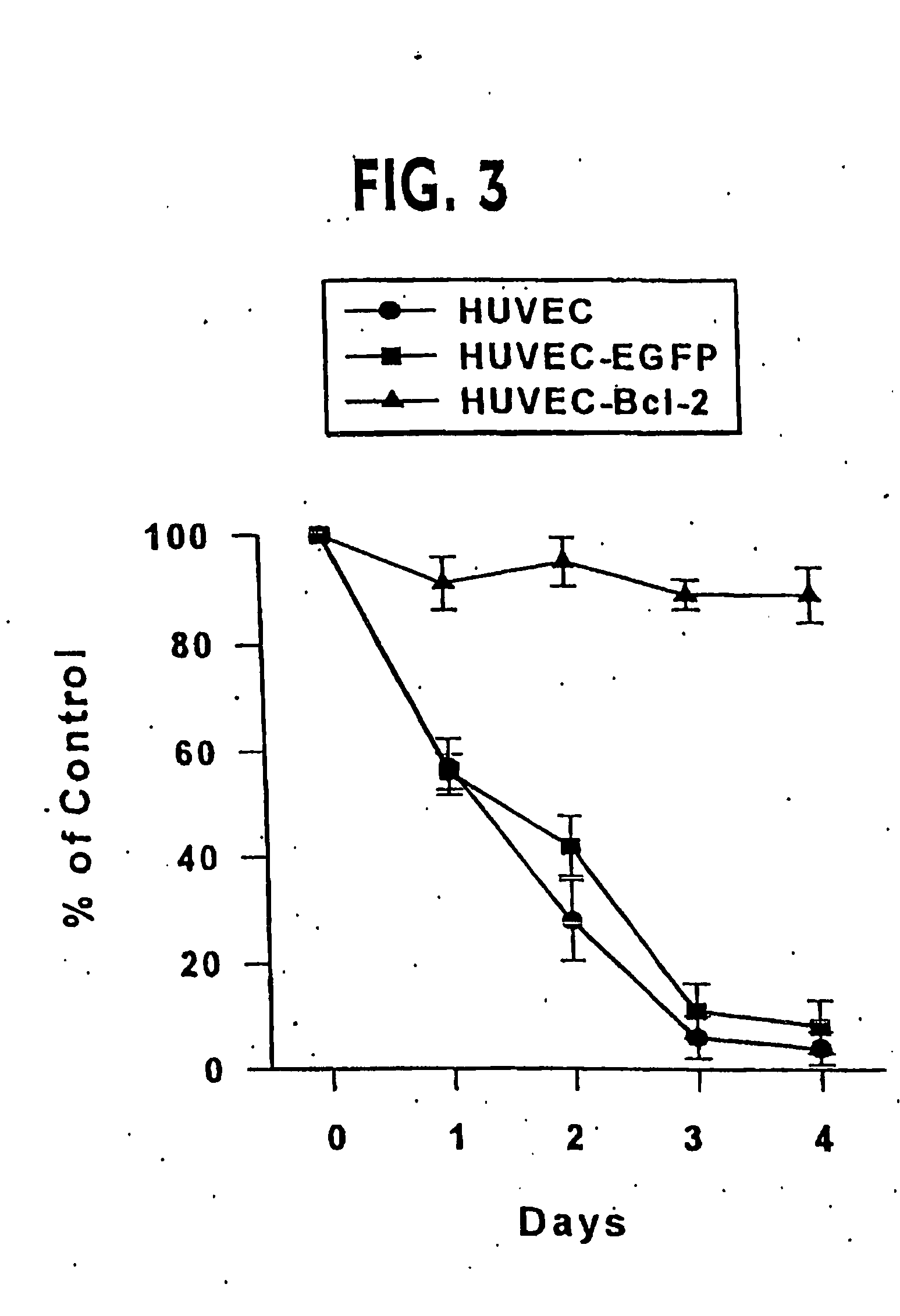
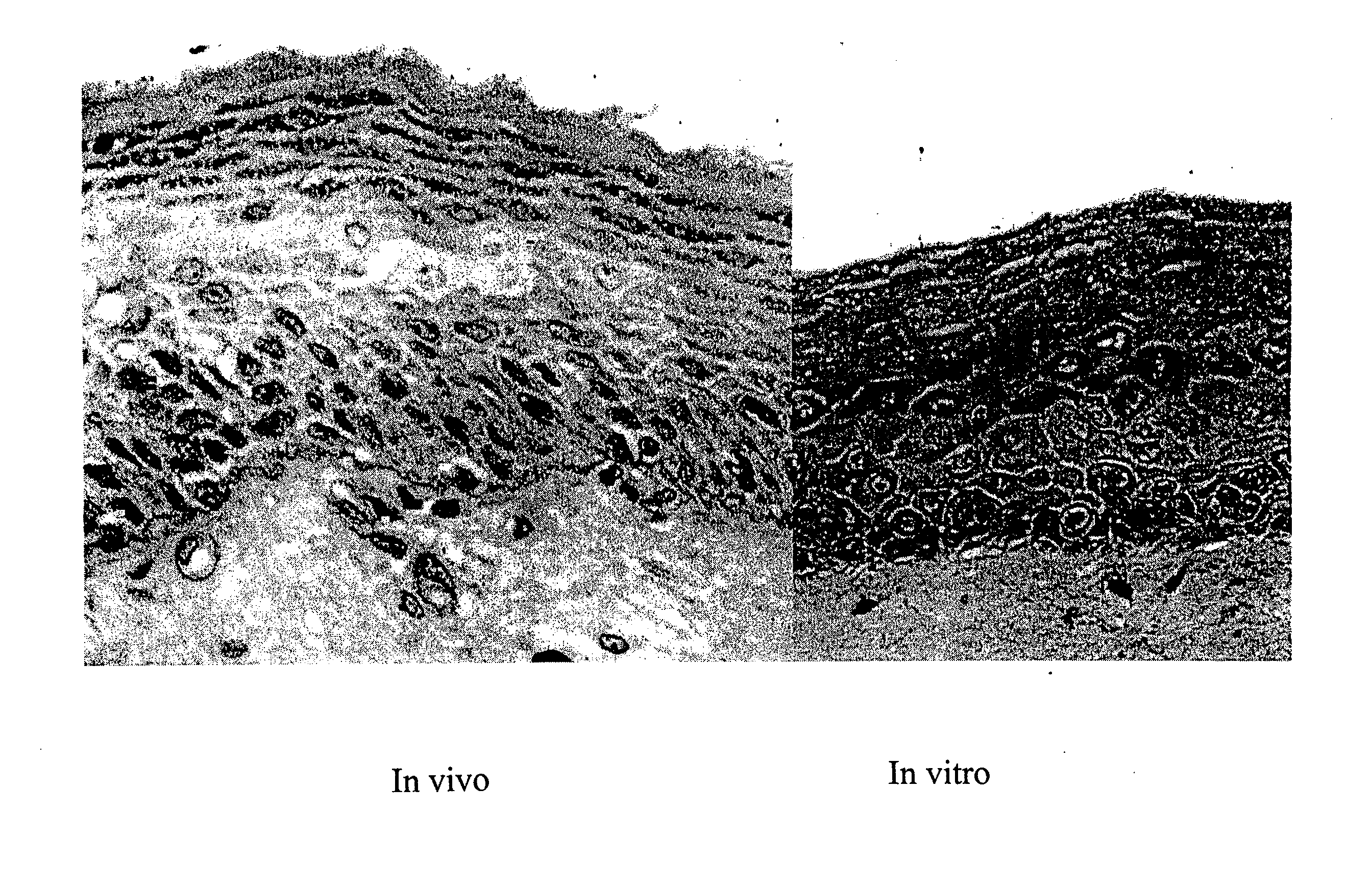
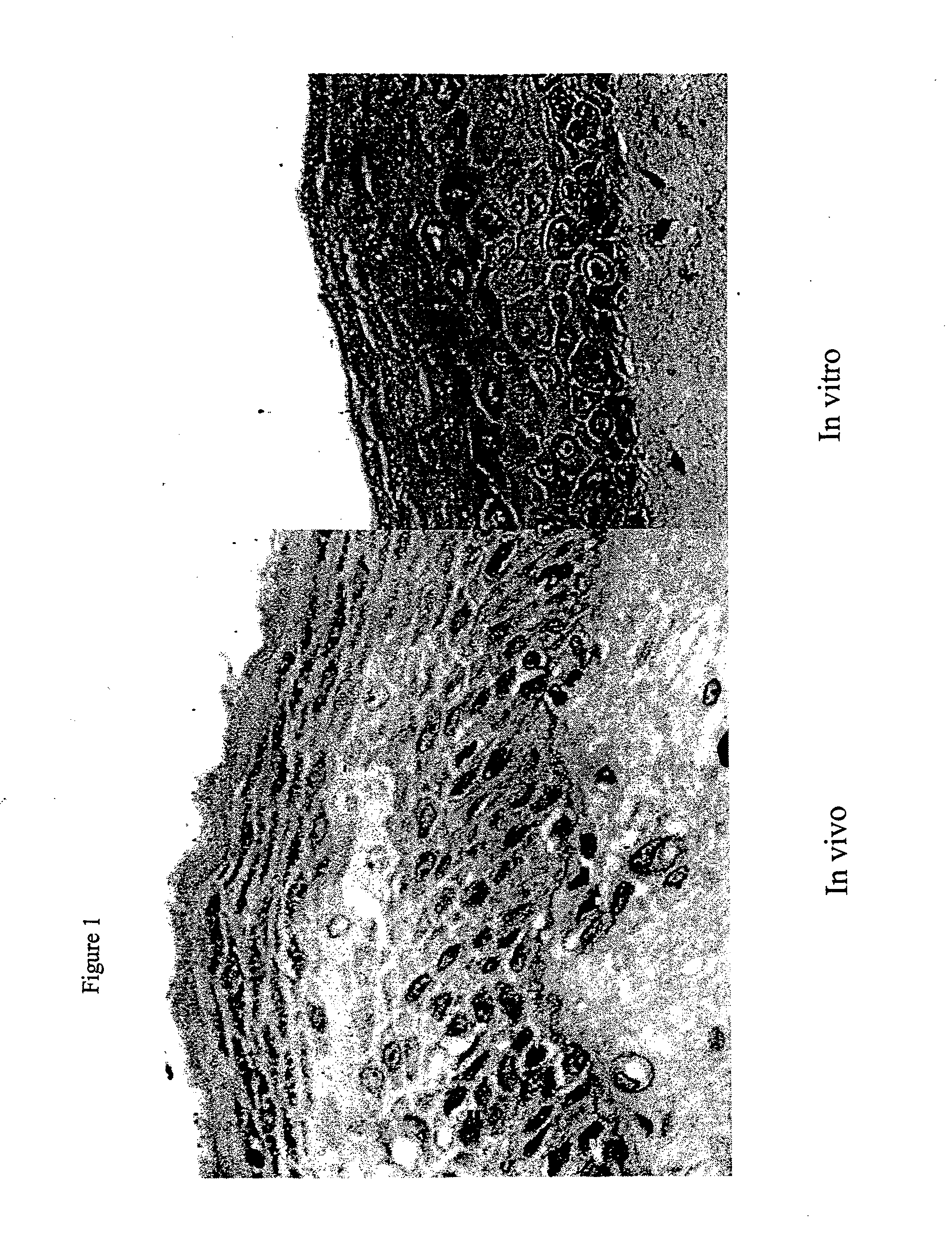
Clinical performance of currently available human skin equivalents is limited by failure to develop perfusion. To address this problem we have developed a method of endothelial cell transplantation that promotes vascularization of human skin equivalents in vivo. Living skin equivalents were constructed by sequentially seeding the apical and basal surfaces of acellular dermis with cultured human keratinocytes and Bcl-2 transduced HUVEC or umbilical cord cells sequentially. After orthotopic implantation of grafts comprising cultured human keratinocytes and Bcl-2 transduced HUVEC cells onto mice, the grafts displayed both a differentiated human epidermis and perfusion through the HUVEC-lined microvessels. These vessels, which showed evidence of progressive maturation, accelerated the rate of graft vascularization. Successful transplantation of such vascularized human skin equivalents should enhance clinical utility, especially in recipients with impaired angiogenesis.
