Preparation method for lithium ion battery cathode material fluorine-doped lithium vanadate with circulatory stability
A lithium-ion battery and negative electrode material technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of high requirements for sintering equipment, rapid material capacity decay, poor cycle performance, etc., and achieve uniform and complete coating effect. The effect of high battery safety performance and improved electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
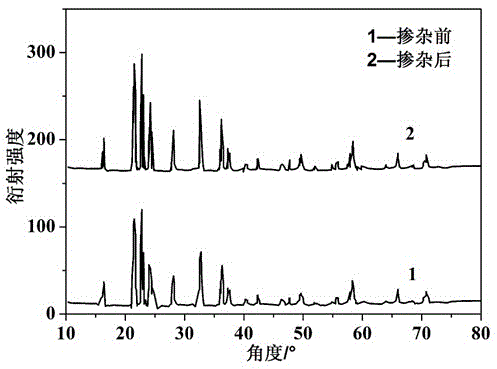
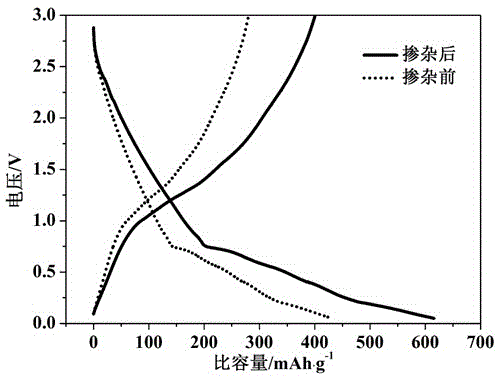
Image
Examples
Embodiment 1
[0034] (1) Weigh 4.68g of ammonium metavanadate and 12.24g of lithium acetate dihydrate according to the molar ratio V:Li=1:3, add them to 50mL of deionized water, and disperse to prepare suspension A;
[0035] (2) According to the molar ratio V:complexing agent=1:2, weigh 16.81g of citric acid monohydrate, add it into 150mL deionized water and stir to obtain a complexing agent solution B with a concentration of 0.1mol / L;
[0036] (3) Control the stirring, add the complexing agent solution B to the suspension A dropwise, and form a clear and transparent solution C after stirring at a temperature of 40°C;
[0037] (4) Weigh 0.026g of lithium fluoride according to the molar ratio V:F=1:0.025, add it to the clear and transparent solution C for stirring, and raise the temperature to 90°C to evaporate the solvent deionized water, and then bake at 120°C Dry for 24 hours to obtain a blue lithium vanadate precursor;
[0038] (5) Grinding and pulverizing the blue lithium vanadate prec...
Embodiment 2
[0045] (1) Weigh 4.68g of ammonium metavanadate and 14.28g of lithium acetate dihydrate according to the molar ratio V: Li=1:3.5, and add them to 50mL of deionized water to prepare suspension A
[0046] (2) According to the molar ratio V:complexing agent=1:2, weigh 16.81g of citric acid monohydrate and add it to 150mL deionized water to obtain complexing agent solution B;
[0047] (3) Control the stirring temperature at 50°C, add the complexing agent solution B to the suspension A dropwise, and form a clear and transparent solution C after stirring;
[0048] (4) Weigh 0.026g of lithium fluoride according to the molar ratio V:F=1:0.025, add it to the clear and transparent solution C in step (3), evaporate the solvent after the stirring temperature rises to 90°C, and bake in the air at 120°C for 24h Obtain blue lithium vanadate precursor;
[0049] (5) After grinding the blue lithium vanadate precursor, under the protection of carbon dioxide, it was pre-fired at 500 ° C for 5 ho...
Embodiment 3
[0051] Weigh 4.68g of ammonium metavanadate and 12.24g of lithium acetate dihydrate according to the element molar ratio V: Li=1:3, and add them to 50mL of deionized water to prepare suspension A
[0052] According to the molar ratio V:complexing agent=1:1.5, weigh 12.61g of citric acid monohydrate and add it to 150mL deionized water to obtain complexing agent solution B;
[0053] Control the stirring temperature at 40°C, add the complexing agent solution B to the suspension A dropwise, and form a clear and transparent solution C after stirring;
[0054] Weigh 0.052g of lithium fluoride according to the molar ratio V:F=1:0.05, add it to the clear and transparent solution C, evaporate the solvent after stirring the temperature to 90°C, and dry in the air at 100°C for 24 hours to obtain blue lithium vanadate Precursor;
[0055] Grind the blue lithium vanadate precursor and pre-calcine at 500°C for 5h under the protection of Ar to obtain the gray lithium vanadate precu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com