Primer probe group and kit for combined detection of mycoplasma pneumoniae and chlamydia pneumoniae based on fluorescence RMA method
A technology of mycoplasma pneumoniae and chlamydia pneumoniae, applied in the direction of microorganism-based methods, DNA/RNA fragments, microorganisms, etc., can solve the problems of easy pollution, poor sensitivity, multiple false positive results, etc., to avoid false positives and environmental pollution, Reduce reaction time and temperature, avoid cross-reaction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
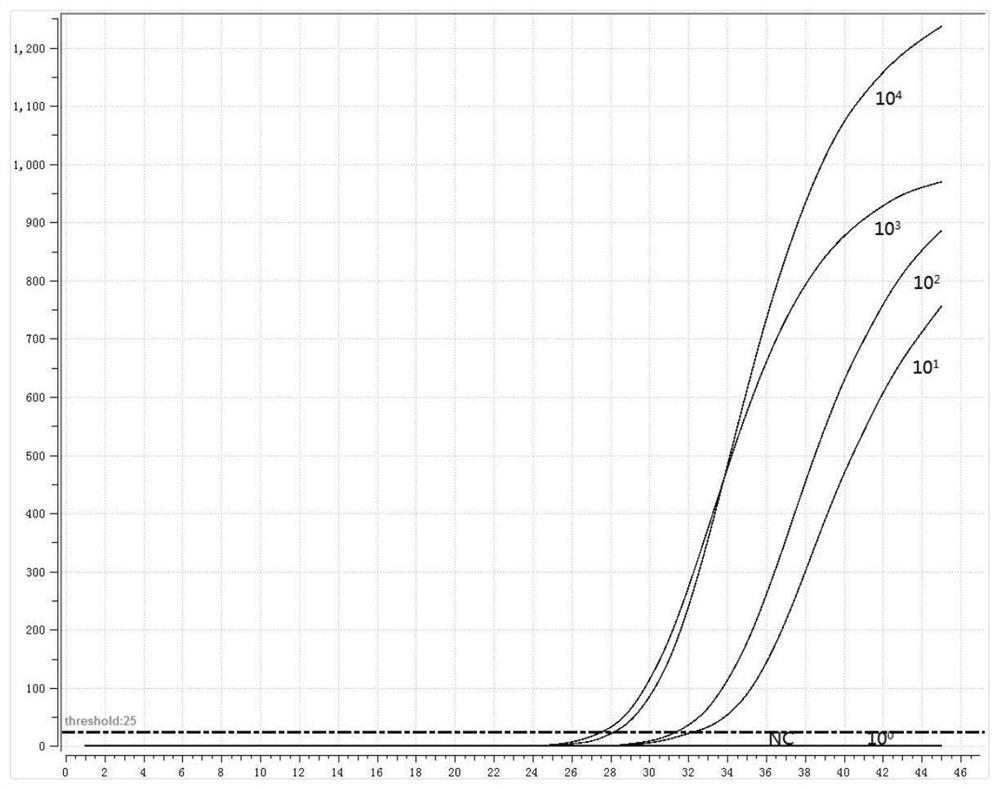
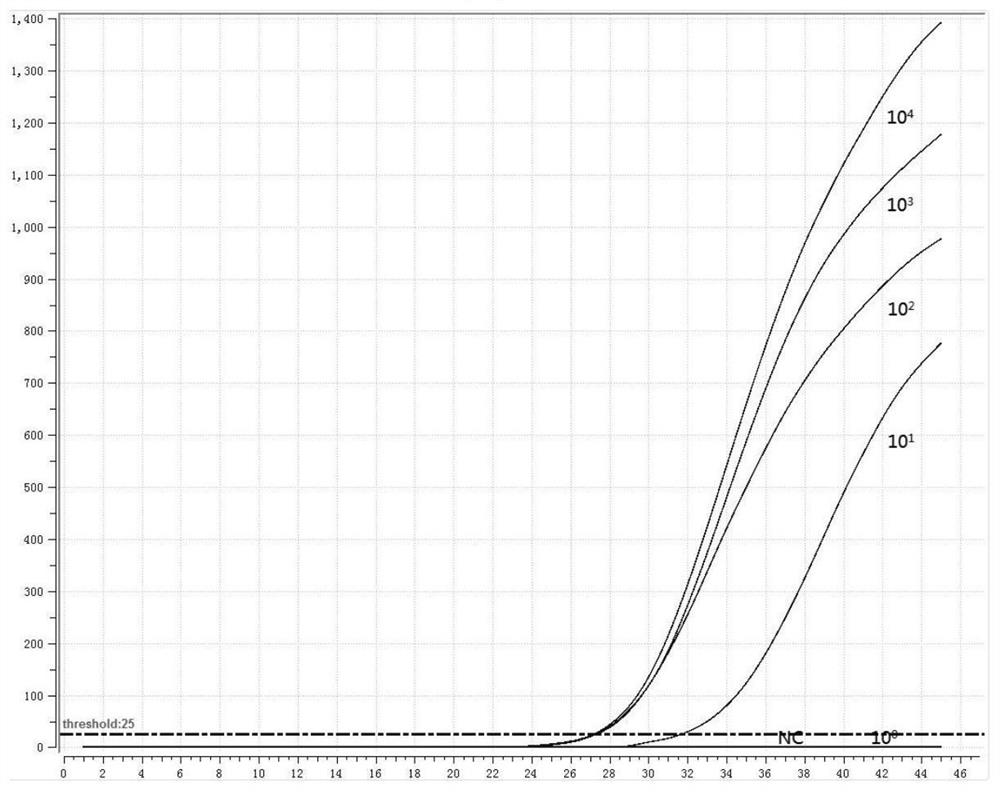
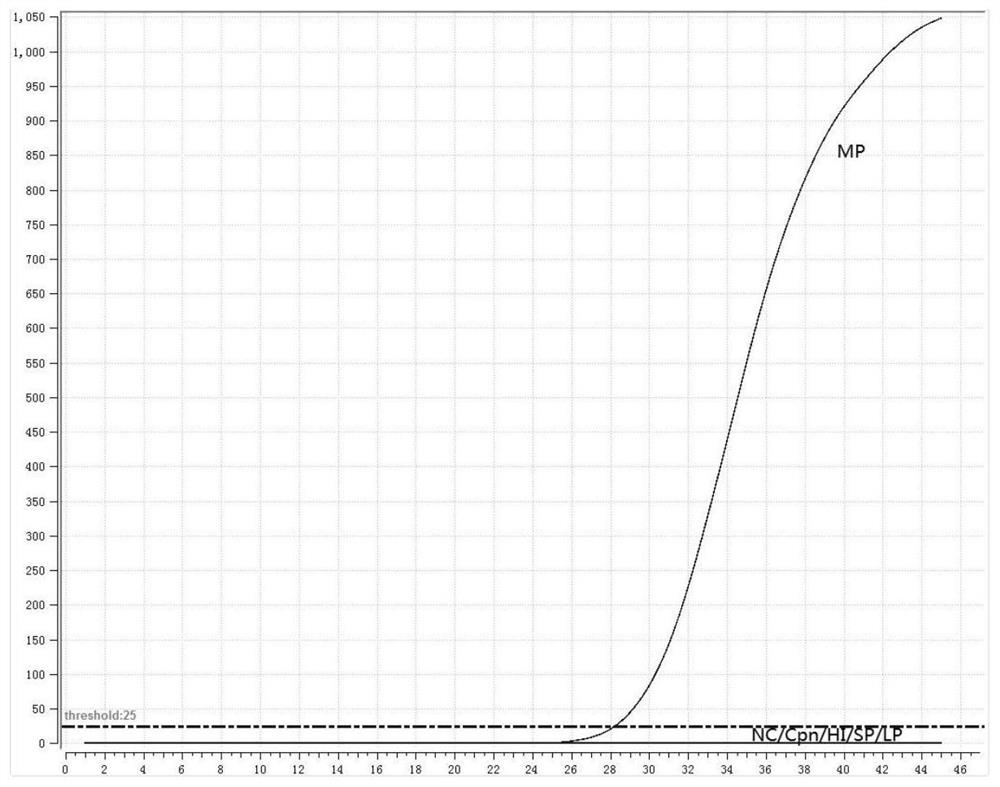
[0045] 1. Preparation of positive standard plasmid
[0046] The RNA of MP and Cpn was extracted as a template, and the adhesion protein P1 gene of MP and the outer membrane protein A gene of Cpn were amplified by PCR. The PCR amplification products were subjected to 1% agarose gel electrophoresis, recovered by tapping gel, and cloned into pMD18 -T vector, transformed into Escherichia coli competent cells, blue-white screening, picking white colonies, and colony PCR verification. Send the positive recombinant bacteria to the company for sequencing, culture the correctly sequenced recombinant bacteria overnight, extract plasmid DNA, and obtain positive plasmids (P-MP, P-Cpn).
[0047] 2. Design of fluorescent RMA primers and probes
[0048] Fluorescent RMA primers and probes were designed for the adhesion protein P1 gene of MP and the outer membrane protein A gene of Cpn, as shown in Table 2:
[0049] Table 2 Primer and Probe Sequences
[0050]
[0051] Note: The fluoresce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com