Owner:YALE UNIV
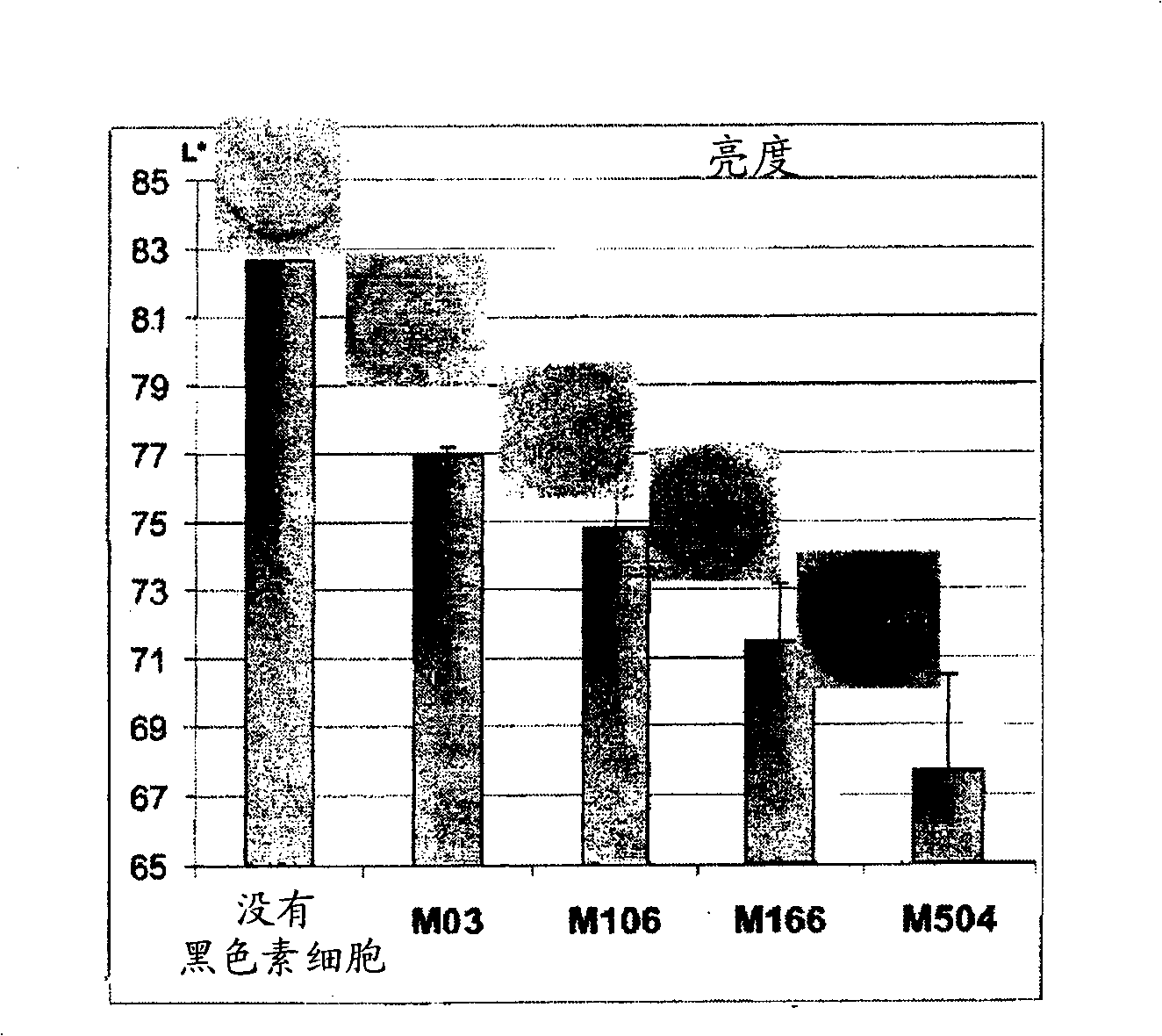
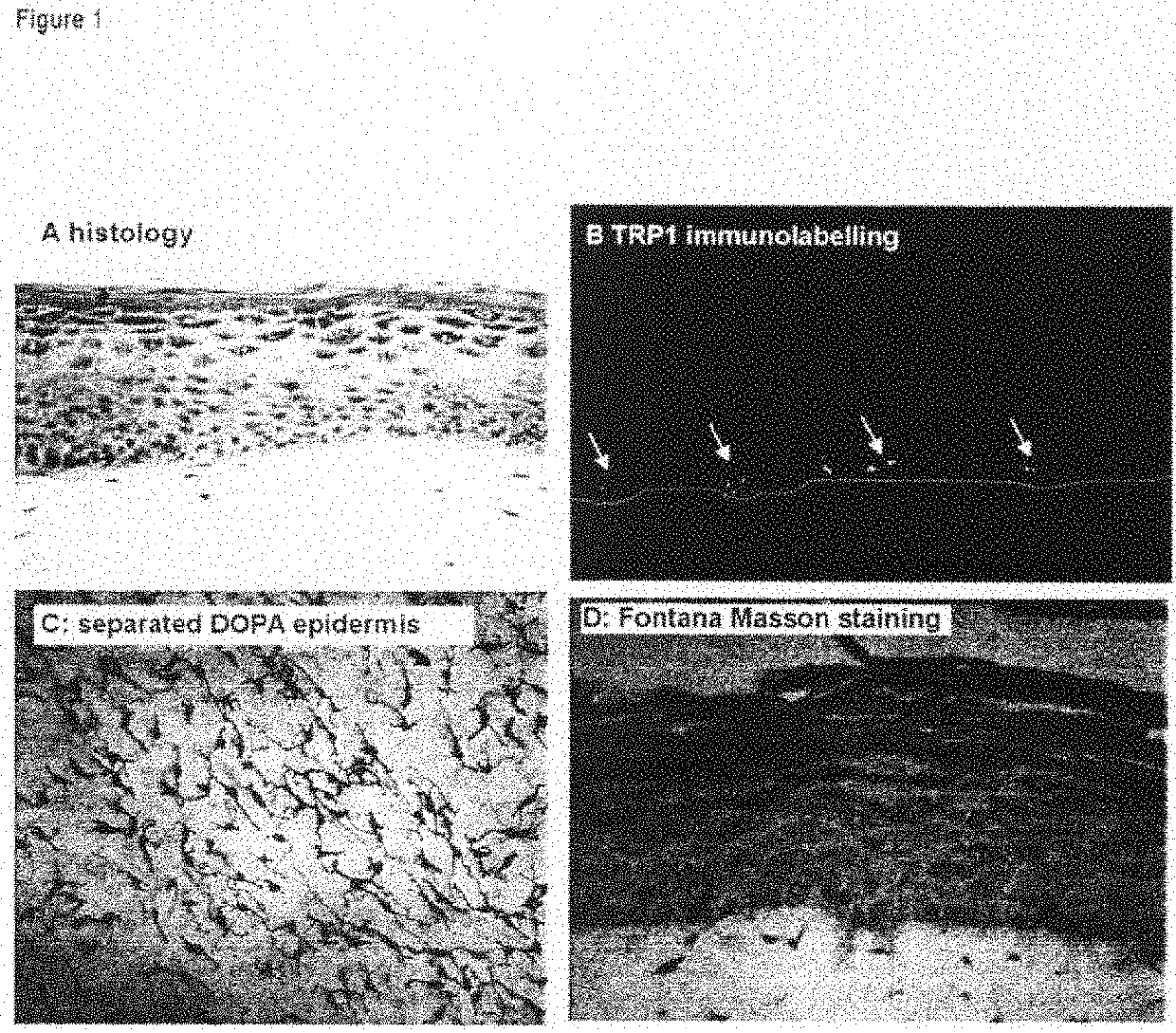
Analogue model of skin with pigments for selecting stripping agent and construction method thereof
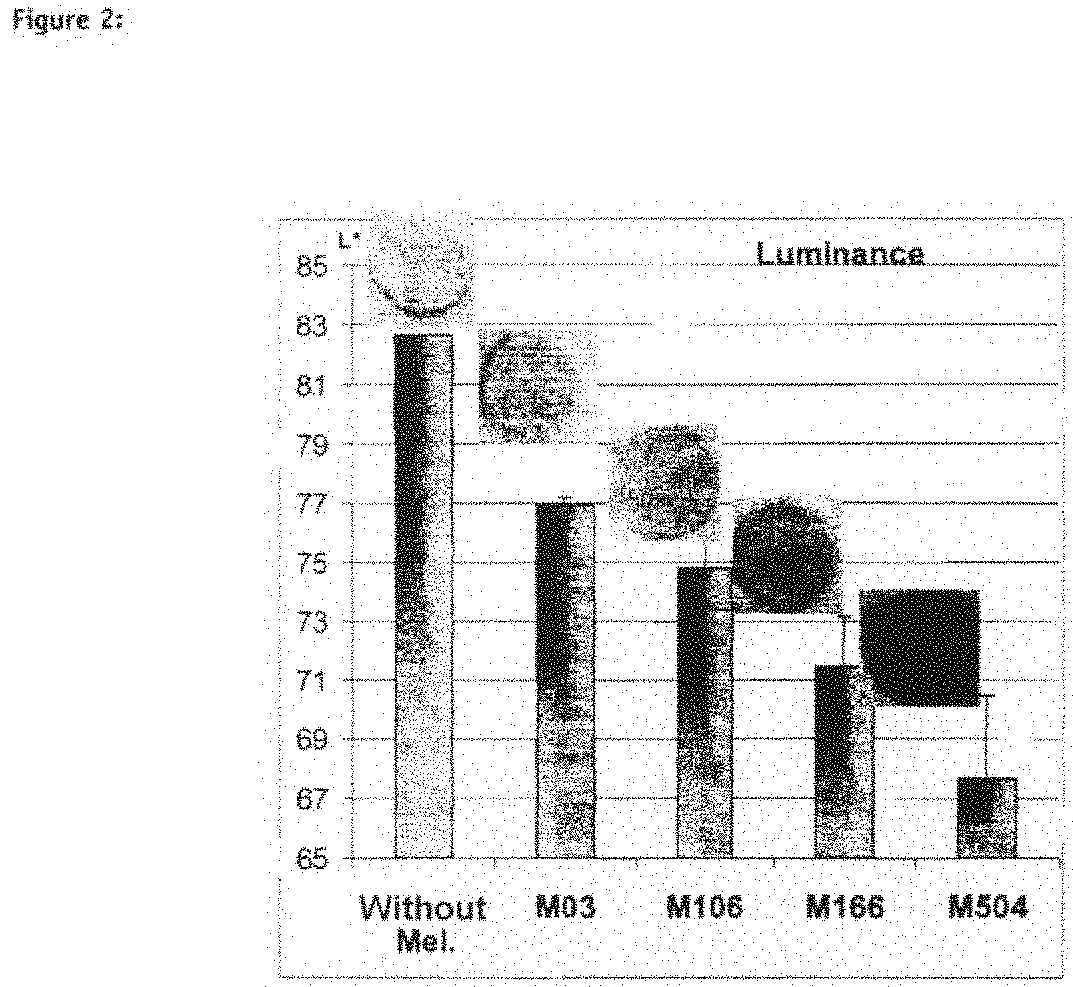
The invention belongs to the biotechnology filed and relates to a pigmented skin equivalent used for screening decolorizing agents and a constructing method thereof. A dermic skin equivalent layer is constructed by skin fibroblasts that are cultured in vitro and collagen, and the equivalent is constructed after inoculating keratinocyte and melanocyte on the skin equivalent layer by using gas-liquid plane three-dimensional culture technology. The obtained pigmented skin equivalent that is similar to the structure of skin can imitate the transportation process of melanin bodies, be used for screening the decolorizing agents and provide a more scientific model for the study of melanocyte in vitro biology and the screening of decolorizing agents and whitening cosmetics.
