Primer probe combination and detection kit for detecting mycoplasma pneumoniae, chlamydia pneumoniae and adenovirus
A technology for Mycoplasma pneumoniae and Chlamydia pneumoniae, which is applied in the biological field, can solve problems such as unreasonable involvement of primers and probes, and achieve the effects of good specificity, cost saving and sensitive identification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Preparation of mycoplasma pneumoniae, chlamydia pneumoniae and adenovirus triple nucleic acid detection kit
[0057] The primer and probe sequences in the kit are shown in Table 1 below:
[0058] Table 1 Primer, probe sequence
[0059]
[0060]
[0061] The kit also includes: 10mM dNTPs, 1U / μL UDG enzyme, 5U / μL Taq enzyme, 50mM MgCl 2 . The kit also includes a negative control (sterile water) and a positive control (artificially synthesized at a concentration of 1×10 6 Copies / mL of pseudovirus).
Embodiment 2
[0062] The detection method of embodiment 2 kit of the present invention
[0063] Detection method of the present invention is Real time RT-PCR, and Real Time RT-PCR reaction process is:
[0064] The composition of each detection system is shown in Table 2:
[0065] Table 2 Detection system
[0066]
[0067]
[0068] Fluorescence detection channel selection:
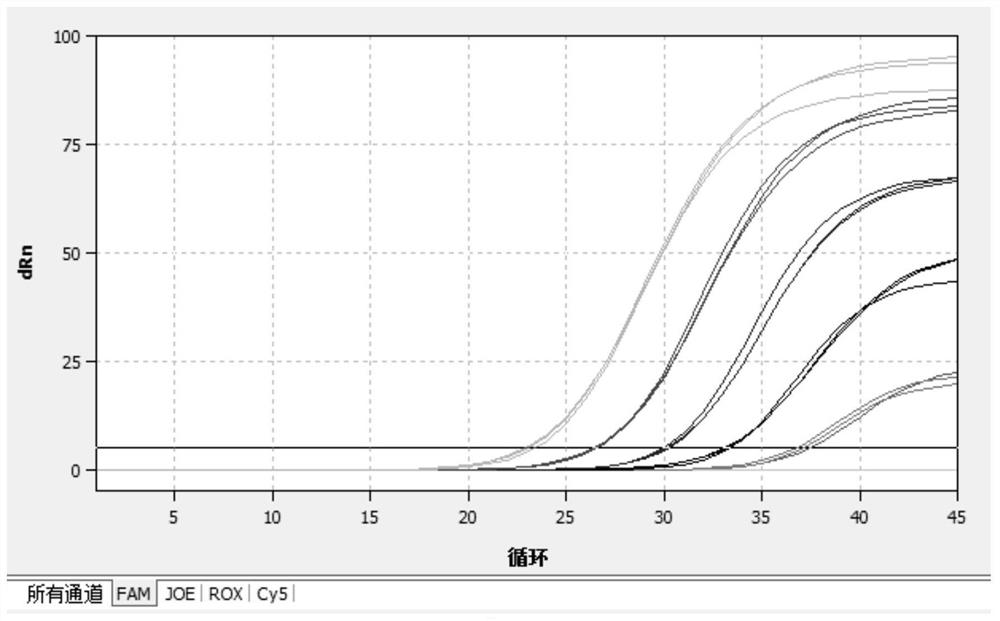
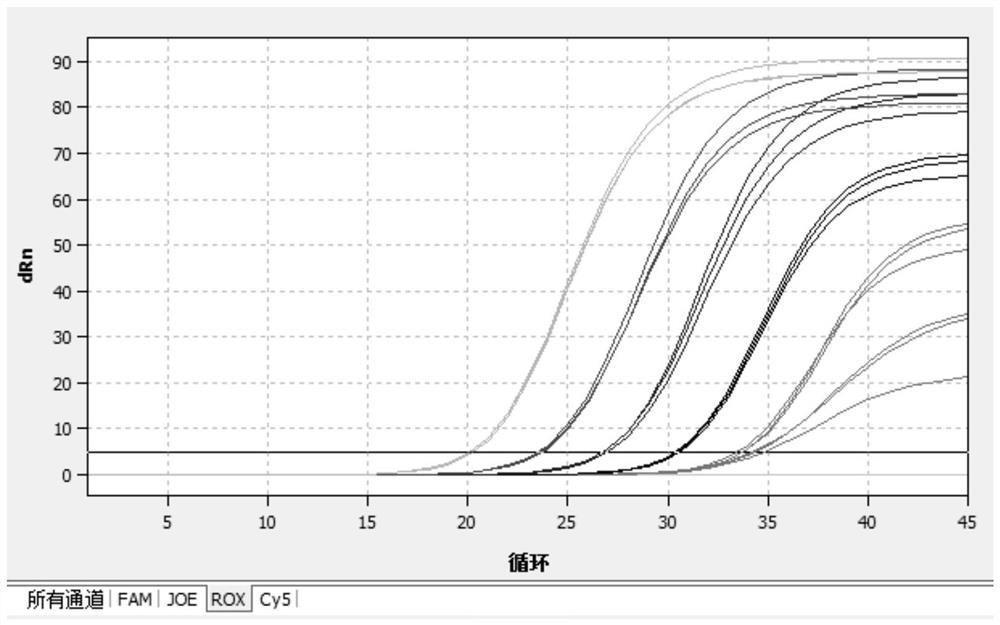
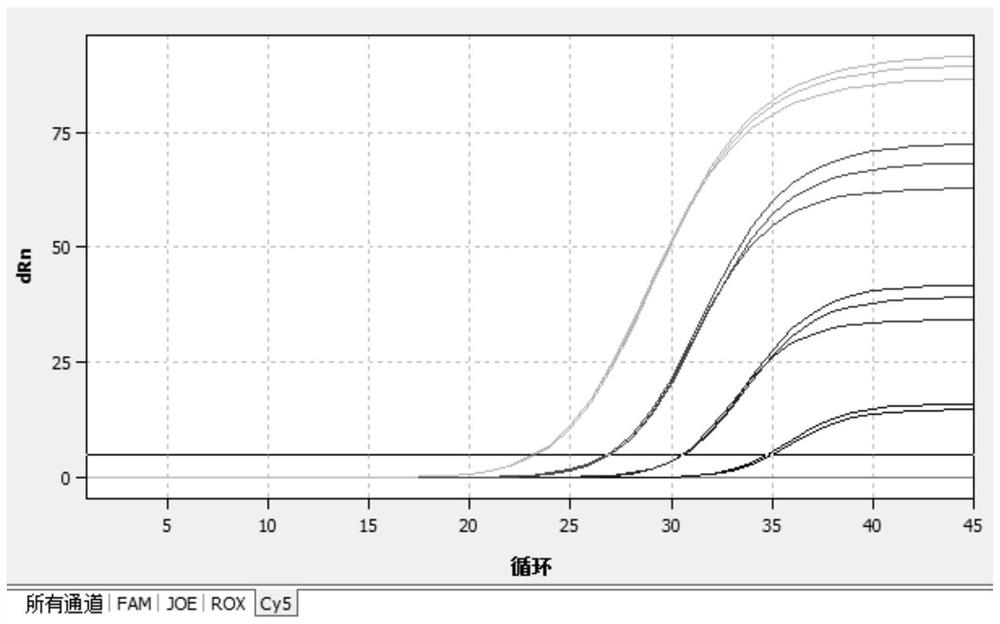
