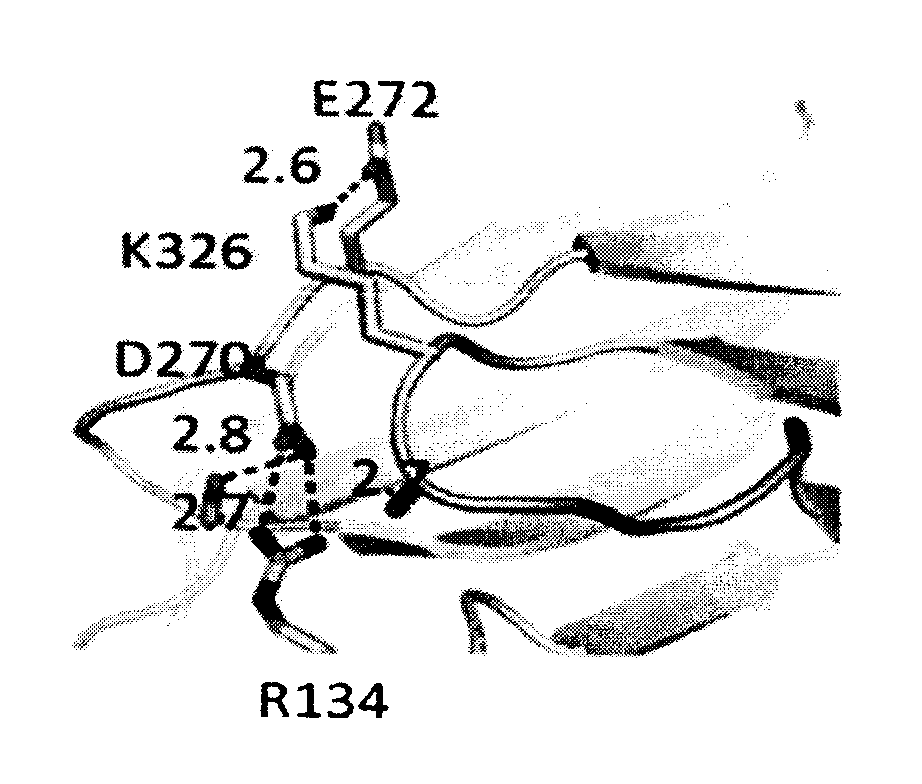
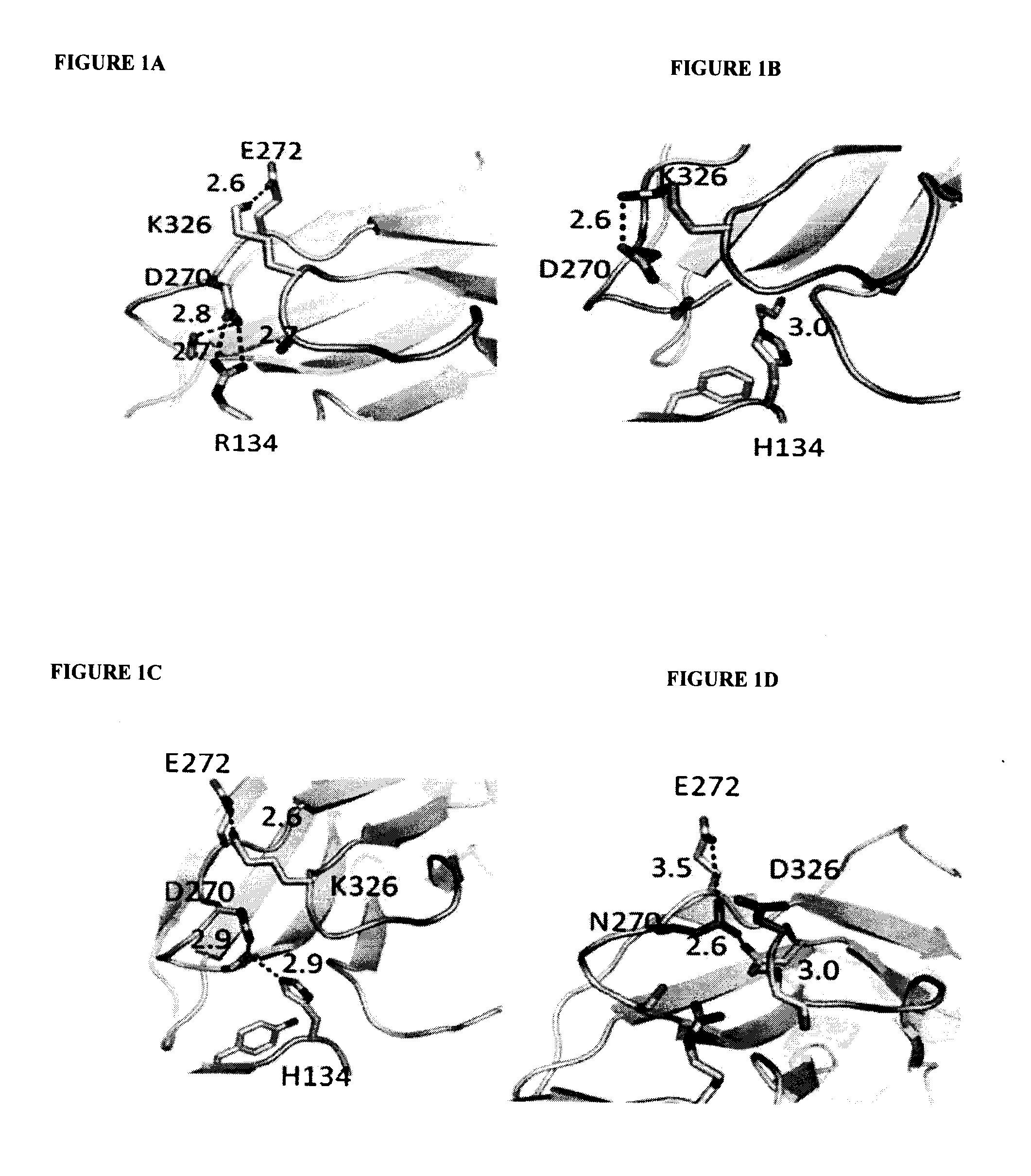
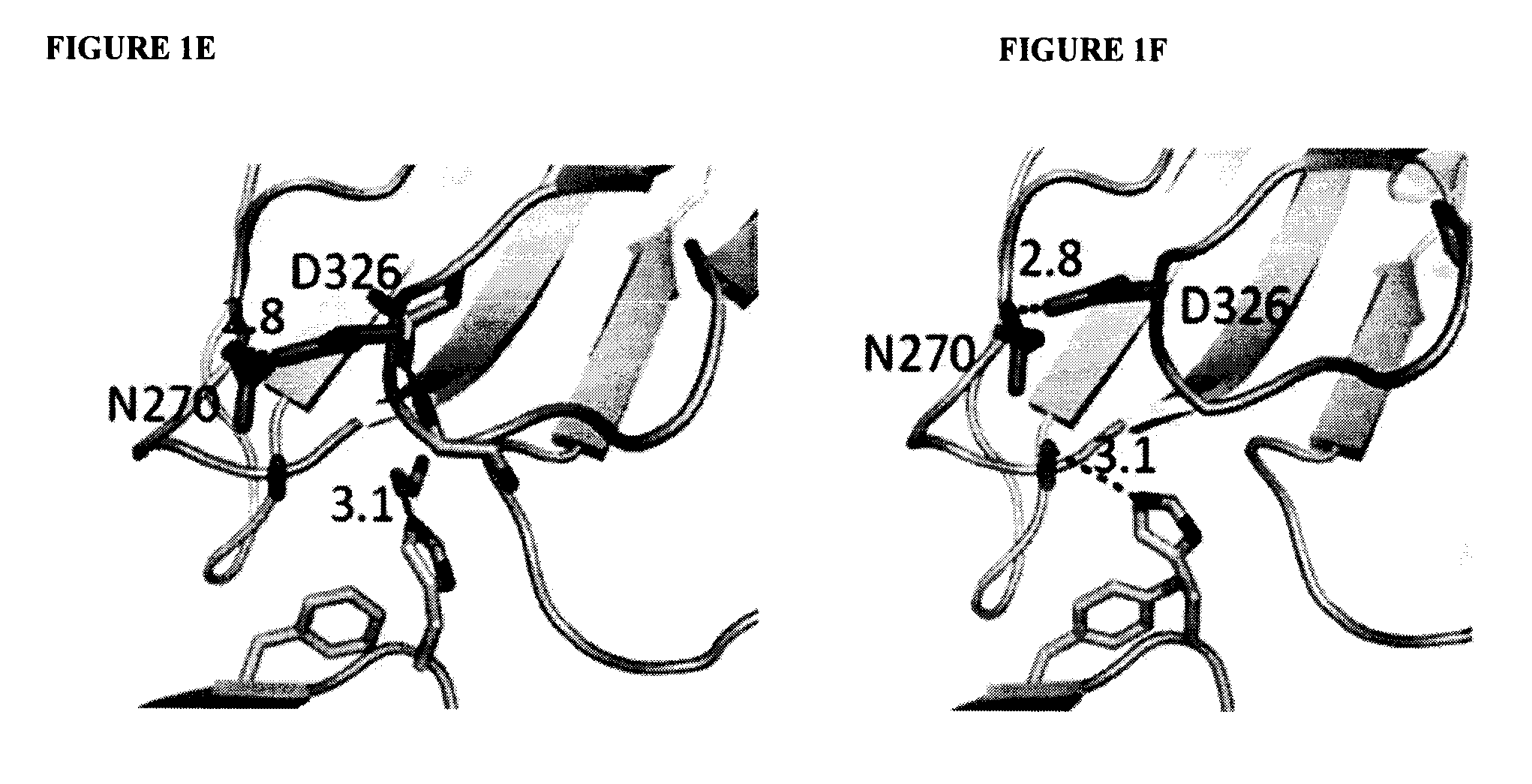
Antibodies with Enhanced or Suppressed Effector Function
a technology of effector function and antibody, which is applied in the field of antibodies, fusion proteins and polypeptides, can solve the problems of not removing foreign antigens, not evenly distributed variability through the variable domains of antibodies, and previous studies failing to describe the effect of simultaneous changes to multiple amino acids, etc., to achieve less binding and higher selectivity in binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Illustration Of Rational Design Of Polypeptides Having A Combination Of Mutations Selected For Producing Selected FcγR Binding Profile
[0212]Antibodies targeting the HER2 / neu receptor:
[0213]The Human Epidermal growth factor Receptors (HERs) are proteins embedded in the cell membrane and communicate molecular signals from outside the cell to inside the cell, and turn genes on and off. The HER proteins regulate cell growth, survival, adhesion, migration, and differentiation—functions that are