Owner:XI AN JIAOTONG UNIV
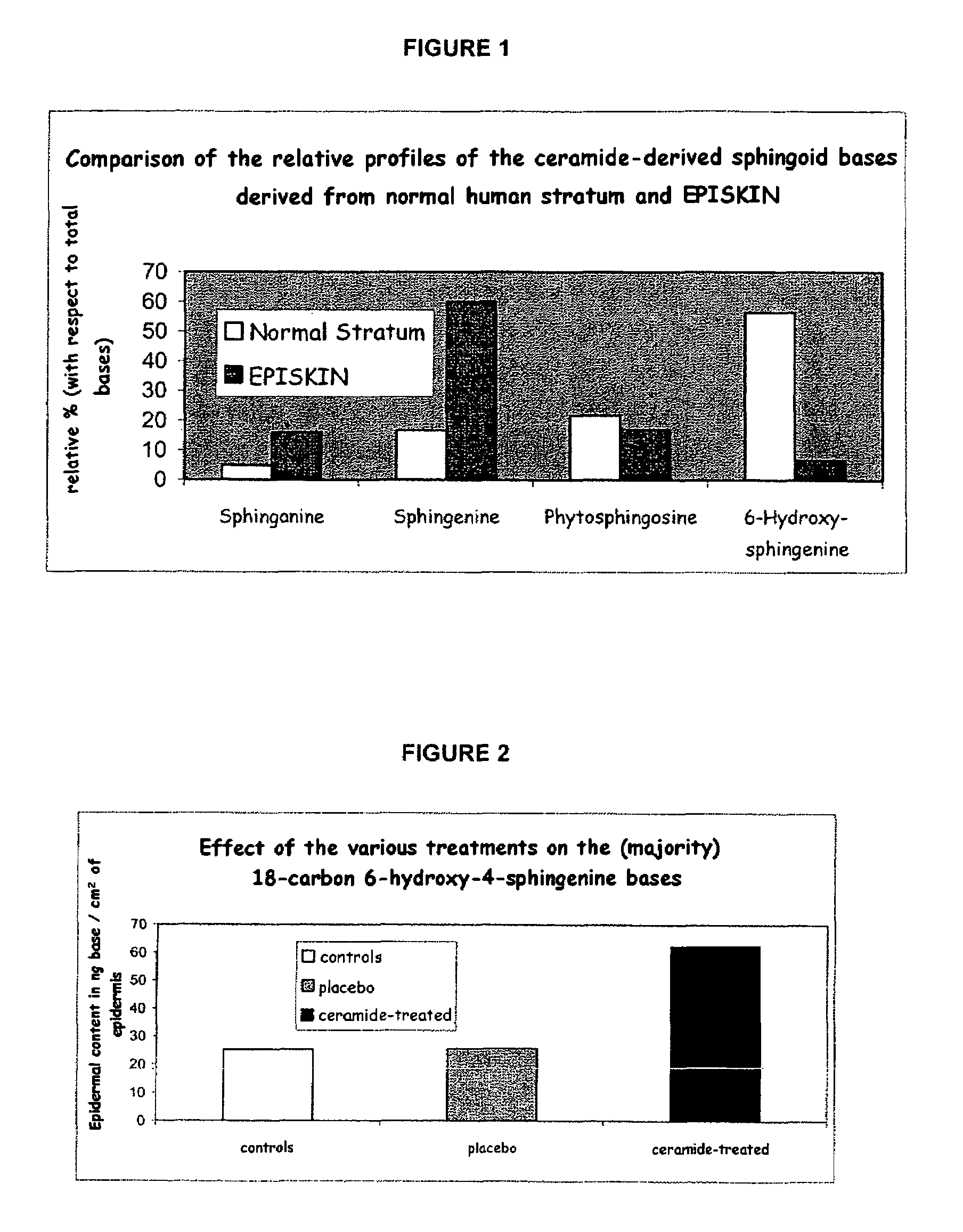
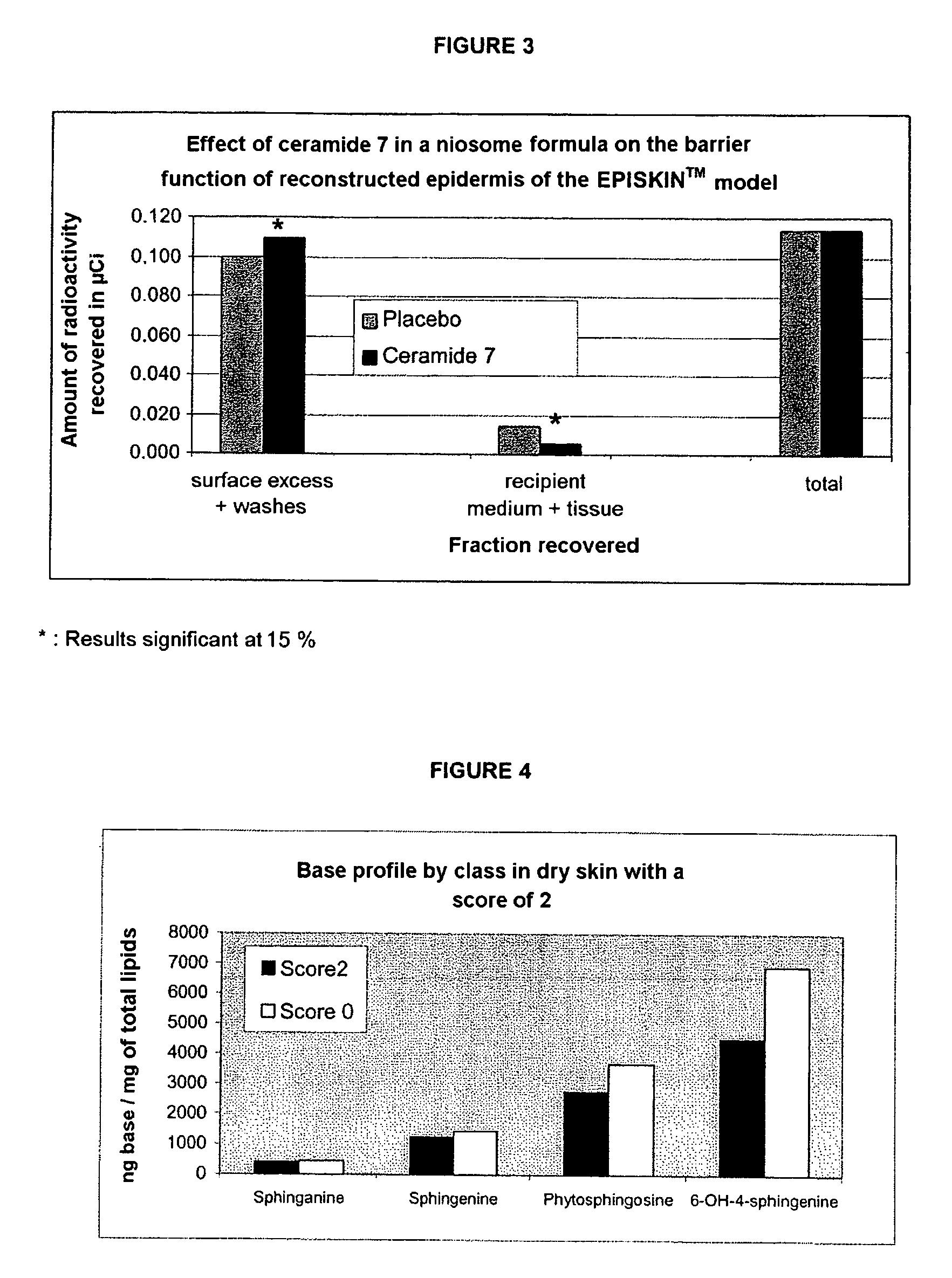
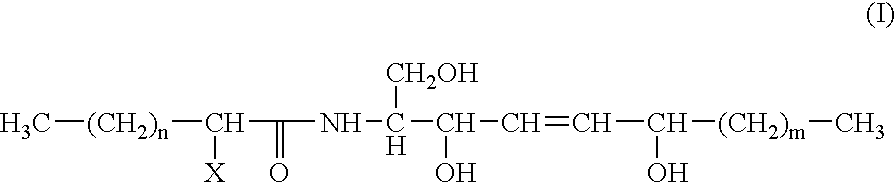
Reconstructed epidermis/skin equivalent comprising a ceramide 7 and /or 5.5 and lipid lamellar vesicular compositions comprising ceramide 7 and/or 5.5 compounds
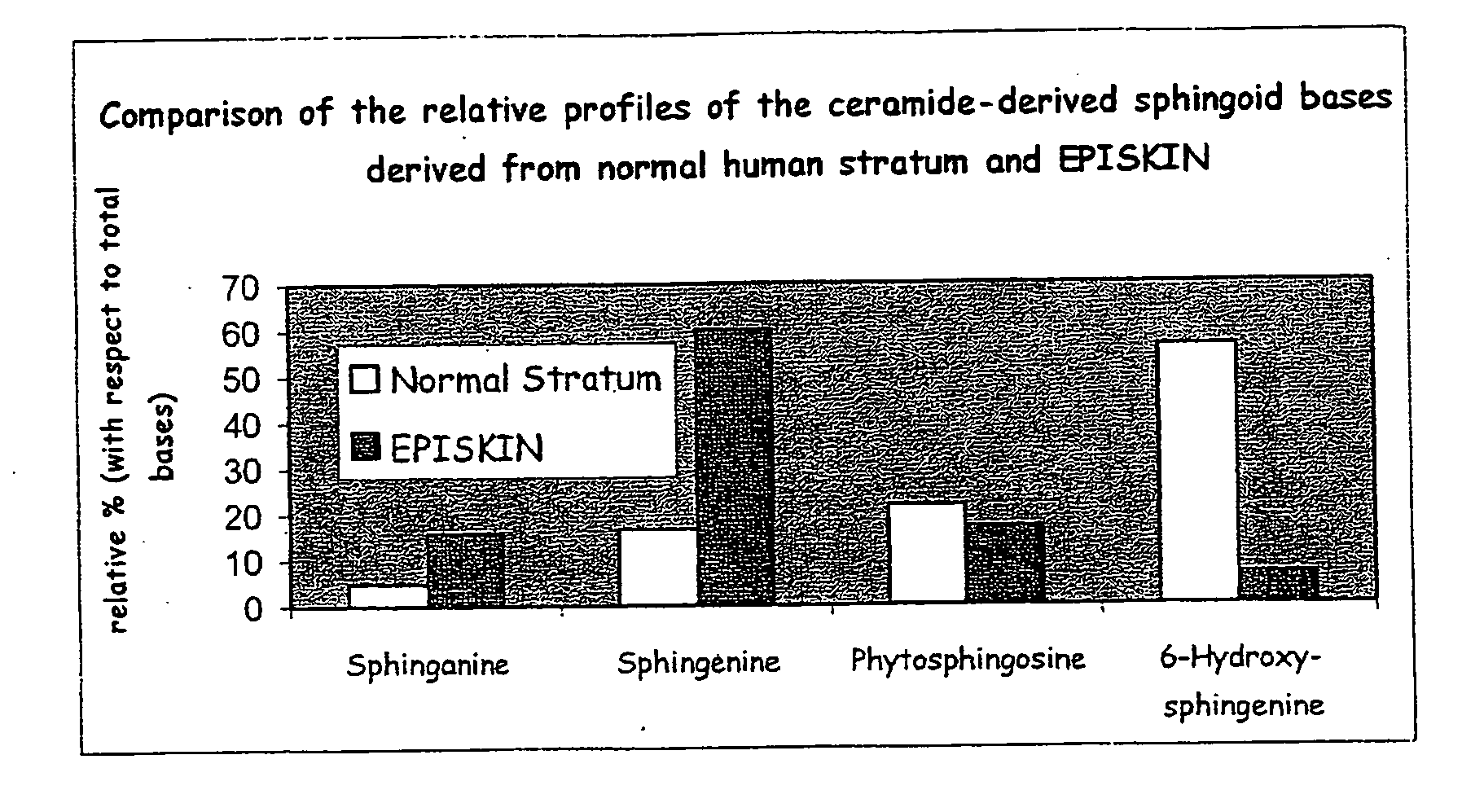
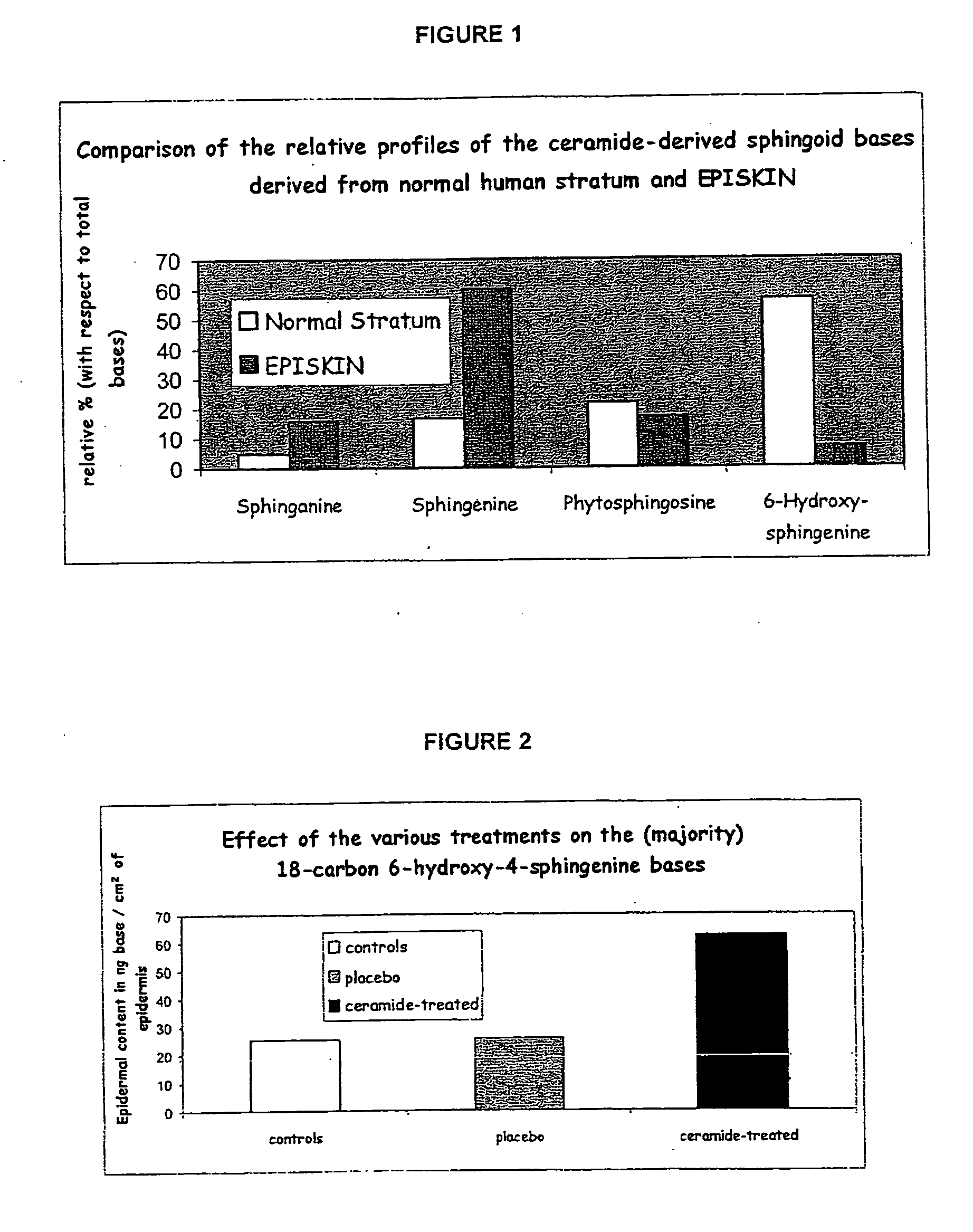
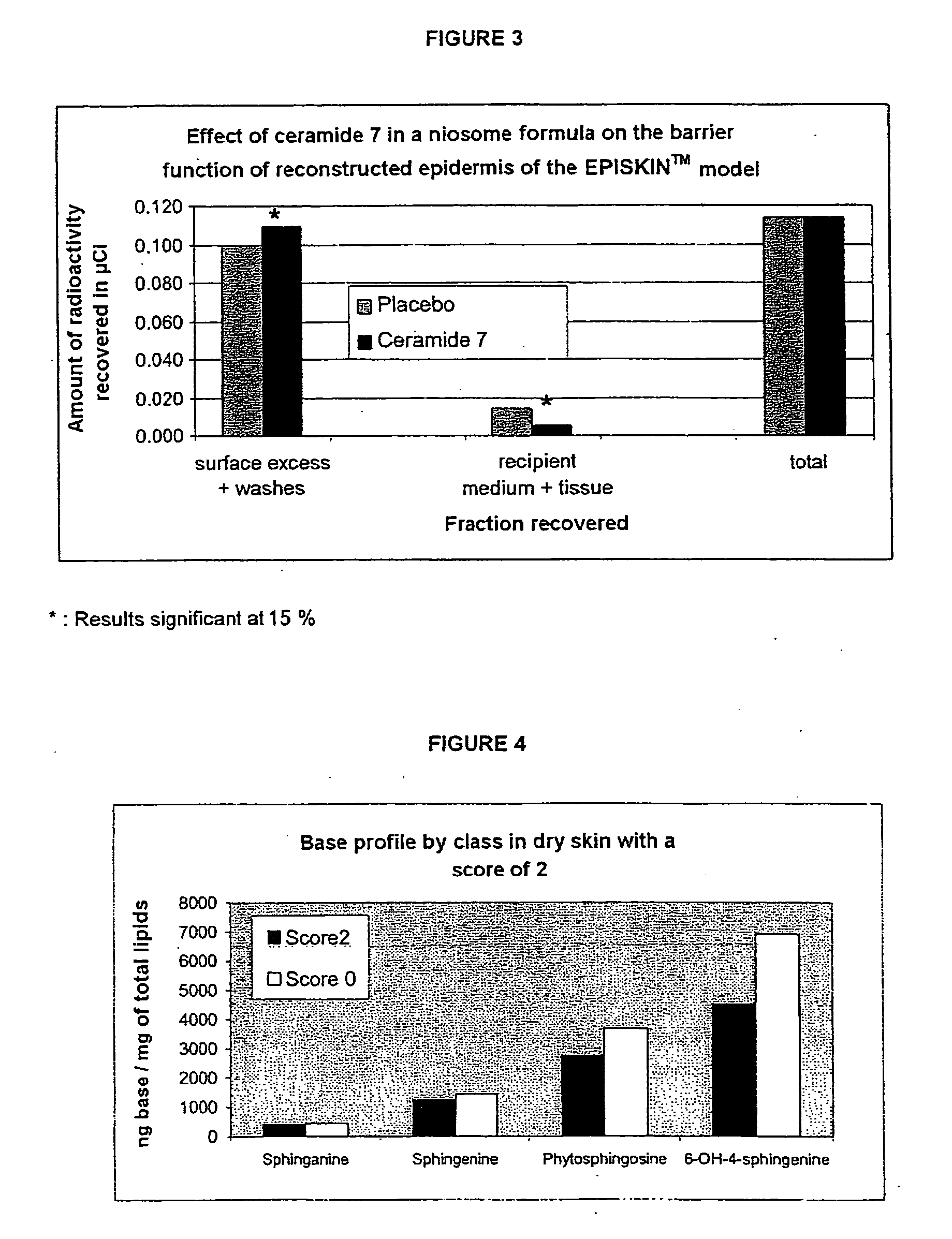
A reconstructed epidermis / skin equivalent supplemented with at least one ceramide 7 and / or 5.5 compound, suited for reinforcing the barrier function of normal human epidermis and for improving the barrier function of dry skin and of reconstructed skin or skin