[0069] (1) Select the FAM channel (ReporTer: FAM, QuenCher: none) to detect Mycoplasma pneumoniae; (2) Select the CY5 channel (ReporTer: CY5, QuenCher: none) to detect Chlamydia pneumoniae; (3) Select the ROX channel (ReporTer: ROX , QuenCher: none), detect adenovirus; (4) select HEX channel, detect internal standard; (5 reference fluorescence (PAssiveReferenCe) is set to none. Fluorescent quantitative real-time reaction conditions are shown in Table 3 below.
[0070] Table 3: Fluorescent quantitative real-time PCR reaction conditions
[0071]
[0072] After the reaction, the instrument automatically saves t...
Embodiment 3
[0084] The feasibility test of embodiment 3 kit of the present invention
[0085] 1. Sensitivity evaluation test
[0086] (1) Preparation of triple nucleic acid detection reagents for Mycoplasma pneumoniae, Chlamydia pneumoniae and adenovirus: The triple nucleic acid detection reagent for Mycoplasma pneumoniae, Chlamydia pneumoniae and adenovirus was prepared by adopting the method in Example 1.
[0087] (2) Pathogen sample extraction
[0088] Use a pipette to mix the 5 different concentrations of mycoplasma pneumoniae, chlamydia pneumoniae and adenovirus human throat swab eluate in the tube, take out 100 μl into a new centrifuge tube, centrifuge at 12000rpm for 5min, and carefully discard the supernatant; Add 200 μL of bacterial lysate (from Beijing Biolab Technology Co., Ltd.) to the precipitate, mix well, bathe in water at 100°C for 5 minutes, and centrifuge at 10,000 rpm for 5 minutes for later use.
[0089] (3) Sample detection
[0090] Add 25 μL of the processed speci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com