Functional pigmented skin equivalent
An equivalent, skin technology, applied in the direction of epidermal cells/skin cells, microorganisms, animal cells, etc., can solve the problems that models cannot be displayed, melanin is not observed, and lack of control of epidermal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Example 1: Preparation of skin equivalents
[0163] Preparation of lattices (D-4)
[0164] Dermis equivalents were prepared using bovine type I collagen and human fibroblasts derived from the dermis.
[0165] Bovine type I collagen and human Fibroblasts prepare dermal equivalents. The Petri dish was pre-coated with MEM containing 10% serum.
[0166] A solution of laminin (3% / final volume), type IV collagen (1.5% / final volume), and nestin (0.35% / final volume) was added to the mixture before pouring into the collagen preparation containing cells, vigorously Stir. Place the suspension in an incubator (37 °C-5% CO 2 ) to enable the grid to shrink.
[0167] Inoculation of keratinocytes and melanocytes (D0)
[0168] After retraction of the lattice, keratinocytes and melanocytes were seeded on dermal equivalents.
[0169] For this, cells were expanded for 7 days in their respective growth media before seeding:
[0170] Melanocytes were expanded in the medium for...
Embodiment 2
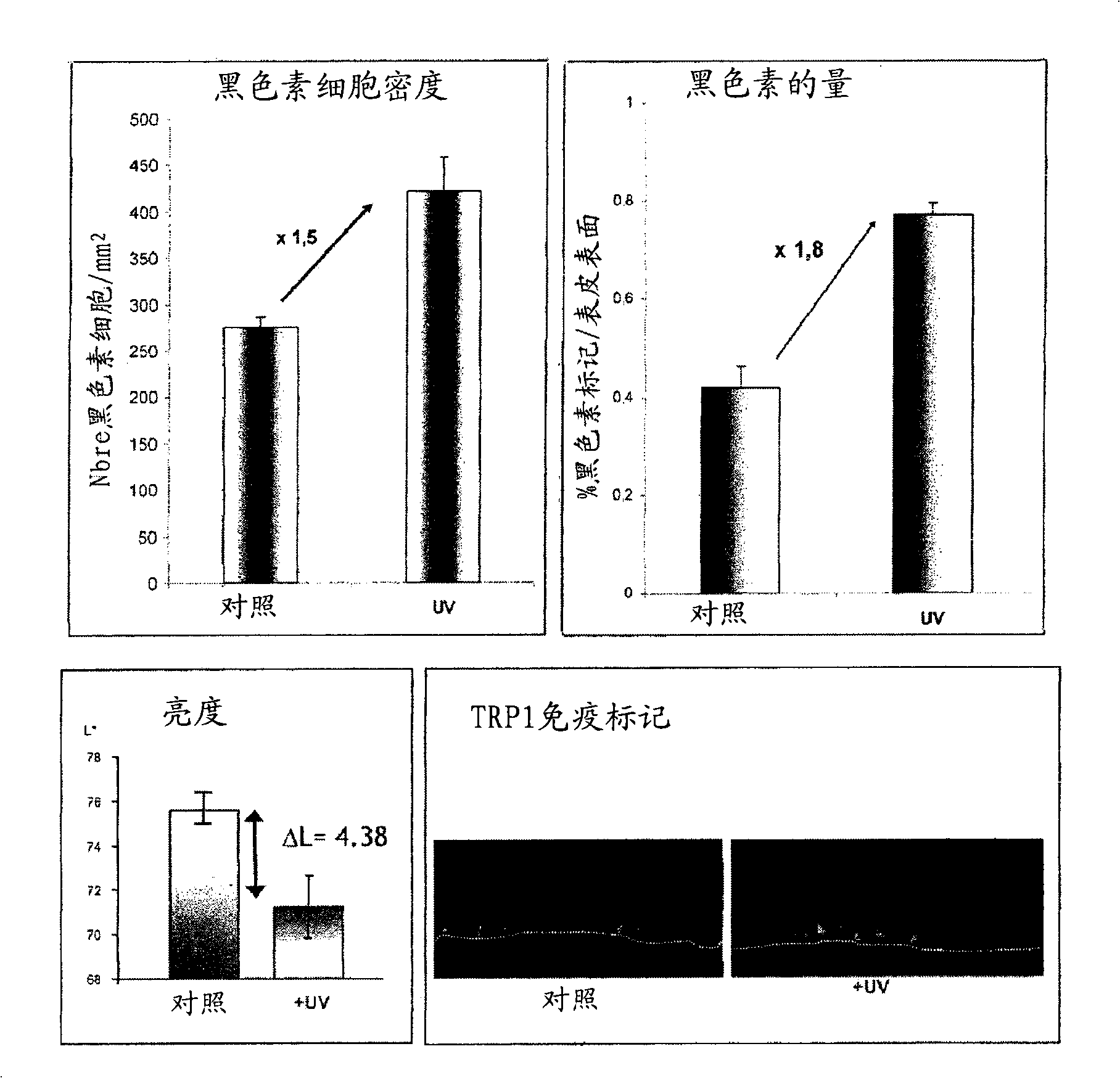
[0180] Example 2: Human Reconstituted Pigmented Skin Containing 3 Cell Types: Melanocytes, Keratinocytes and fibroblasts ( figure 1 )
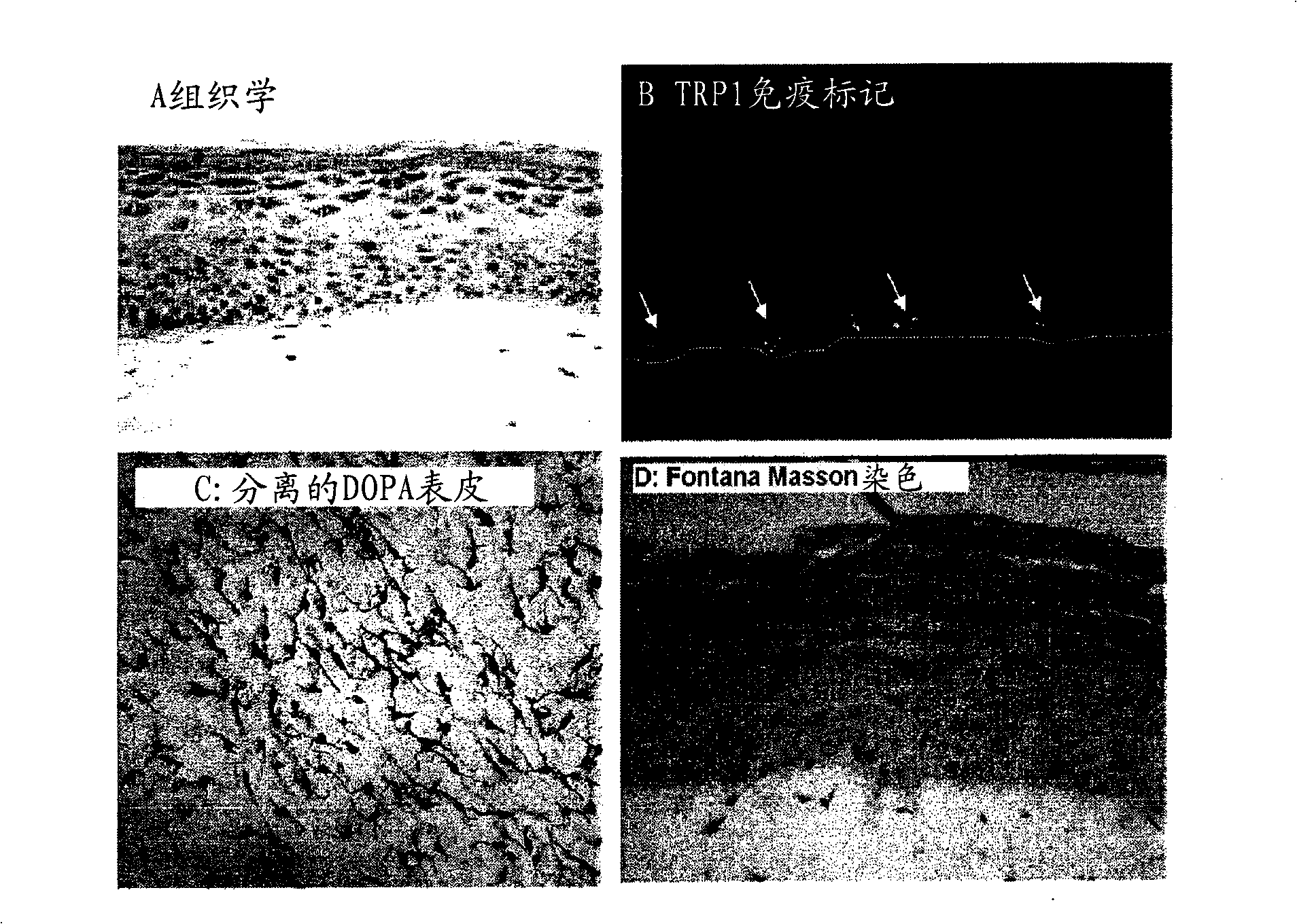
[0181] After 7 days of emergence, the skin exhibited a histology and three-dimensional structure similar to human skin.
[0182] It has a stratified and differentiated epidermis composed of various cell layers (basal, spinous-cell, granular) and stratum corneum.
[0183] The dermis contains viable fibroblasts embedded in a collagen matrix ( figure 1 A).
