Once-weekly oral administration of aripiprazole
a technology of aripiprazole and once-weekly oral administration, which is applied in the direction of drug composition, metabolism disorder, nervous disorder, etc., can solve the problems of time-consuming and expensive supervised administration, patients may consider once-a-day oral administration dosage regime too frequent for many patients, etc., to reduce the indirect human cost of drugs, improve patient convenience and compliance, and increase adverse events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Aripiprazole Compositions
[0285]30 mg direct compression (DC) and wet granulation (WG) controlled release tablets were manufactured as described below.
[0286]Direct Compression Tablets
[0287]The ingredients set out in Table 1 below were blended together in a planetary mixer for 5 minutes. The blend was compressed on a rotary tabletting machine, using 7.0 mm diameter round n / c punches. The tablet breaking strength was 2.5 kp to 3.5 kp.
TABLE 1Direct Compression CompositionDJ / 1 / 27 / ADCIngredient%tablet mgbatch gAripiprazole2030100Methocel K4M3552.5175Avicel PH 2004466220Sodium Stearyl Fumarate11.55100150500
[0288]Wet Granulation Tablets
[0289]The ingredients set out in Table 2 below except for sodium stearyl fumarate were blended together in planetary mixer for 5 minutes prior to wet granulation with purified water. The moist powders were dried in a fluid bed drier at an inlet temperature of 70° C. for 15 minutes. The dried granule had a loss on drying value of 2.5% w / w. The granules were si...
example 2
In Vitro Release Experiments
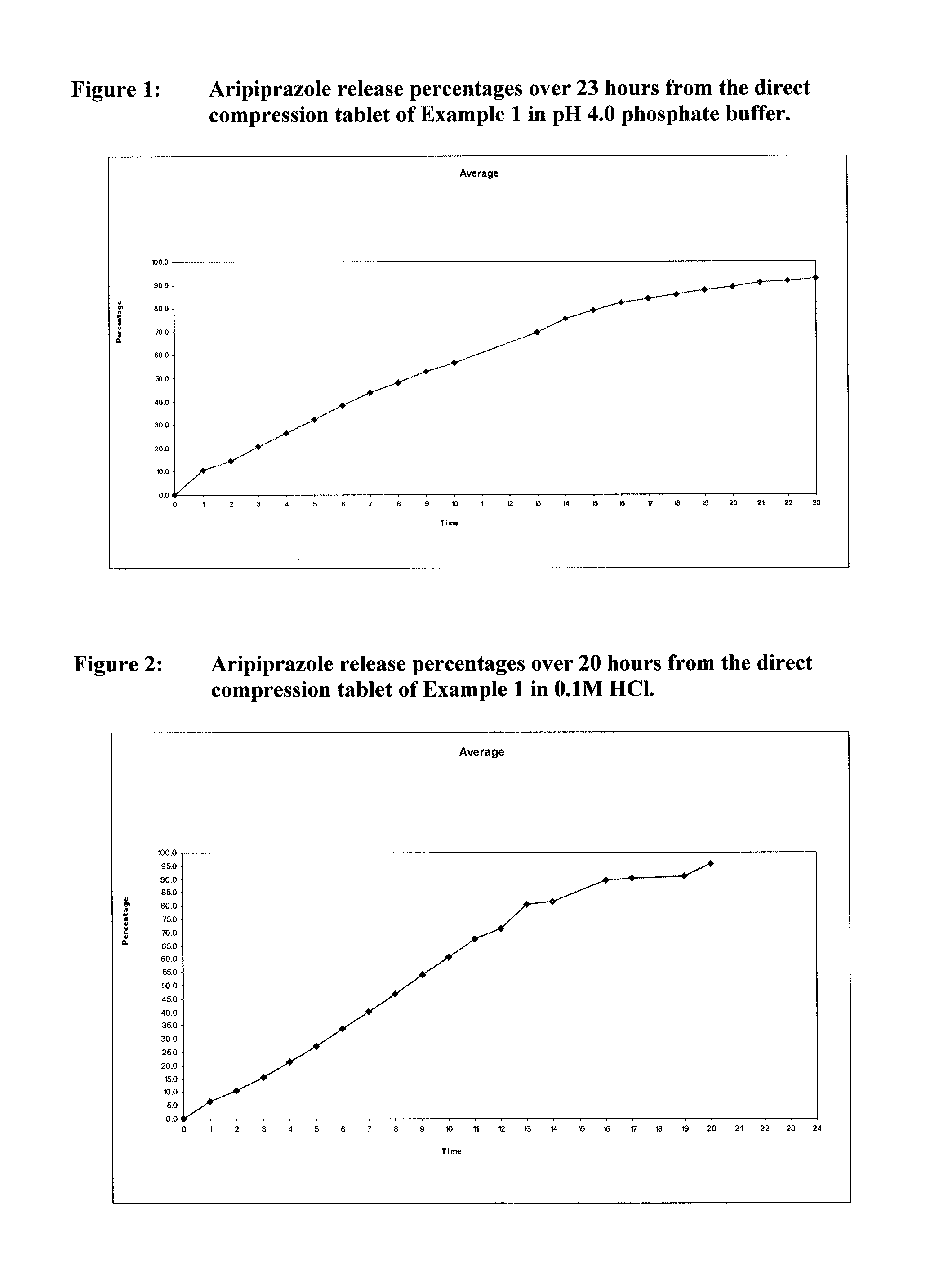
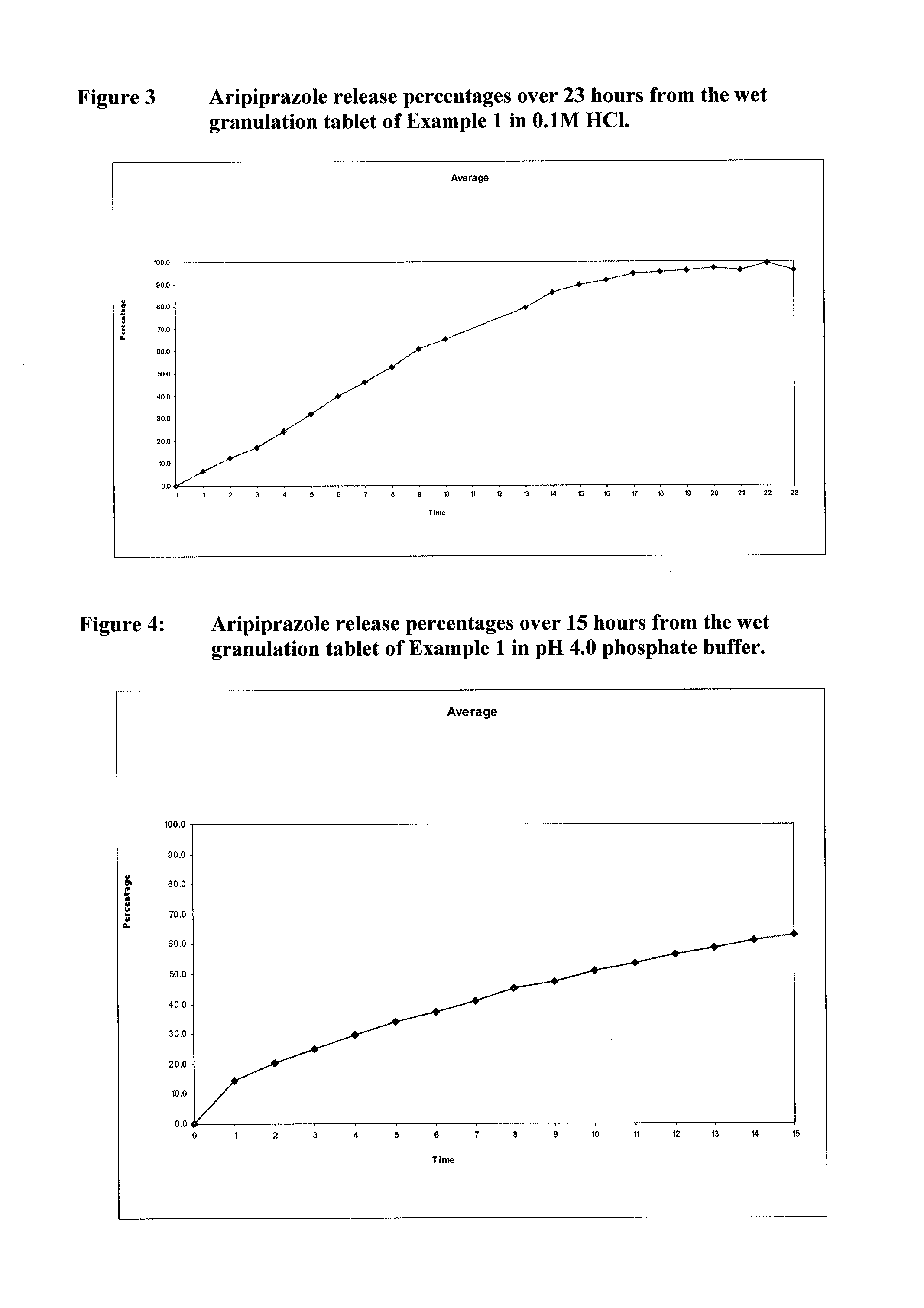
[0291]The release profiles of Aripiprazole from the DC and WG tablets described in Example 1 were studied in pH 4.0 phosphate buffer and 0.1M HCl, as described in more detail below.
[0292]Dissolution System
Dissolution medium0.05M Phosphate Buffer (pH 4.0)or 0.1M Hydrochloric AcidApparatusUSP II (Paddles)Volume900 mlSpeed100 rpmTemperature37° C.
[0293]7 litres 0.05M Phosphate buffer (pH 4.0) was prepared by dissolving 47.8 g potassium phosphate in 6.75 litres of water, then adding portions of 60% orthophosphoric acid solution to obtain a pH of 4.0 (+ / −0.05). The solution was made up to 7 litres with water and the pH adjusted as necessary with sodium hydroxide or phosphoric acid).
[0294]The 0.1M HCl was prepared by diluting 3.5 litres of 0.2M hydrochloric acid to 7 litres with purified water.
