Exendins to lower cholesterol and triglycerides
a technology of triglyceride and exendin, which is applied in the field of medicine, can solve the problems of greater risk of coronary heart disease, and achieve the effect of reducing total cholesterol and total cholesterol levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0129]In order that the invention described herein may be more fully understood, the following examples are set forth. It should be understood that these examples are for illustrative purposes only and are not to be construed as limiting this invention in any manner.
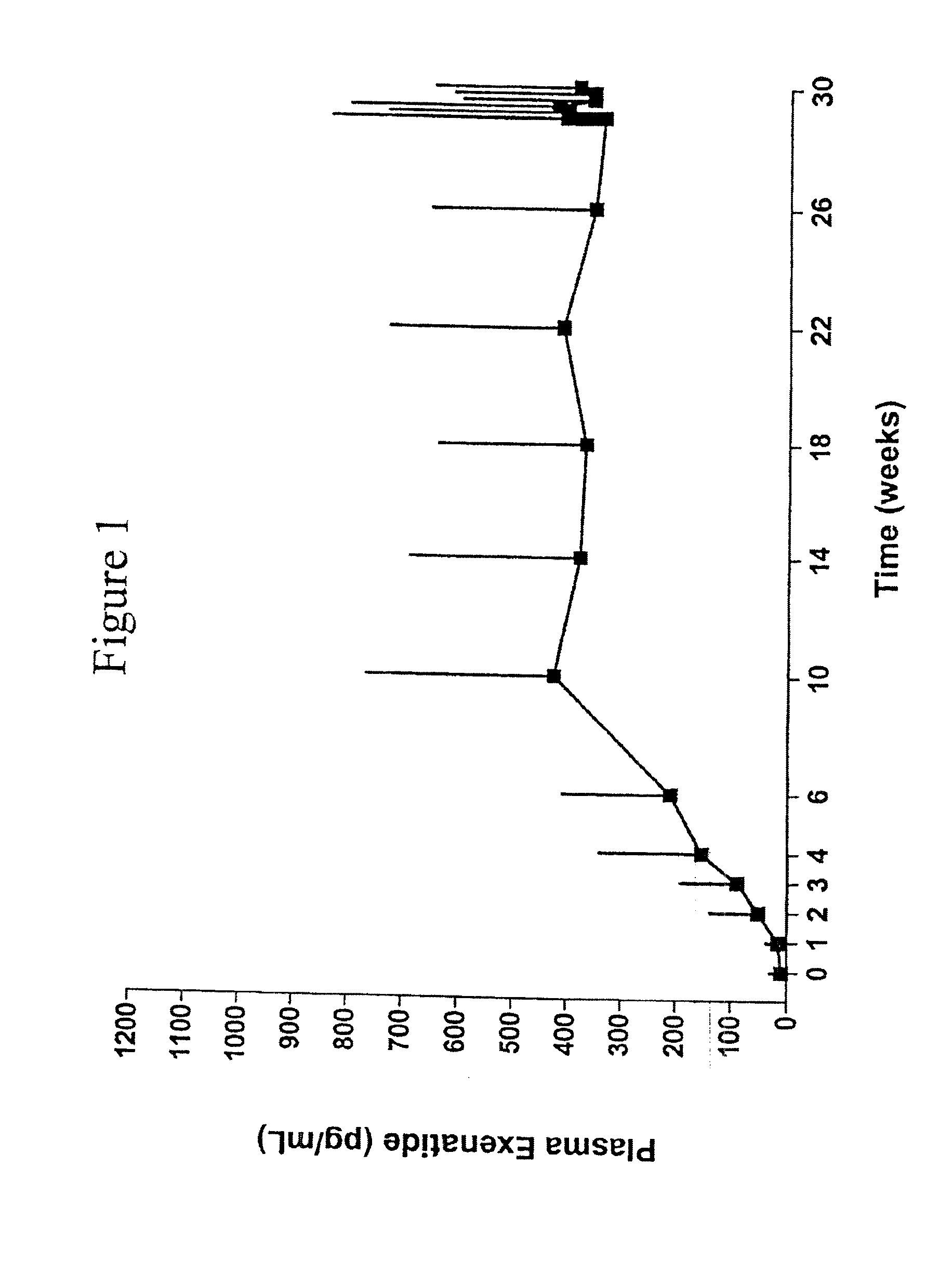
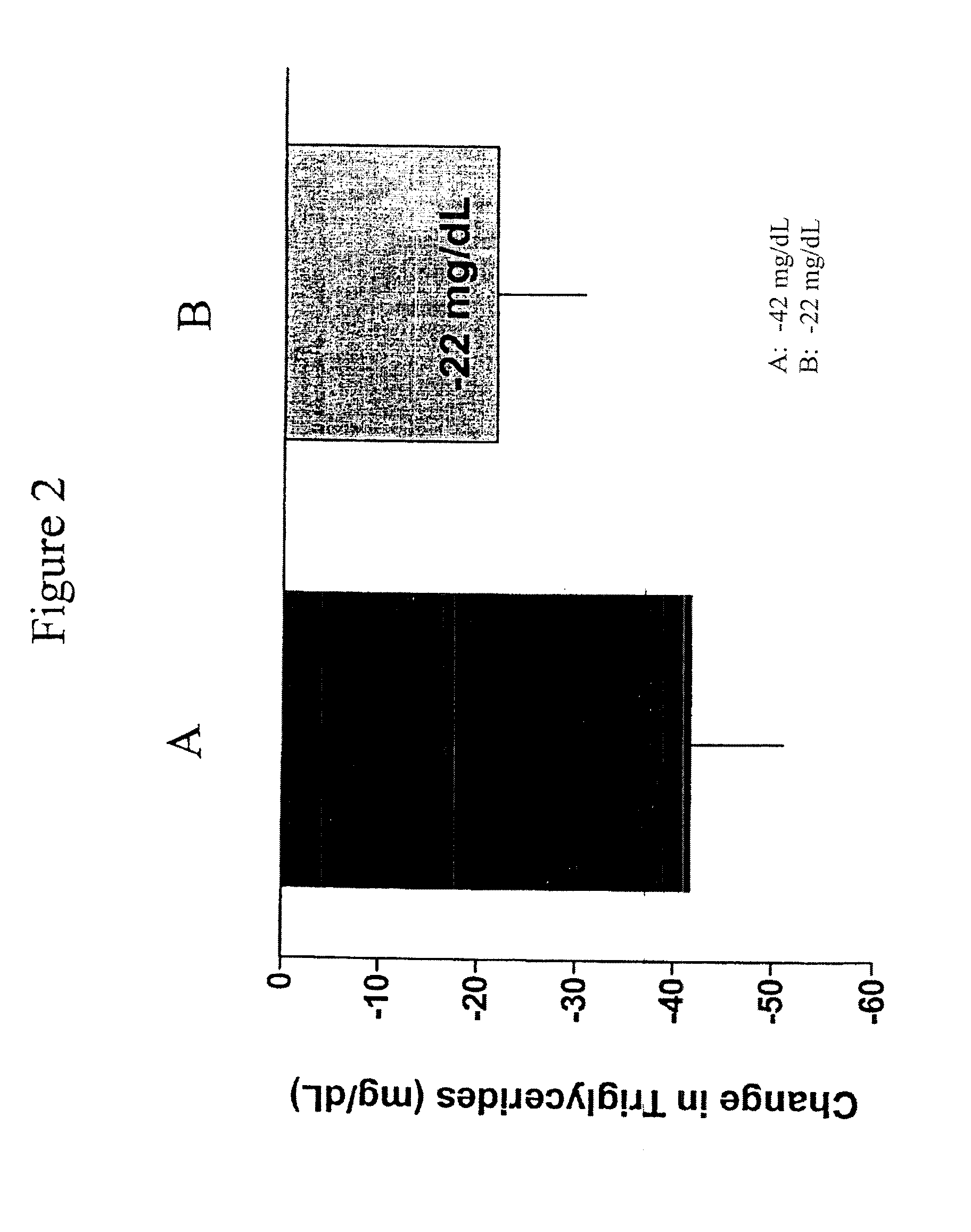
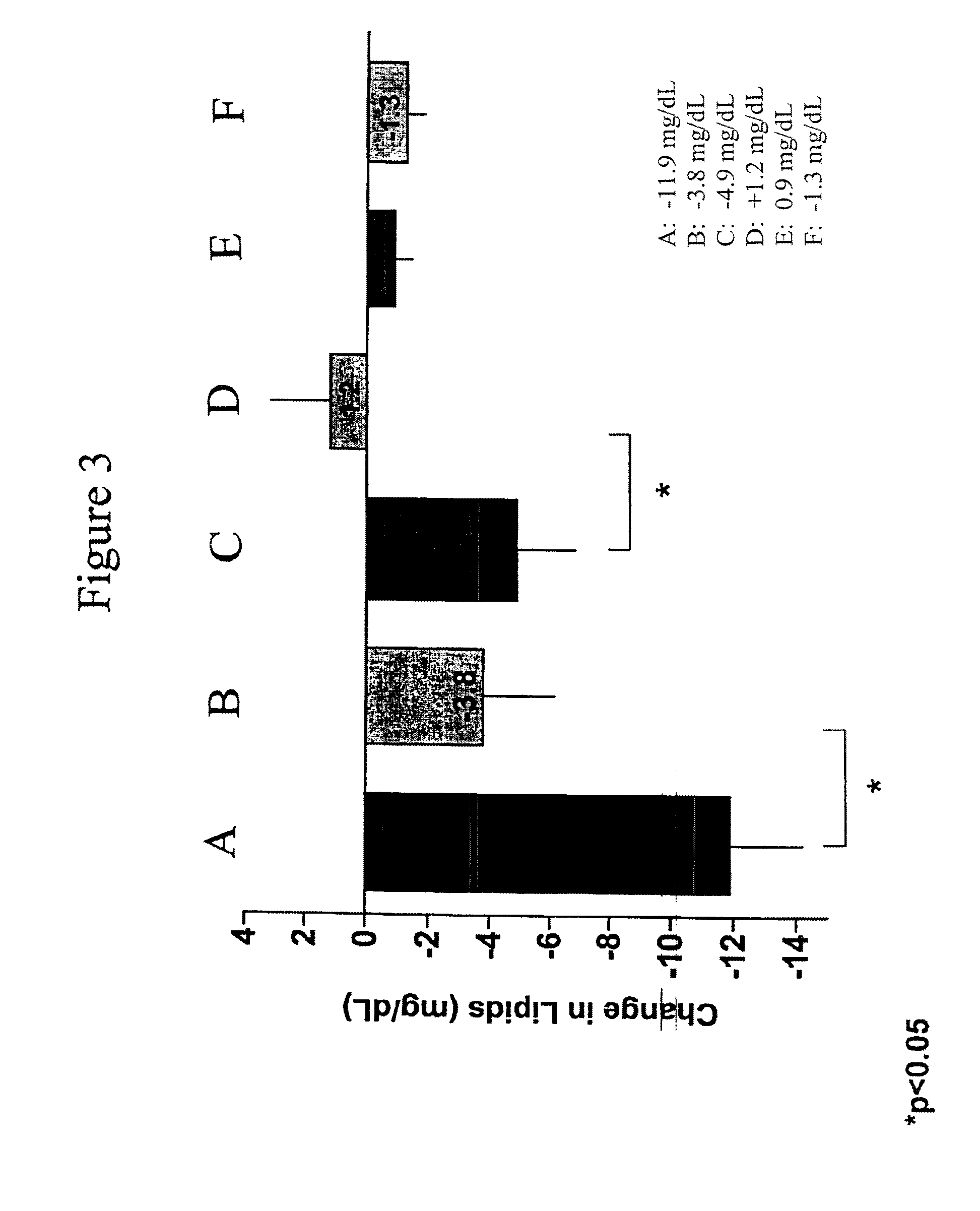
[0130]The pharmacokinetics of a long-acting release formulation of exenatide was evaluated in a study in patients with Type 2 diabetes. The study population consisted of individuals with type 2 diabetes treated with a stable regimen of oral diabetes medications or managed with diet modification and exercise. Patients, male or female, had a mean age of 55±10 years with a mean body mass index (BMI) of about 34.9 kg / m2 at screening, and a mean HbA1c of 9.3±1.0% at screening. The 30 week study compared a 10 μg formulation of exenatide administered twice daily (BID) by subcutaneous (SC) injection and a 2 mg formulation of exenatide administered once weekly by subcutaneous (SC) injection. The study was also conducted, inter al...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com