amplified or weakened in cancer cells. Human Epidermal growth factor Receptor 2″—a member of the epidermal growth factor receptor family, is a protein giving higher aggressiveness in breast cancers. HER2 / neu has also been designated as CD340. Approximately 15-20 percent of breast cancers have an amplification of the HER2 / neu gene or overexpression of its protein product. Overexpression of this receptor in breast cancer is associated with increased disease recurrence and worse prognosis. Because of...
example 2
In Vitro And Ex Vivo Validation Of Designed Antibodies
[0221]Once designed in silico individual antibodies are tested according to the methods described in Stavenhagen et al. (2007) Cancer Res. 67:882 and Stavenhagen et al. (2008), Adv. Enzyme Regal., 48:152.
[0222]Briefly, the gene for each mutant is constructed by standard chemical synthesis using, for example, Trastuzumab IgG1 as the wild-type framework. After cloning into a suitable vector, the mutant Fc polypeptides are expressed in mammalian HEK293 cells. The FcγRIIa, FcγRIIb and FcγRIIIa are also cloned and expressed in HEK293 cells. The binding affinities of the antibodies to each of the three receptors is then determined by surface plasmon resonance.
[0223]Surface Plasmon Resonance Analysis: Surface Plasmon Resonance Analysis: Affinity of Fcγ receptors to antibody Fc was measure by SPR (surface Plasmon resonance) using a ProteOn XPR36 system from BIO-RAD. HER-2 in buffer (10 mM Hepes pH 6.8) was immobilized on CM5 chip through...
example 3
[0228]Additional antibodies comprising modifications based on the in silico methods described above are summarized in Table 2.
TABLE 2in silico ΔΔG solv [kcal / mol]IIIa (F)IIIa (V)IIa (H)IIa (R)IIb (F)IIb (Y)Mutations Compared to WildtypevariantvariantvariantvariantvariantvariantWildtype Trastuzumab++++++++++++++++++L235YG236A———+++++++L235FG236A———+++++++++++++G236AD270E———+++++++++G236AD270L———++++++++H268FD270E———++++++++++++++G236DD270E———+++++++S239DD270L———++++++++++++++++++++++++++++++S239DD270K———+++++++++++++++++++++++++++++D270EY300L———+++++++++++++++D270ES298A———++++++++++++++++++++ ++++ −5 to −2 kcal / mol;+++ Between −2 and 2 kcal / mol;++ 2 to 5 kcal / mol;+ >5 kcal / molNB: no binding;ND: Not Determined
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com