equivalents, is prepared by introducing the at least one ceramide 7 and / or 5.5 compound into the culture medium of such reconstructed epidermis / skin equivalent and / or topically applying onto the face surface of such reconstructed epidermis / skin equivalent a composition which comprises lipid lamellar vesicles incorporating at least one ceramide 7 and / or 5.5 compound.
Owner:LOREAL SA
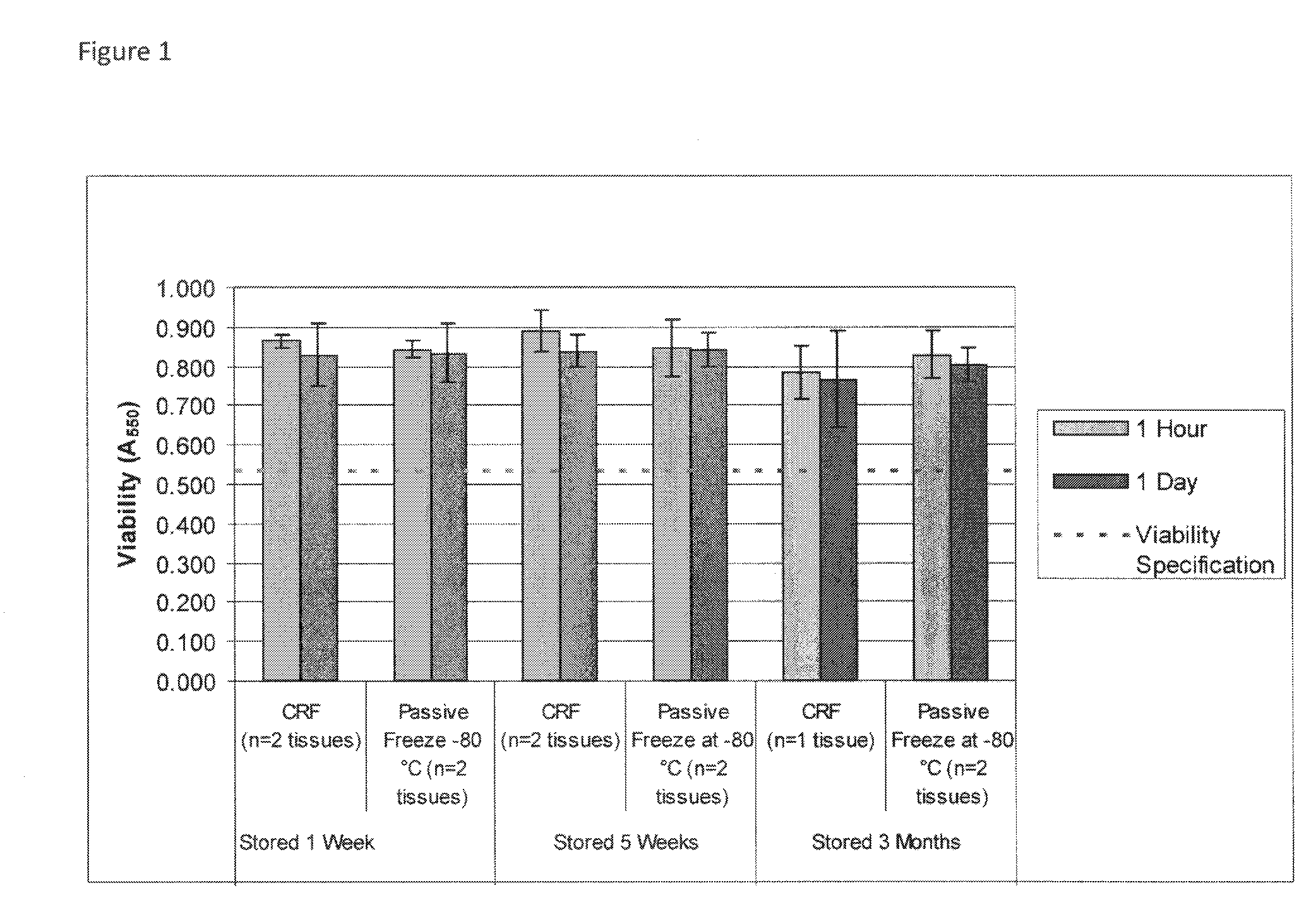
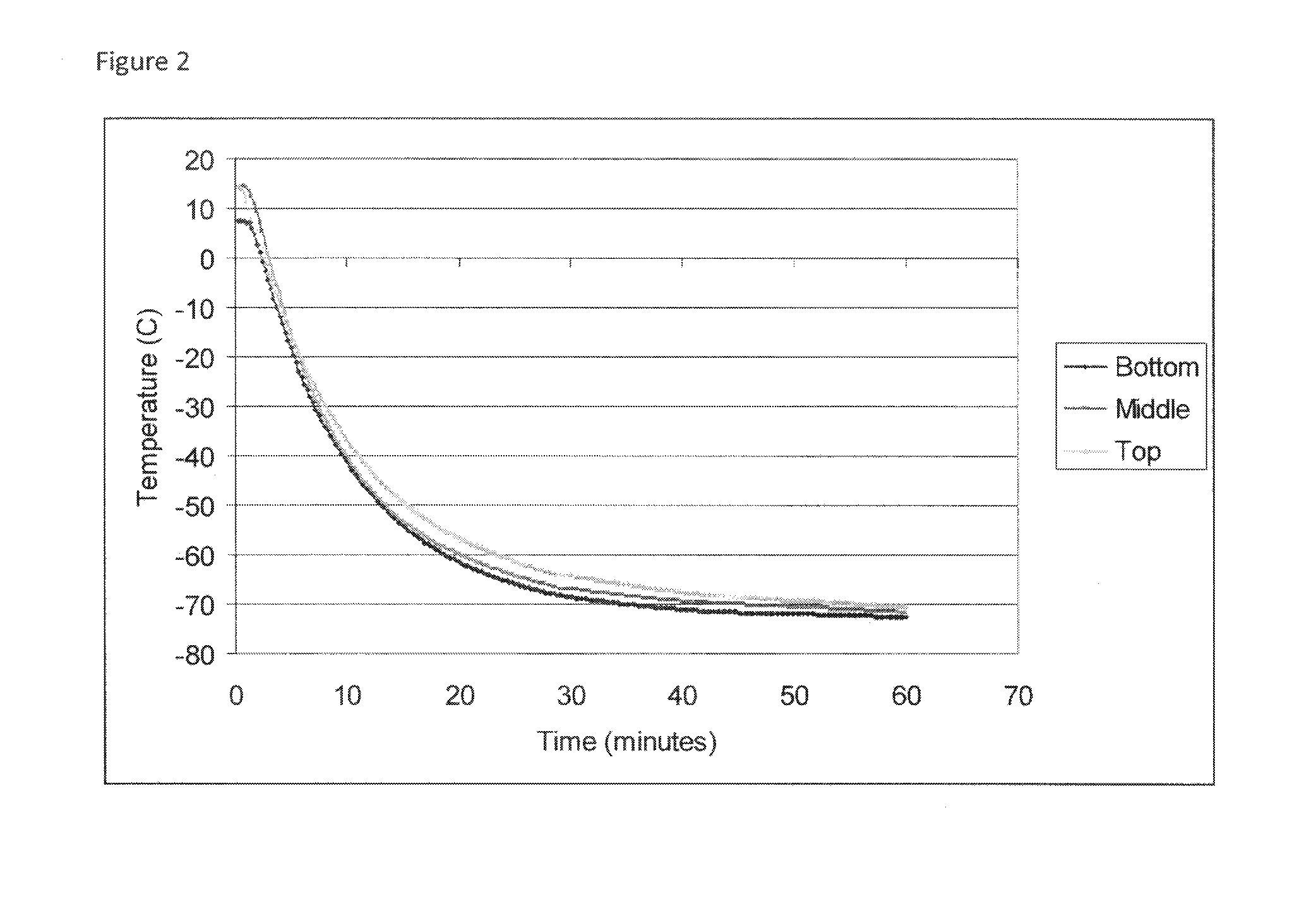
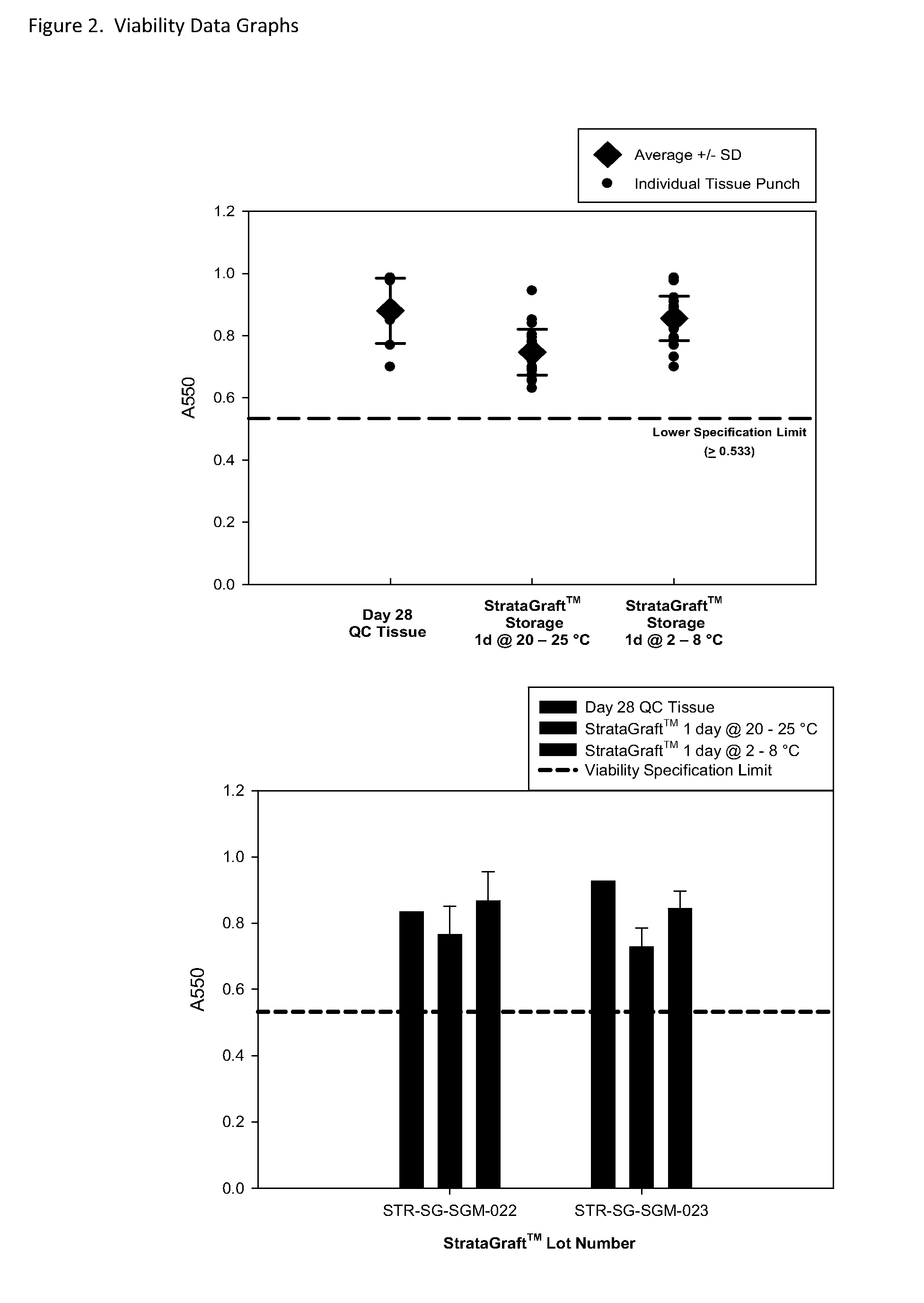
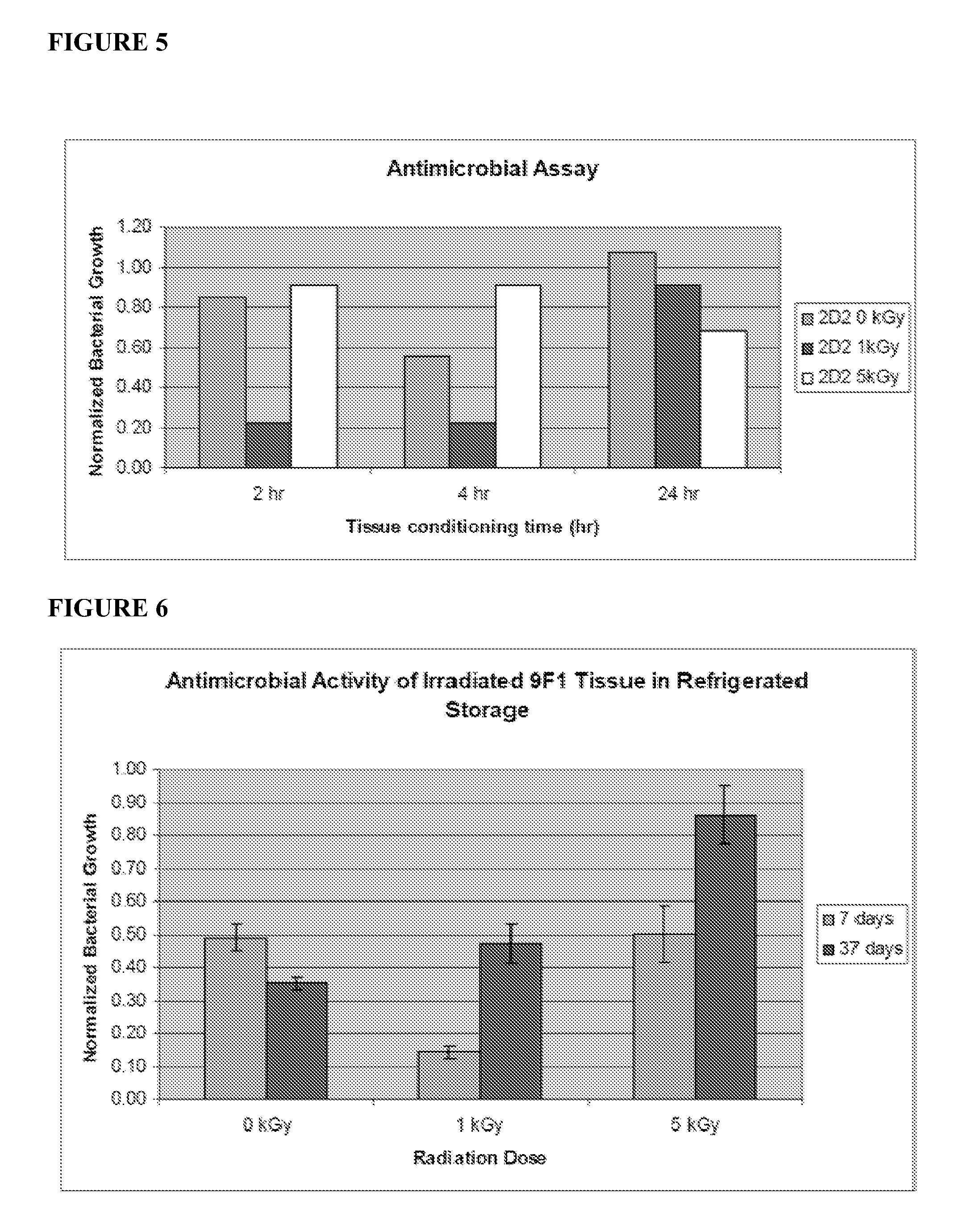
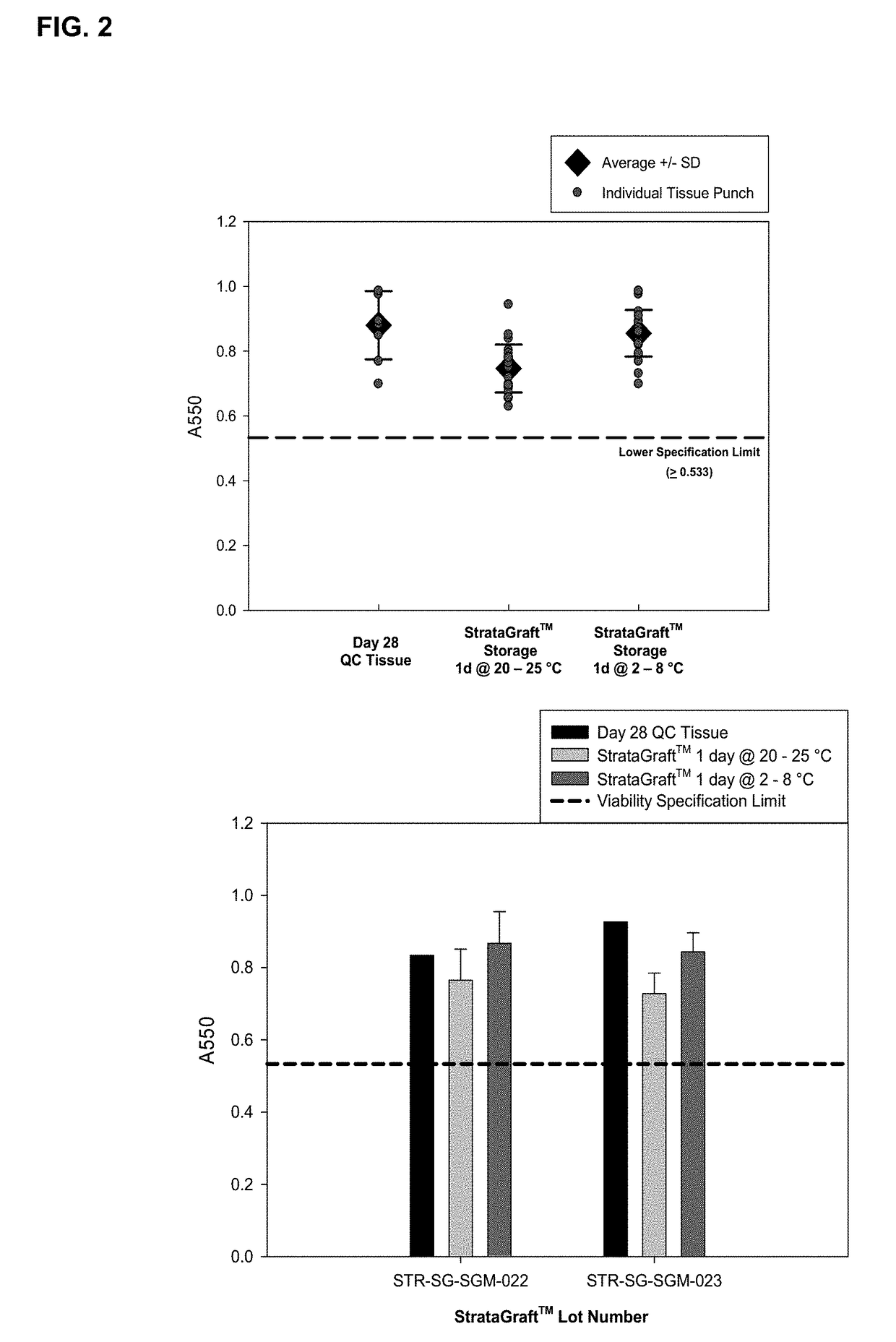
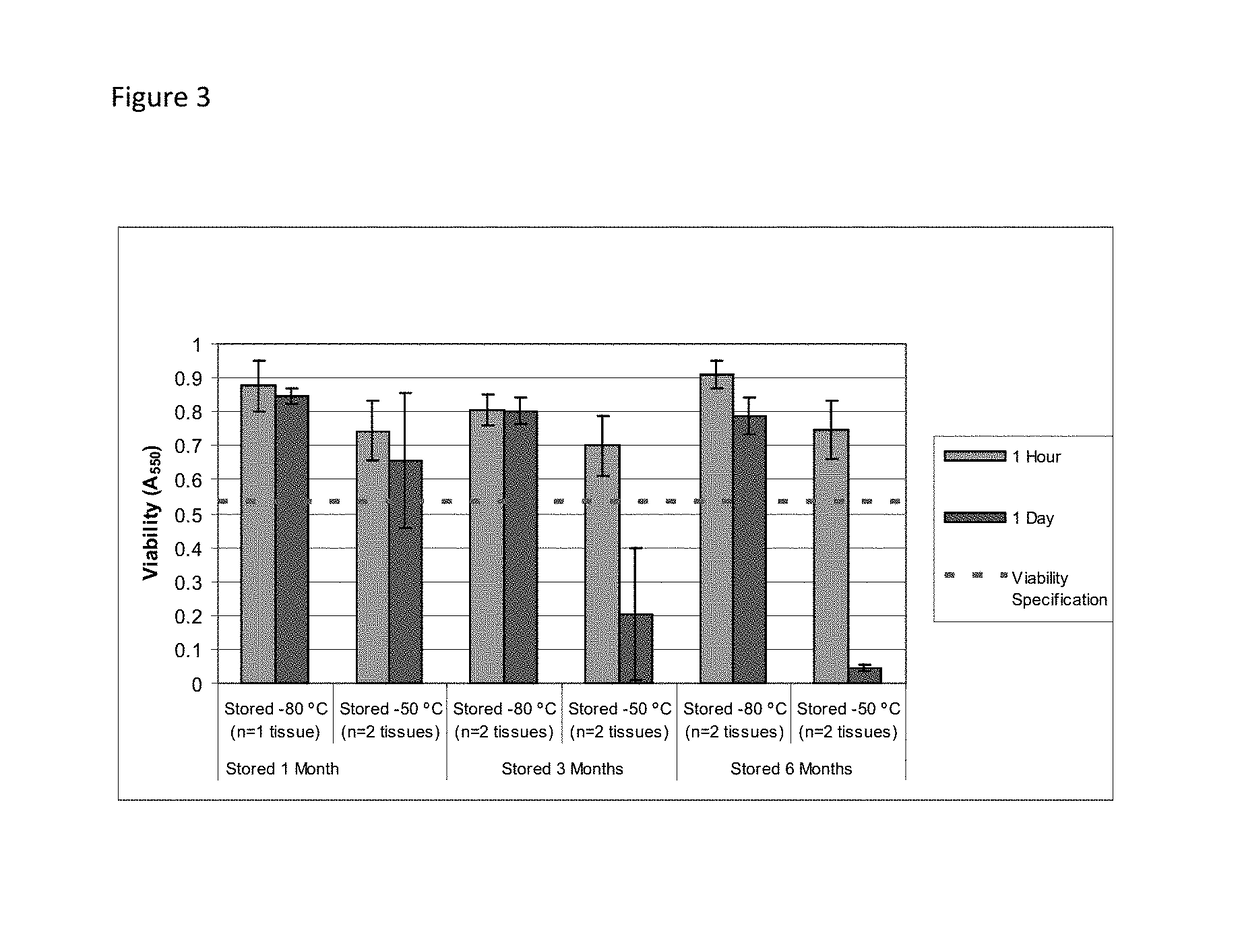
Cold storage of organotypically cultured skin equivalents for clinical applications
InactiveUS20090145087A1Improve availabilityImprove clinical utilityPackage sterilisationVertebrate cellsSkin equivalentDegree Celsius
The present invention relates generally to systems and methods for storing, shipping and using skin equivalents made by organotypic culture. In particular, the present invention relates to systems and methods for producing, transporting, storing and using skin equivalents produced by organotypic culture at reduced temperatures, preferably from 2-8 degrees Celsius. The methods include sterile packaging of the grafts so that the sterility and integrity of the package is maintained until the time of use for grafting purposes.