[0184] Melanocytes are integrated into the epidermis and display a morphology and density similar to normal skin ( figure 1 C, DOPA response on isolated epidermis). They are clearly localized in the basal layer ( figure 1B, Immunolabeling of TRP1, the dotted white line indicates the dermal-epidermal junction), and the production of melanosomes and melanin, which are transferred to nearby keratinocytes ( figure 1 D, using Fontana-Masson to stain melanin).
Embodiment 3
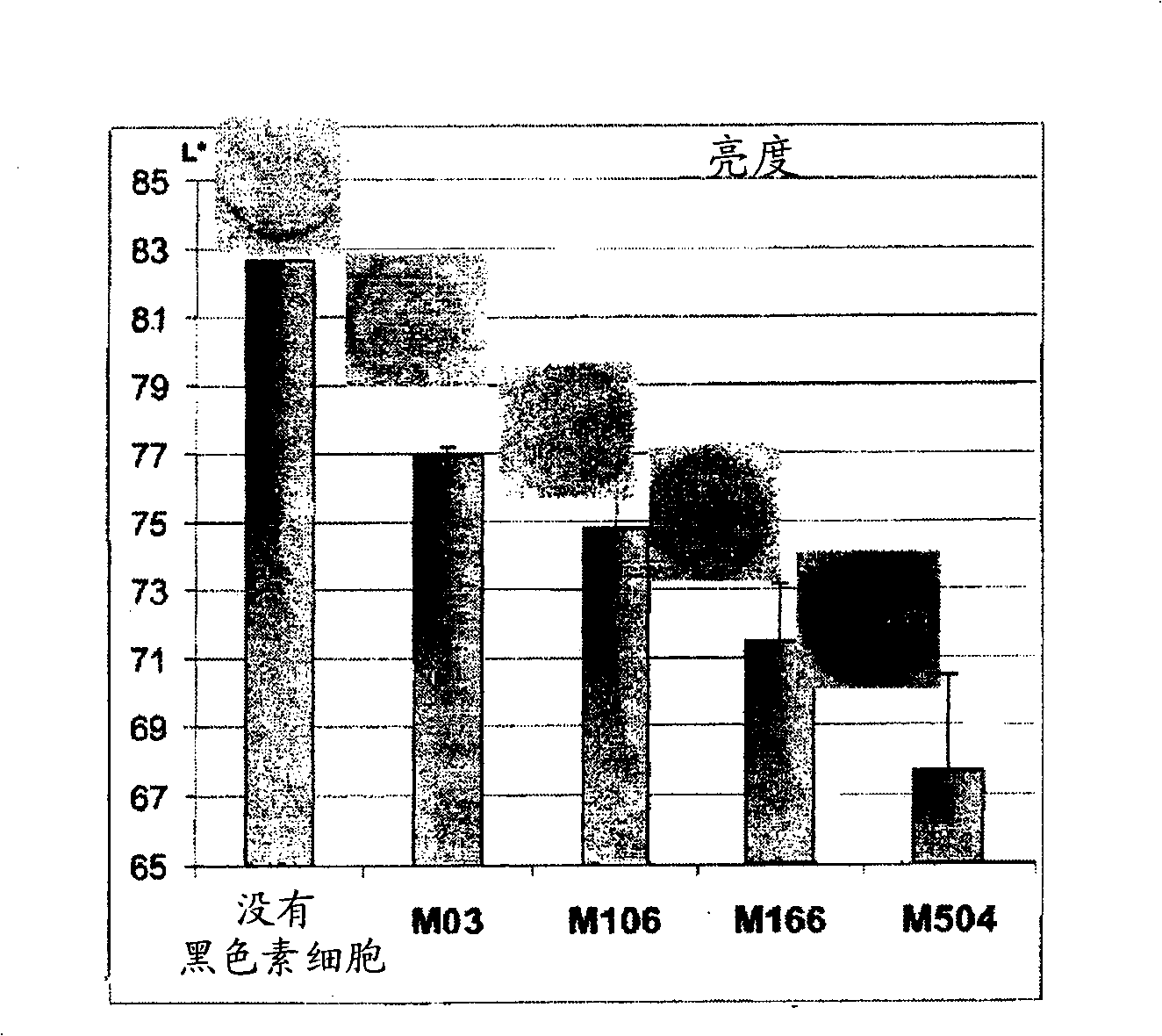
[0185] Example 3: Human Reconstituted Pigmented Skin with Constitutive Pigment Shape Associated with Melanocytes to make( figure 2 )
[0186] The model was able to integrate various melanocyte lines from the least pigmented to the most highly pigmented (sound model), the phenotype of the more or less pigmented reconstructed skin corresponded to the melanocyte type used ( Physical maintenance): In fact, the more pigment the melanocyte line has (M03-M504), the darker the macroscopic color of the skin, and the greater the amount of melanin visible on the slice. This macroscopic pigmentation is reflected by a decrease in brightness ( figure 2 ,brightness).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com