Truncated glp-1 derivaties and their therapeutical use
a technology of truncated glucagon and derivate, which is applied in the field of new truncated glucagonlike peptide1 (glp1) analogues, can solve the problems that the fear of injection may become a serious obstacle to the widespread use of clinically very promising compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[Glu22,Arg26]GLP-1(7-33)amide
[0782]
H—H A E G T F T S D V S S Y L E E Q A A R E F I A W L V—NH2
[0783]Preparation method: B
[0784]The peptide was eluted at 64% acetonitrile.
[0785]Structure confirmed by MALDI-MS
[0786]Calculated MW=3056.4
example 2
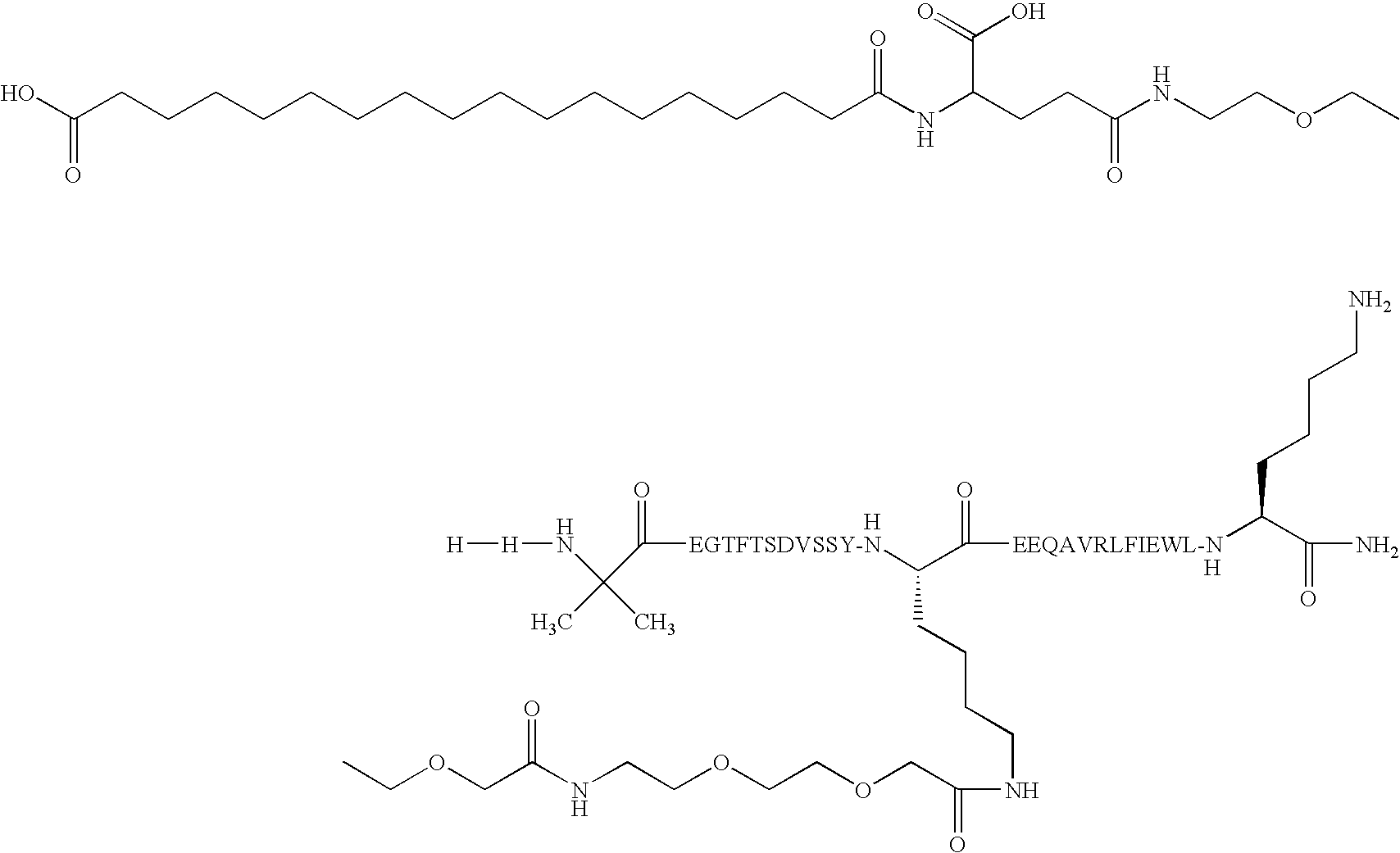
N epsilon20 {2-(2-{2-[2-(2-{2-[4-Carboxy-4-(17-carboxy-heptadecanoylamino)butyrylamino]ethoxy}ethoxy)acetylamino]ethoxy}ethoxy)acetyl}-(Aib8,Lys20,Glu22,Val25,Arg26,Leu27,Glu30,Lys33)GLP-1(7-33)amide
[0787]
[0788]Preparation method: Method C except that the peptide was prepared on an Apex396 from Advanced Chemtech using a molar excess of 8-10 fold amino acid, DIC and HOAt / HOBt (1:1) and the Mtt group was deprotected with hexaflouroisopropanol. The final product was characterized by analytical HPLC and MALDI-MS.
[0789]HPLC (METHOD 02_B6—1):
[0790]RT=32 min
[0791]MALDI-MS=3901
[0792]Calculated MW=3900.5
example 3
N epsilon20 {2-(2-{2-[2-(2-{2-[4-Carboxy-4-(17-carboxy-heptadecanoylamino)butyrylamino]ethoxy}ethoxy)acetylamino]ethoxy}ethoxy)acetyl}-(Aib8,Lys20,Glu22,Arg26,Glu30)GLP-1(7-33)amide
[0793]
[0794]Preparation method: Method C except that the peptide was prepared on an Apex396 from Advanced Chemtech using a molar excess of 8-10 fold amino acid, DIC and HOAt / HOBt (1:1) and the Mtt group was deprotected with hexaflouroisopropanol. The final product was characterized by analytical HPLC and MALDI-MS.
[0795]HPLC (METHOD 02_B6—1):
[0796]RT=32.9 min
[0797]MALDI-MS=3858.7
[0798]Calculated MW=3859.3
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com