Owner:STRATATECH
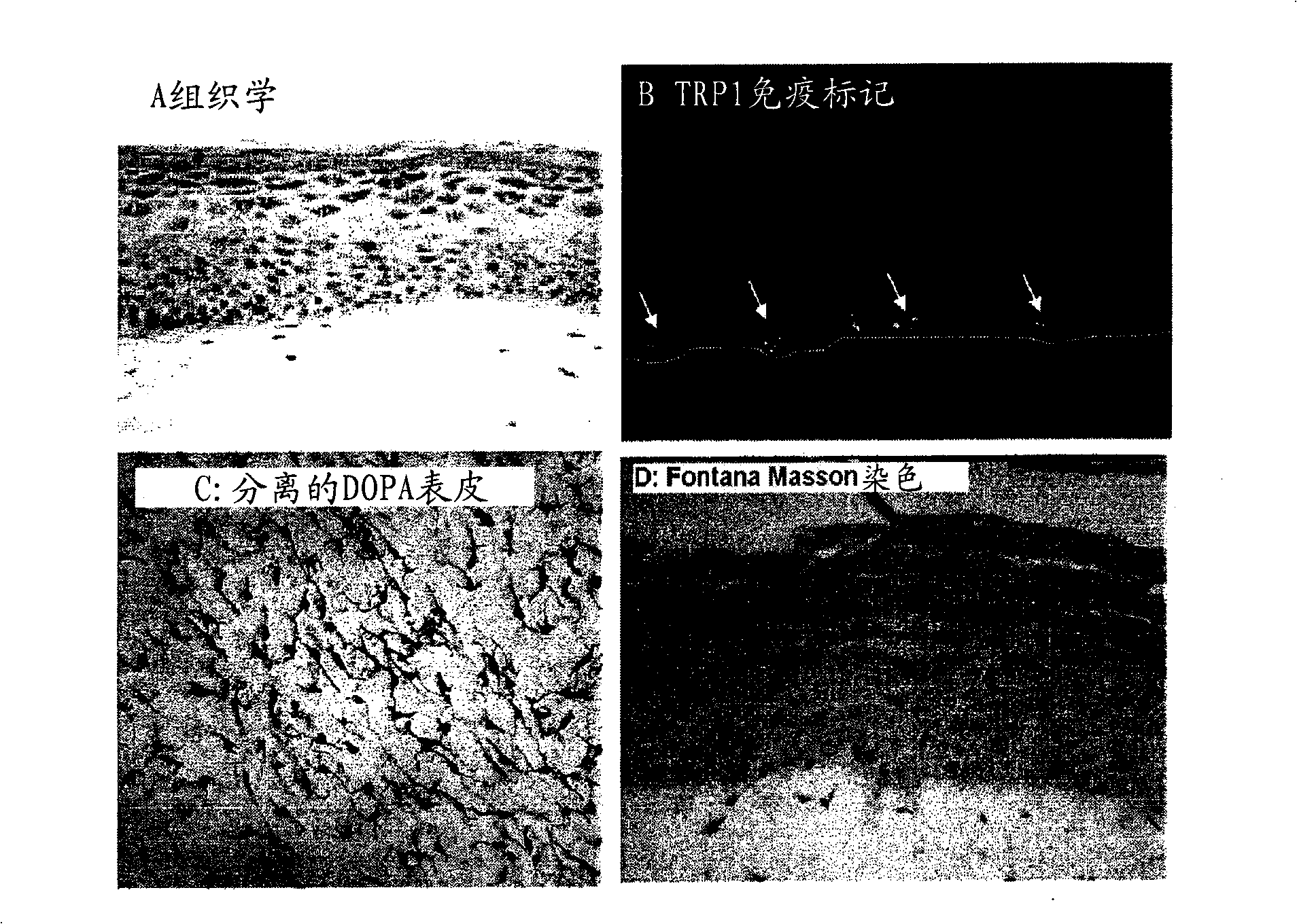
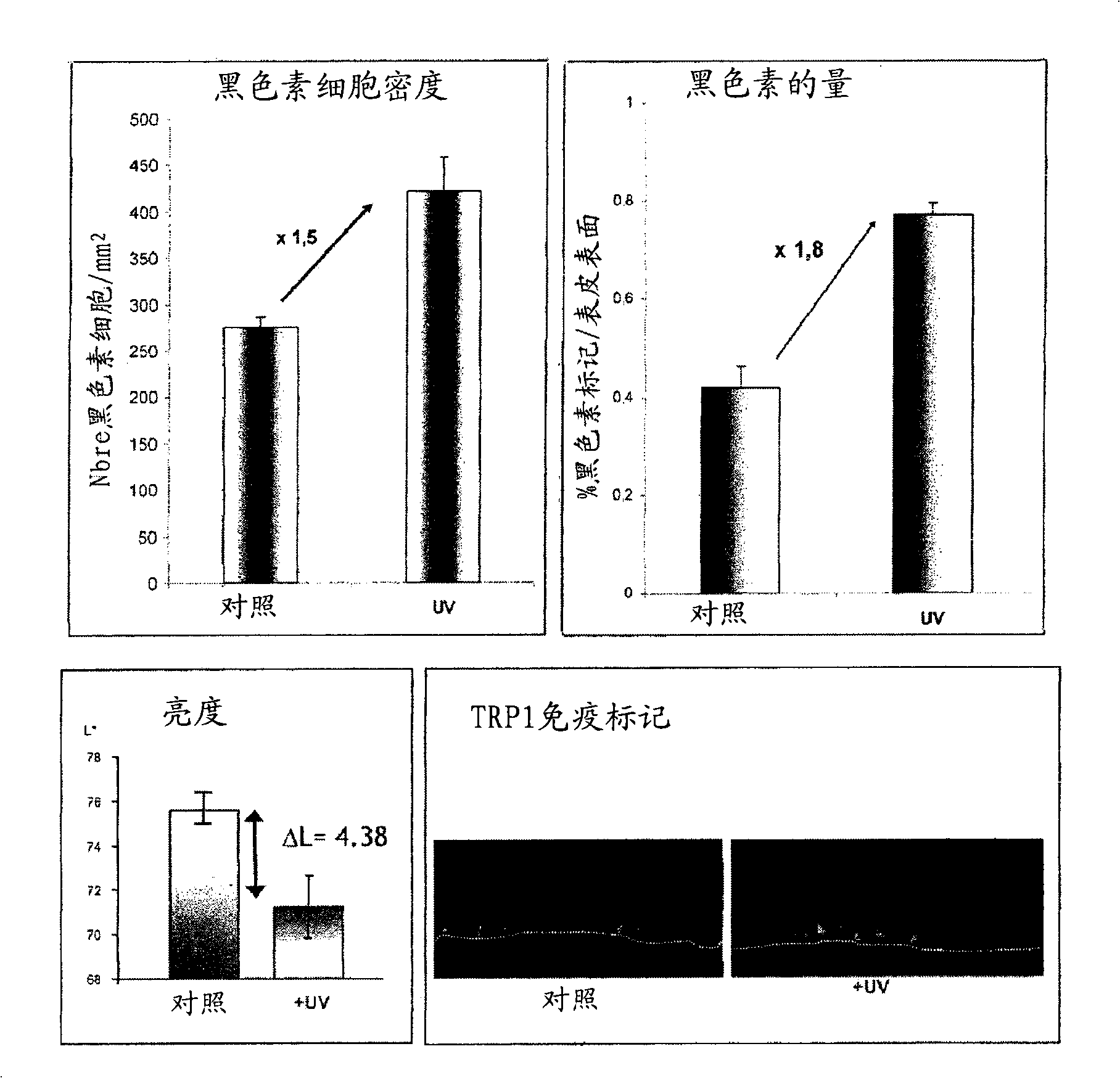
Functional pigmented skin equivalent
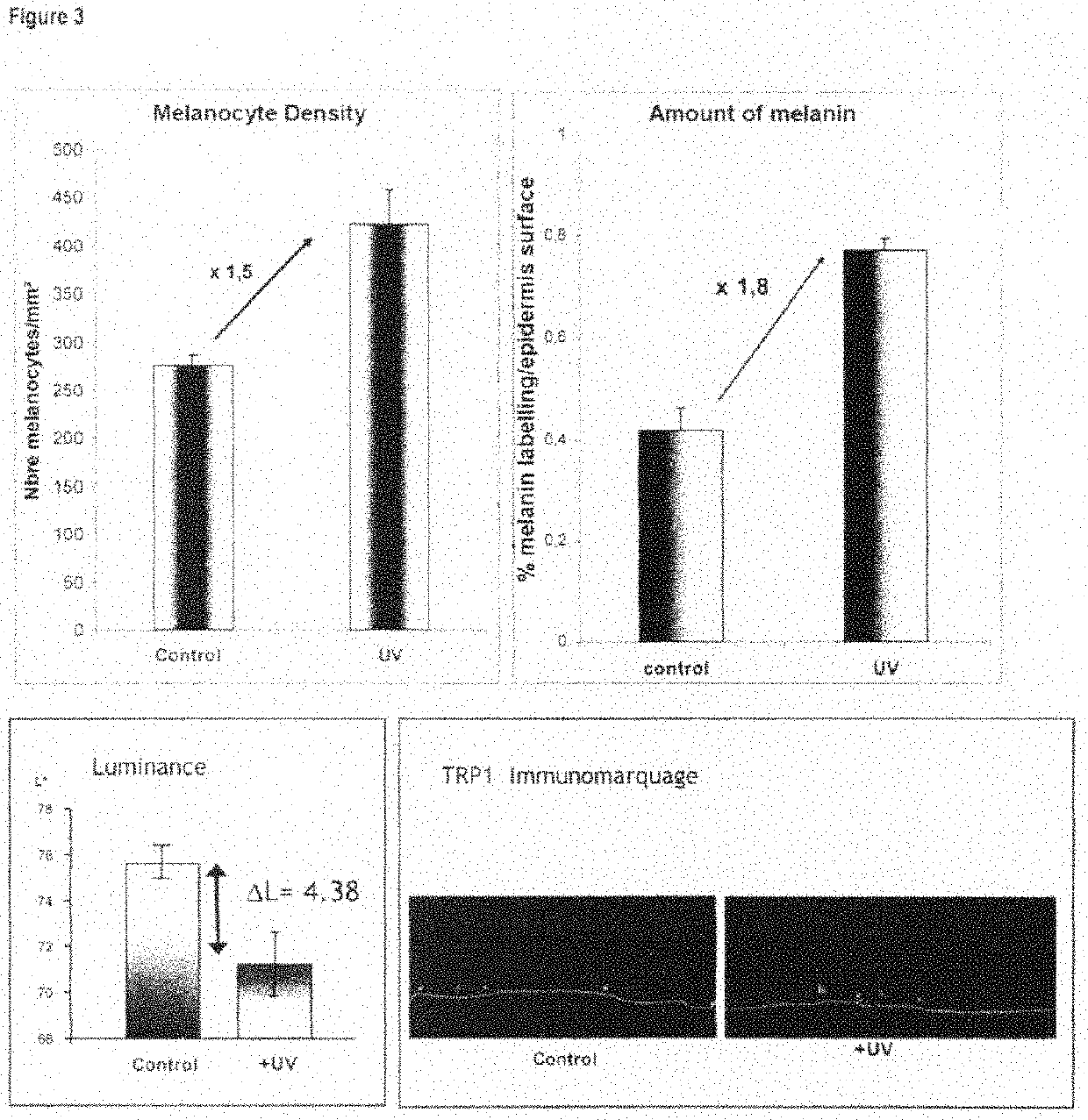
ActiveCN101538555AChange stimulusAbility to regulate stimuliMicrobiological testing/measurementEpidermal cells/skin cellsCuticleSkin equivalent
The present invention relates to a functional pigmented skin equivalent. The invention relates to an in vivo skin equivalent which is characterized by comprising at least one epidermis equivalent and at least one dermis equivalent. The in vivo skin equivalent further comprises melanocytes constitutively producing melanin and fibroblasts. The invention also relates to a method for preparing the skin equivalent.