[0295]Dissolution Procedure
[0296]Aliquots were taken from each dissolution vessel at the indicated (e.g. hourly) hourly intervals. The UV absorbance of each aliquot at 215...
example 3
Pharmacokinetic Modelling
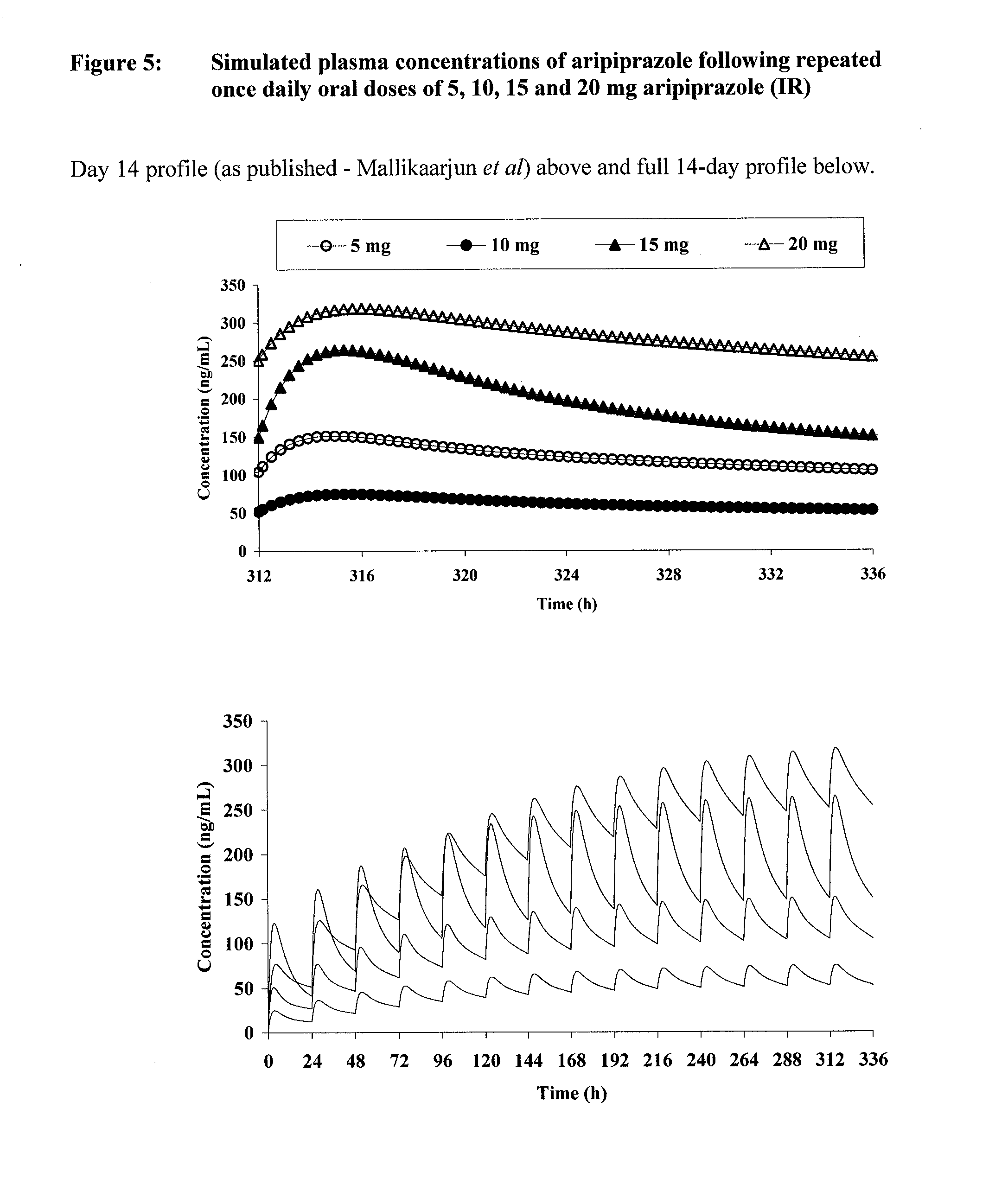
[0303]Multiexponential functions were fitted to the published plasma concentration-time profiles of immediate-release (IR) aripiprazole after repeated once-daily oral doses of 5, 10, 15 and 20 mg (Mallikaarjun S, Salazar D, Braner S, “Pharmacokinetics, tolerability and safety of aripiprazole following multiple oral dosing in normal healthy volunteers”, J. Clin. Pharmacol., 2004; 44:179-187).
[0304]The function (representing first-order absorption with two-compartment disposition) was of the form:
C(t)=A·e−α(t)+B·e−β(t)+C·e−k01(t)
where A, B, α, β and k01 are constants, C=−(A+B) and C(t) is the plasma concentration at time, t.
[0305]The published model (Mallikaarjun et al, see above) was used to simulate plasma concentrations of aripiprazole after various dose regimens of the IR formulation. In addition, various dose regimens of a sustained-release (SR) formulation were simulated assuming that the release was zero-order for examples ranging from 10 h to 18 h. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Biodegradability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com