Owner:LOREAL SA
Reconstructed epidermis/skin equivalent comprising a ceramide 7 and/or 5.5 and lipid lamellar vesicular compositions comprising ceramide 7 and/or 5.5 compounds
InactiveUS20070148771A1Enhanced barrier functionCosmetic preparationsToilet preparationsCompound aLipid formation
Owner:LOREAL SA
Skin substitutes and uses thereof
The present invention relates to in vitro cultured skin substitutes, and in particular to improved methods for organotypic culture of skin substitutes. In some embodiments, the dermal equivalent of the skin substitute is lifted to air interface of the culture prior to seeding with keratinocytes. In other embodiments, increased concentrations of collagen are used to form the dermal equivalent. In still other embodiments, optimized media are utilized to maintain the skin equivalents.
Owner:STRATATECH
Cellular scaffold
ActiveUS20120077272A1Epidermal cells/skin cellsArtificial cell constructsCell based assaysMesenchymal stem cell
A cellular scaffold that is suitable for tissue regeneration, cell culture and in vitro assays. The invention relates to a layered cell scaffold that is seeded with mesenchymal and ectodermal cells. The layered cellular scaffold comprises an inoculum of mesenchymal cells and ectodermal cells positioned between two opposing scaffolds in a sandwich configuration. The layered cell scaffold provides a functional skin equivalent that is suitable for transplantation and in vitro cell-based assays.
Owner:STEMEDICA CELL TECH
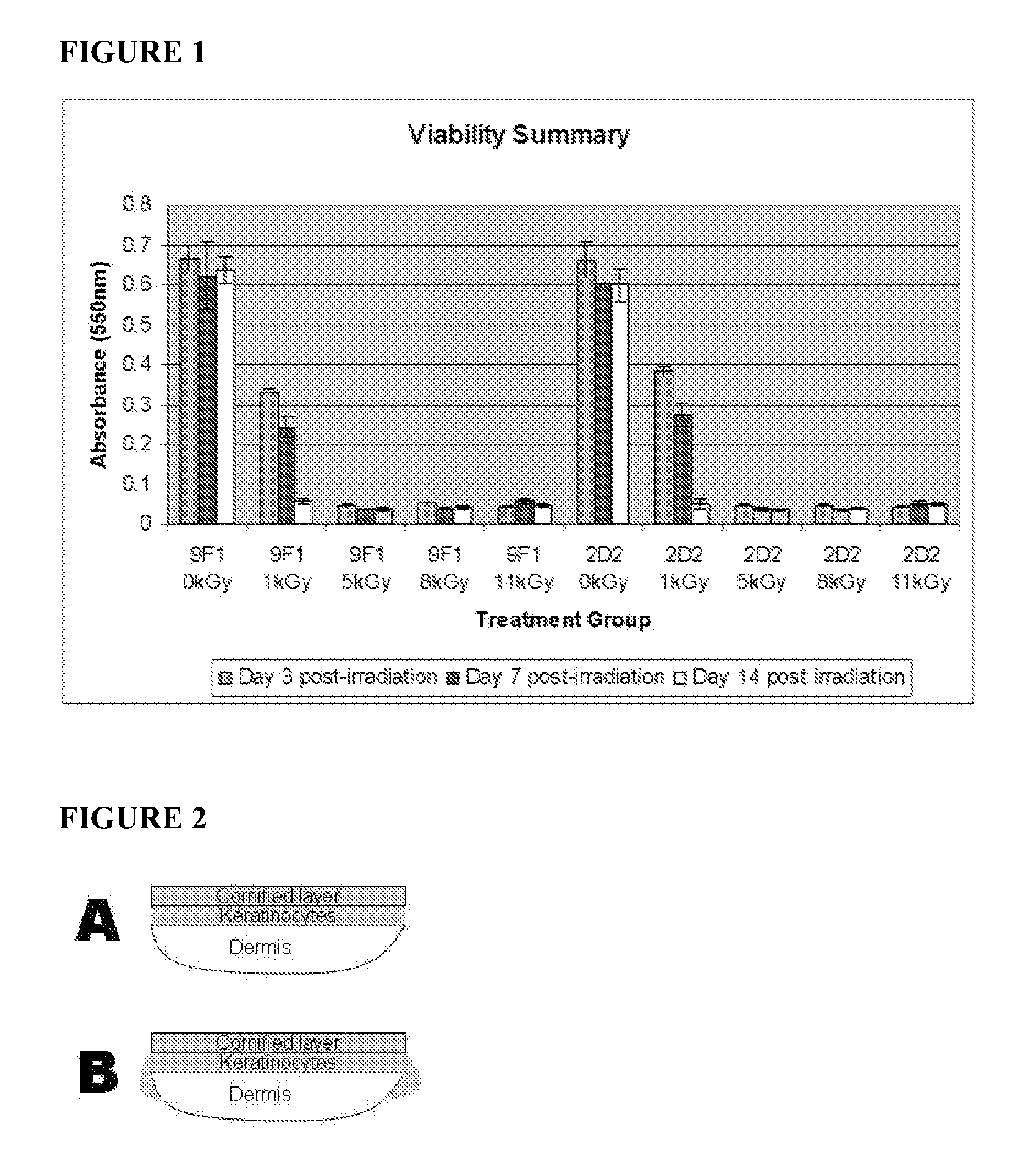
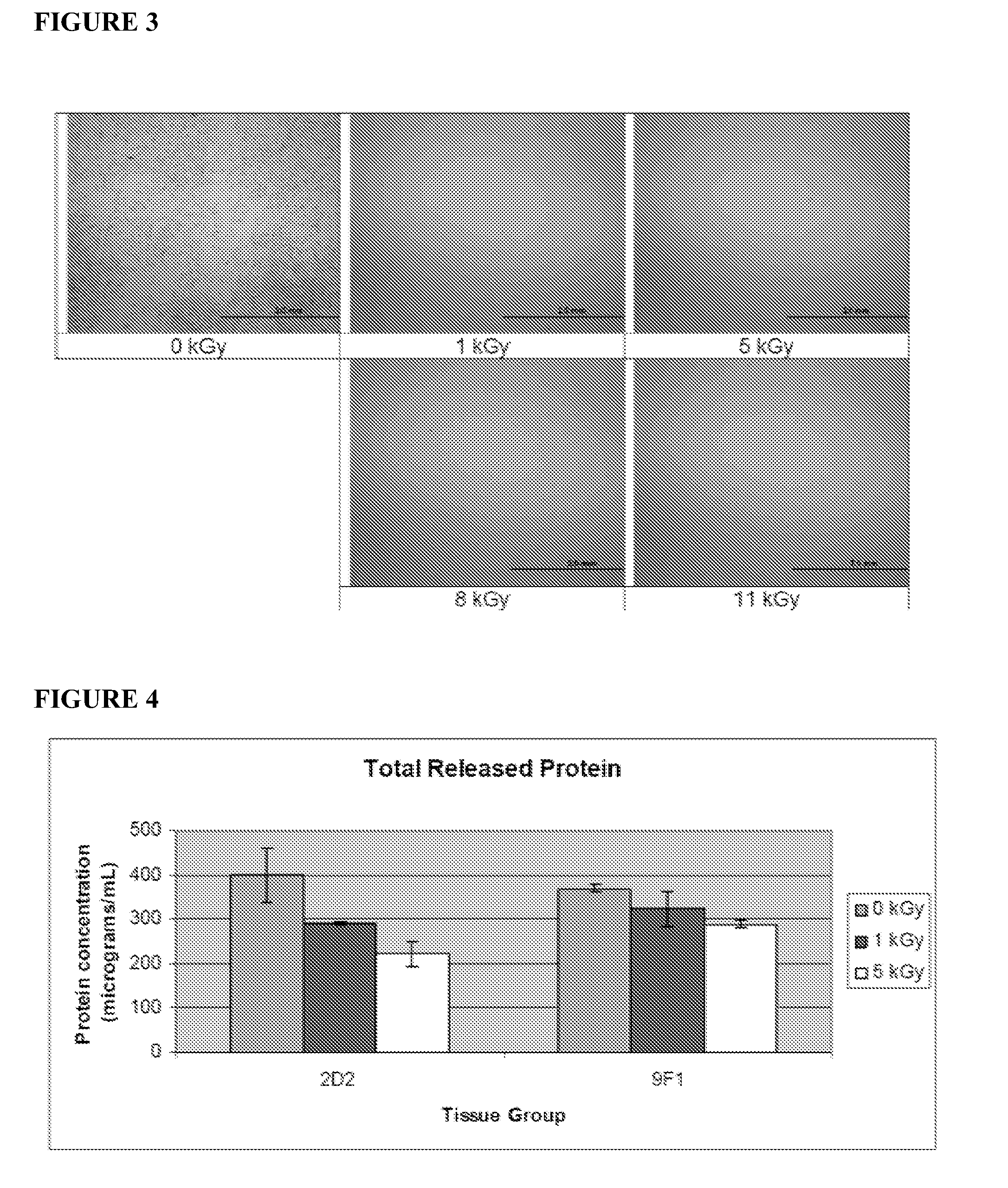
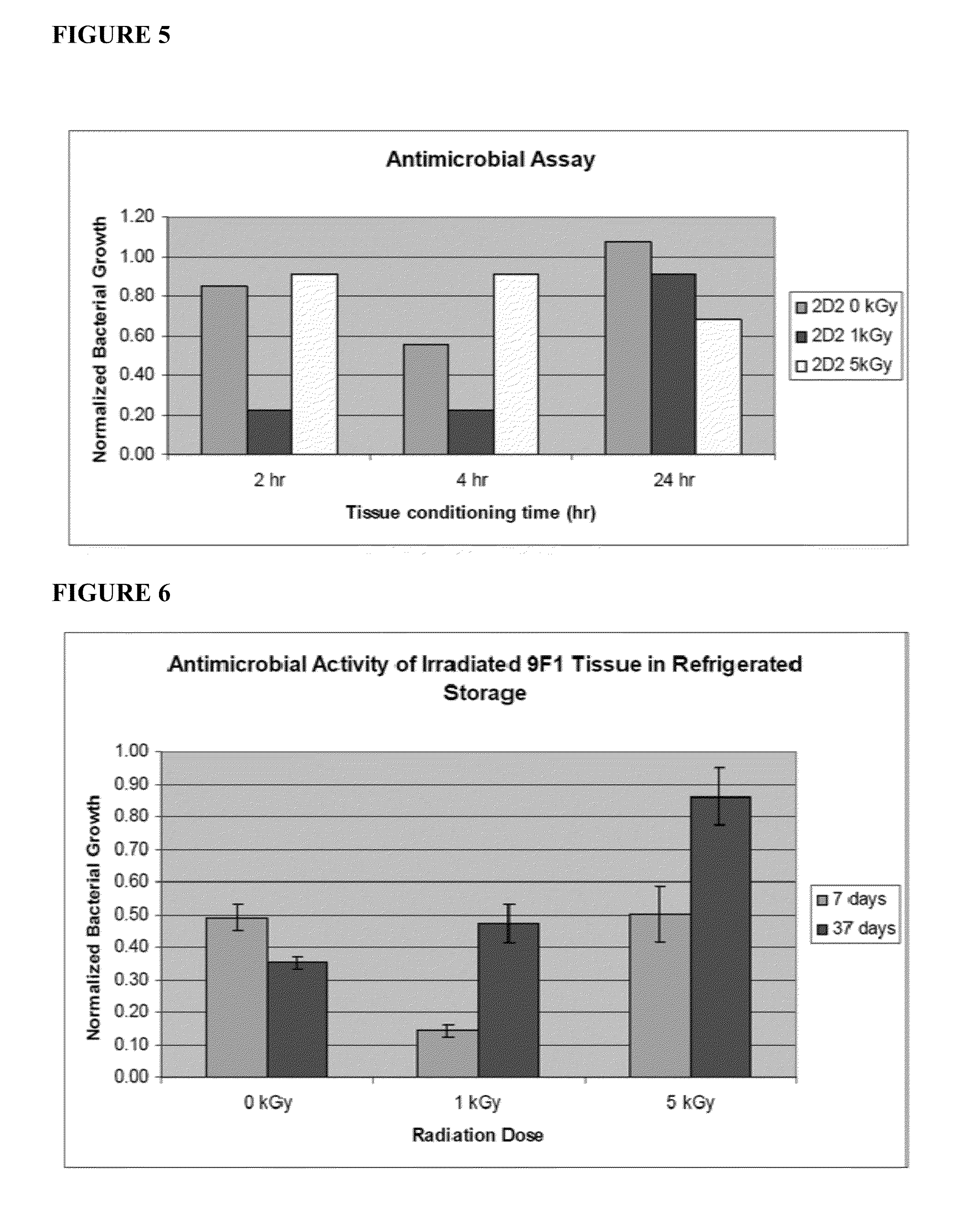
Dried and irradiated skin equivalents for ready use
ActiveUS20100119615A1Prevented tissue movementRestored protein accessibilityDiagnosticsSurgical needlesCelsius DegreeSkin equivalent
The present invention relates generally to systems and methods for preparing, storing, shipping and using skin equivalents made by organotypic culture. In particular, the present invention relates to systems and methods for producing, transporting, storing and using skin equivalents produced by organotypic culture at reduced temperatures, preferably from 2-8 degrees Celsius to ambient temperature. The methods include sterile packaging of the grafts so that the sterility and integrity of the package is maintained until the time of use for grafting purposes.
Owner:STRATATECH
Antibodies specific for papillary fibroblasts as markers for skin quality
Whether or not a sample of skin or of a skin equivalent contains such amount of a papillary fibroblast population as to be considered a normal skin is determined by labelling said skin or skin equivalent with at least one antibody specific for papillary fibroblasts and evaluating the extent of such labelling as a marker for skin or skin equivalent quality.
Owner:LOREAL SA
Cellular scaffold
A cellular scaffold that is suitable for tissue regeneration, cell culture and in vitro assays. The invention relates to a layered cell scaffold that is seeded with mesenchymal and ectodermal cells. The layered cellular scaffold comprises an inoculum of mesenchymal cells and ectodermal cells positioned between two opposing scaffolds in a sandwich configuration. The layered cell scaffold provides a functional skin equivalent that is suitable for transplantation and in vitro cell-based assays.
Owner:STEMEDICA CELL TECH
Three-dimensional skin model
InactiveUS20090298042A1High quality resultUniform surfaceBiocideEpidermal cells/skin cellsFiberHuman skin
The present invention relates to methods for cultivating dermal fibroblasts, methods for preparing in vitro dermis equivalents, methods for preparing three-dimensional in vitro skin equivalents, an in vitro dermis equivalent, a three-dimensional in vitro skin equivalent, and methods for determining the effect of a chemical substance or of an agent on human skin cells using the in vitro dermis equivalent and / or the in vitro skin equivalent.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Skin equivalent culture
InactiveUS20110027366A1Provide rigidityProvide strengthPowder deliveryPeptide/protein ingredientsFiberSupport matrix
Disclosed is a method of preparing a collagenous construct comprising casting a support matrix comprising fibrin and viable collagen-producing cells onto a support material, wherein said cells include human dermal fibroblasts, incubating in situ said support matrix in a collagen-inducing medium thereby, inducing or enhancing collagen production by said cells to form a collagenous construct, and degrading said fibrin, and rendering said collagenous construct free of said viable collagen-producing cells.
Owner:SMITH & NEPHEW INC
Epidermis/dermis equivalents and aged skin equivalents shaped therefrom
An aged skin equivalent comprising an epidermis equivalent and an aged dermis equivalent, wherein the aged dermis equivalent comprises glycated collagen.An aged dermis equivalent, the epidermis equivalent obtained and methods of producing the aged skin and / or aged dermis equivalent and / or the epidermis equivalent.
Owner:LOREAL SA
Skin model
ActiveUS20160003856A1Extend your lifeMicrobiological testing/measurementEpidermal cells/skin cellsContact dermatitisAdverse drug reaction
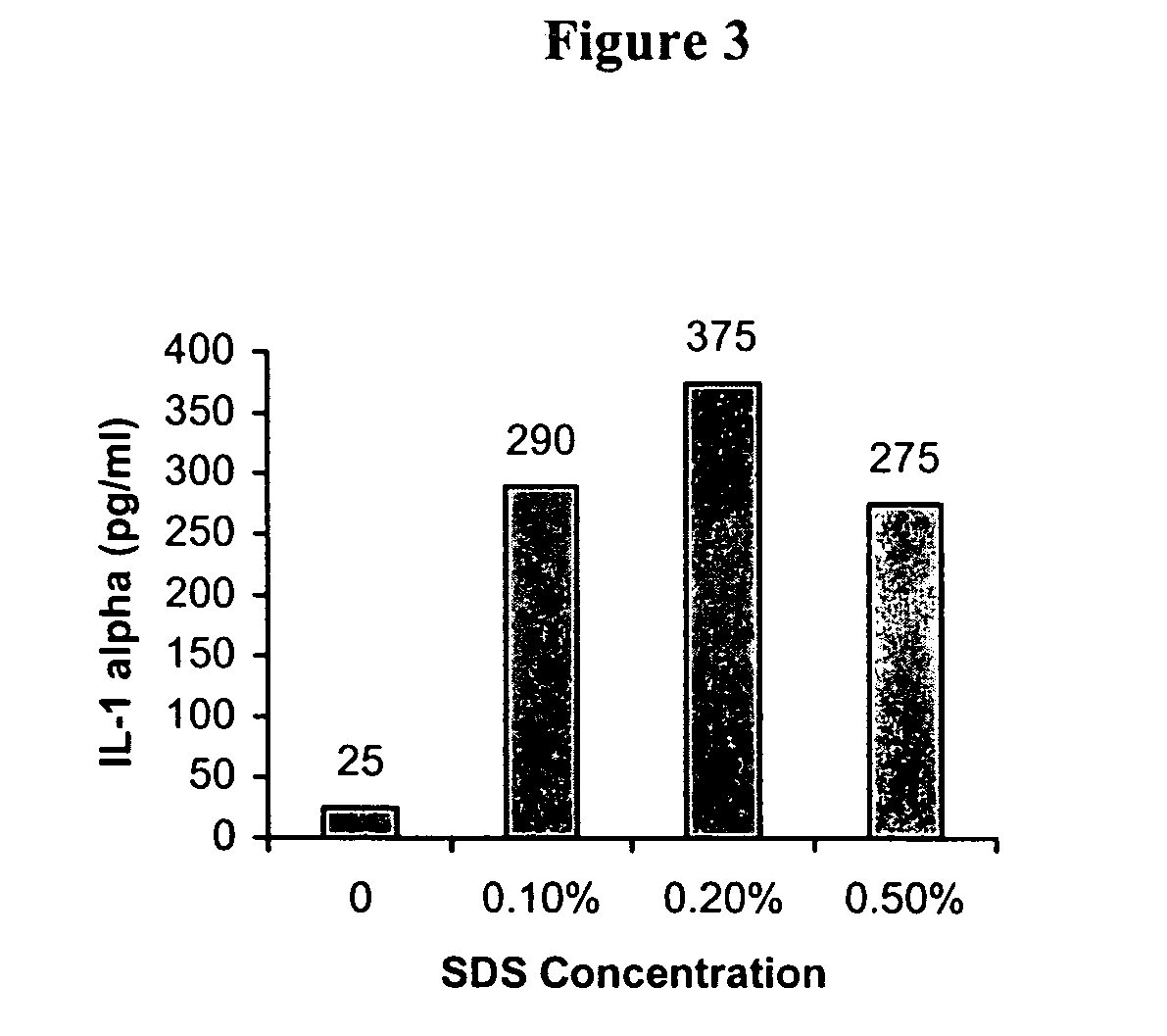
A three dimensional (3-D) model comprising a scaffold and autologous skin cells, the invention also provides methods of predicting immunogenicity and hypersensitivity or allergic or adverse immune reactions to potential therapeutic compounds, biologies, cosmetics and chemical sensitizers using the 3-D model of skin cells. The methods provide an in vitro assay employing autologous blood derived cells in the 3-D skin equivalent model and is of particular utility in the identification and prediction of skin sensitizers and in particular agents that may cause allergic contact dermatitis. The assay of the present invention provides inter alia methods of screening library compounds for sensitizing activity, identifying optimal therapeutics, especially but not exclusively, monoclonal antibodies and kits therefor.
Owner:NEWCASTLE UNIV +1
Three-dimensional skin model
InactiveUS7553664B2High quality resultUniform surfaceMicrobiological testing/measurementBone implantFiberHuman skin
The present invention relates to methods for cultivating dermal fibroblasts, methods for preparing in vitro dermis equivalents, methods for preparing three-dimensional in vitro skin equivalents, an in vitro dermis equivalent, a three-dimensional in vitro skin equivalent, and methods for determining the effect of a chemical substance or of an agent on human skin cells using the in vitro dermis equivalent and / or the in vitro skin equivalent.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Dried and irradiated skin equivalents for ready use
The present invention relates generally to systems and methods for preparing, storing, shipping and using skin equivalents made by organotypic culture. In particular, the present invention relates to systems and methods for producing, transporting, storing and using skin equivalents produced by organotypic culture at reduced temperatures, preferably from 2-8 degrees Celsius to ambient temperature. The methods include sterile packaging of the grafts so that the sterility and integrity of the package is maintained until the time of use for grafting purposes.
Owner:STRATATECH
Cellular scaffold
A cellular scaffold that is suitable for tissue regeneration, cell culture and in vitro assays. The invention relates to a layered cell scaffold that is seeded with mesenchymal and ectodermal cells. The layered cellular scaffold comprises an inoculum of mesenchymal cells and ectodermal cells positioned between two opposing scaffolds in a sandwich configuration. The layered cell scaffold provides a functional skin equivalent that is suitable for transplantation and in vitro cell-based assays.
Owner:STEMEDICA CELL TECH
Cold storage of organotypically cultured skin equivalents for clinical applications
The present invention relates generally to systems and methods for storing, shipping and using skin equivalents made by organotypic culture. In particular, the present invention relates to systems and methods for producing, transporting, storing and using skin equivalents produced by organotypic culture at reduced temperatures, preferably from 2-8 degrees Celsius. The methods include sterile packaging of the grafts so that the sterility and integrity of the package is maintained until the time of use for grafting purposes.
Owner:STRATATECH
Skin equivalents with distinct, juxtaposed dermal compartments
ActiveCN109475666BMicrobiological testing/measurementArtificial cell constructsSkin equivalentBiomedical engineering
Owner:LOREAL SA
Process for producing skin equivalents and their use for in vitro testing and in vivo transplantation
The present invention describes a method for producing a skin equivalent comprising the steps of: a) producing a dermal matrix comprising a first layer and a second layer, comprising the steps of: i) providing said first layer ; ii) providing a polymerizable solution comprising a liquid and at least one polymer selected from collagen, elastin and / or hyaluronic acid; iii) adding said polymerizable solution to said first layer; iv) polymerizing the solution to form the second layer; v) compressing the second layer, wherein due to the compression, the liquid content of the second layer is reduced, and combining the first layer with the second layer layers bonded together to form said dermal matrix; and vi) introducing at least one lumen into said dermal matrix; b) introducing at least one hair follicle and / or hair follicle germ into said dermal matrix produced in step a). at least one cavity to produce a skin substitute; and c) adding at least one additional cell population selected from fibroblasts, endothelial cells, adipocytes, nerve cells, gland cells, melanocytes or keratinocytes to said in a skin substitute to produce the skin equivalent.
Owner:TECH UNIV BERLIN
Method for producing a skin equivalent, and use thereof for in vitro tests and in vivo transplants
The invention relates to a method for producing a skin equivalent, comprising the following steps: a) producing a dermal matrix containing a first layer and a second layer, comprising the steps of i)providing the first layer; ii) providing a polymerizable solution, comprising a liquid and at least one polymer selected from collagen, elastin, and / or hyaluronic acid; iii) adding the polymerizable solution to the first layer; iv) polymerizing the solution in order to form the second layer; v) compressing the second layer, wherein, as a result of the compressing, the liquid content of the secondlayer is reduced and the first layer and the second layer are joined in order to form the dermal matrix; b) inserting at least one hair follicle and / or hair follicle germ into the at least one cavityof the dermal matrix produced in step a) in order to produce a skin surrogate; and c) adding at least one further cell population selected from fibroblasts, endothelial cells, adipocytes, nerve cells,glandular cells, melanocytes, or keratinocytes to the skin surrogate in order to produce the skin equivalent.
Owner:TECH UNIV BERLIN
Skin equivalent culture
InactiveUS20160333320A1Provide rigidityProvide strengthCulture processEpidermal cells/skin cellsFiberSupport matrix
Disclosed is a method of preparing a collagenous construct comprising (i) casting viable collagen-producing human dermal fibroblasts in a support matrix comprising fibrin onto a support material, (ii) incubating in situ said support matrix with said viable collagen-producing human dermal fibroblasts in a collagen-inducing medium thereby (a) inducing or enhancing collagen production by said viable collagen-producing human dermal fibroblasts to form a collagenous construct, and (b) degrading said fibrin, (iii) rendering said collagenous construct free of said viable collagen-producing human dermal fibroblasts, and (iv) cross-linking said collagenous construct with a chemical or exposure to ultraviolet light.
Owner:SMITH & NEPHEW INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com