Application of heterocyclic compound in preparation of medicine for treating pneumonia
A technology of heterocyclic compounds and pneumonia, which is applied in the application field of heterocyclic compounds in the preparation of drugs for the treatment of pneumonia, can solve the problems of mycoplasma pneumonia patients increase, pneumonia recurrence, azithromycin resistance, etc., and achieve excellent anti-pneumonia activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
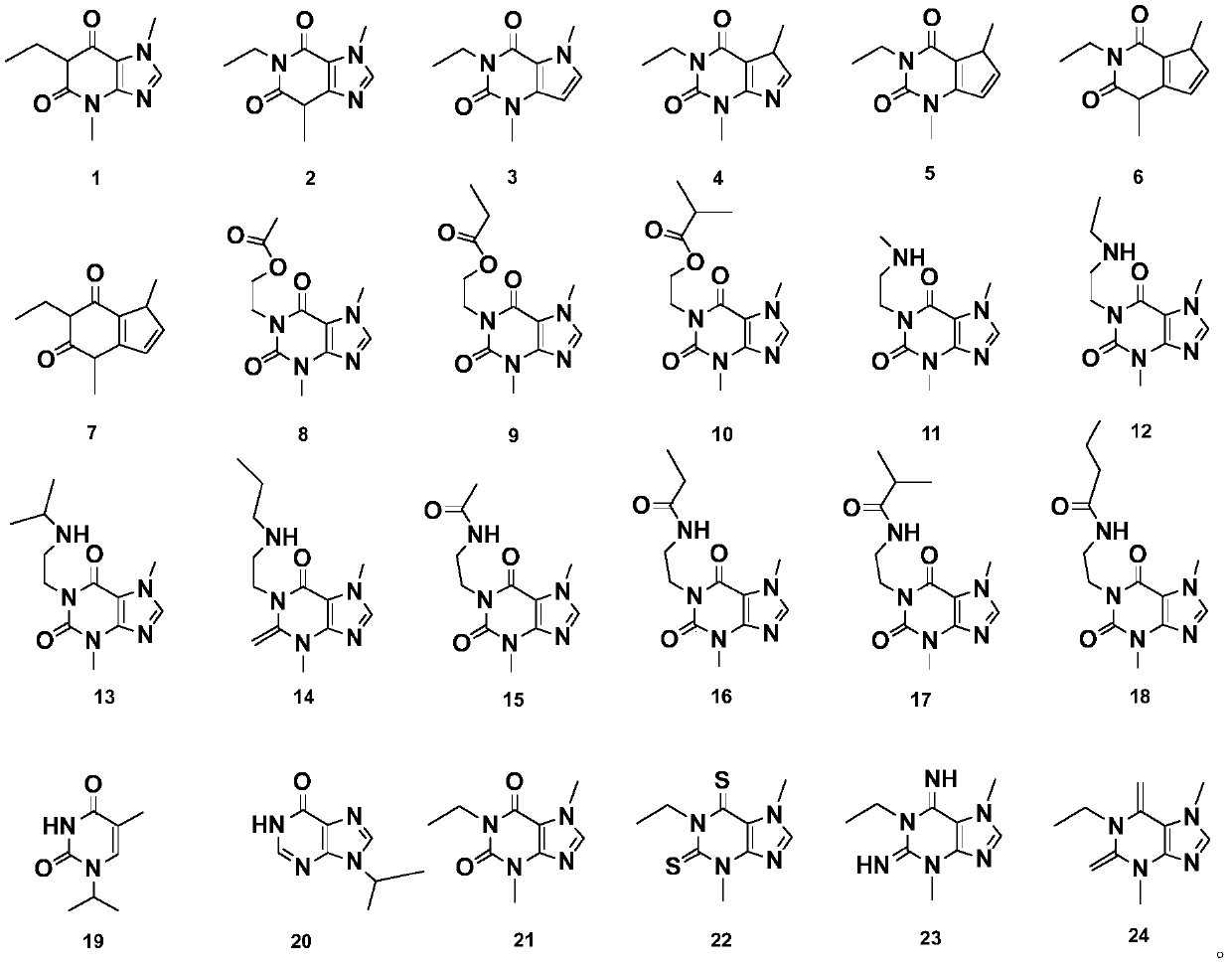
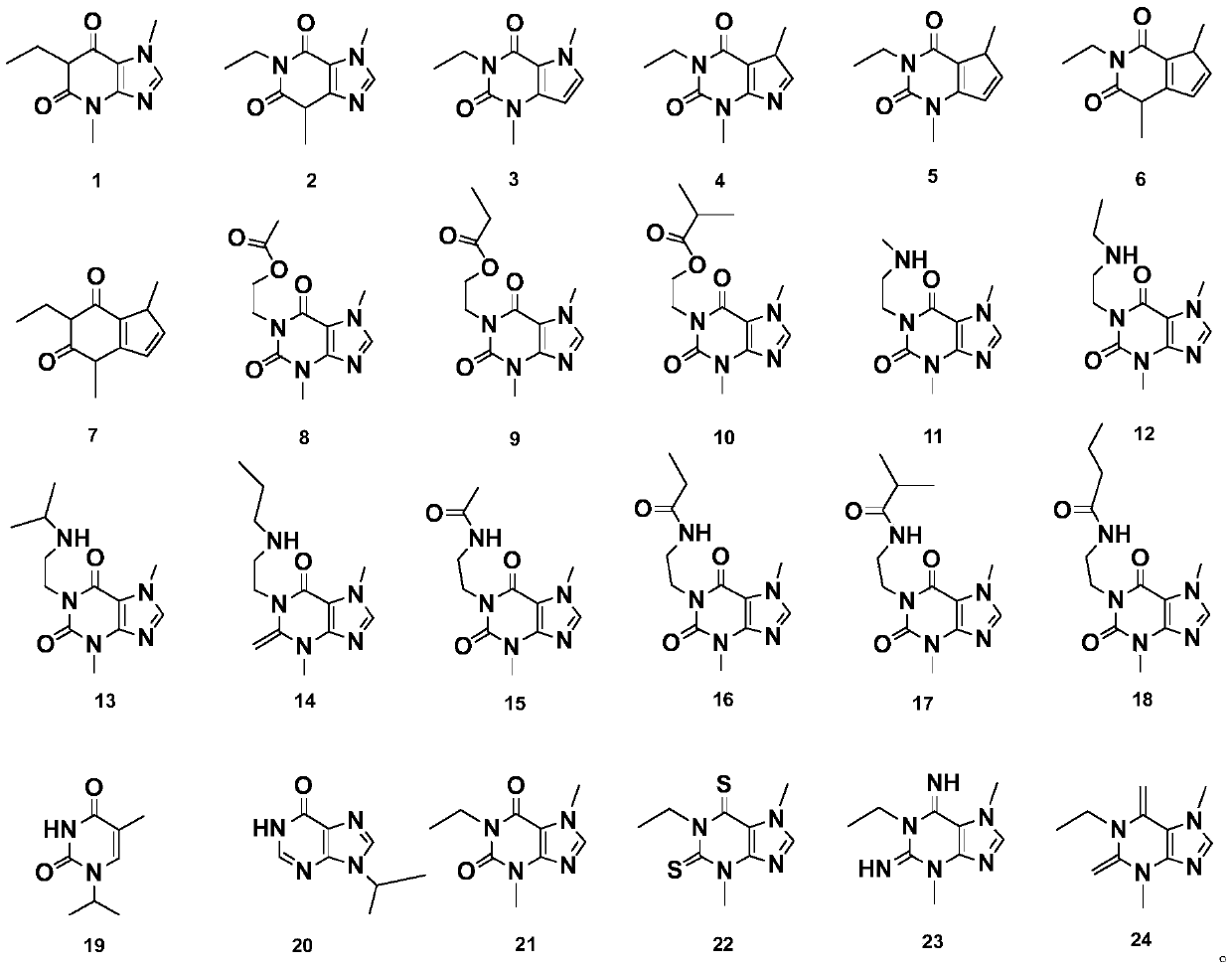
[0027] This example describes the preparation methods of compound 1 to compound 24 in detail.
[0028] According to a preferred embodiment, compounds 1 to 24 are through cyclization reaction, acidification reaction, ethylation reaction, addition reaction, substitution reaction, catalytic reaction, nitrosation reaction, reduction reaction, formylation reaction, and methylation reaction. In one or more reactions.
[0029] This example provides the preparation methods of 24 kinds of heterocyclic compounds. All the prepared heterocyclic compounds have their structures determined by infrared spectroscopy (IR), nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry.
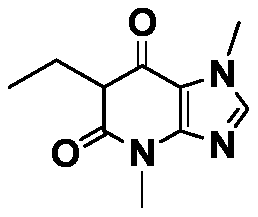
[0030] Preparation of compound 1
[0031] Compound 1 has the following structure:
[0032]
[0033] The preparation process of compound 1 is as follows, and the main starting materials for each reaction step below are calculated as 1 mmol.
[0034] ①1mmol of CNCH 2 COCH 3 (CH 2 CH 3 )CONH 2 The derivative was cycliz...
Embodiment 2
[0121] In this example, the activity of compounds 1-24 prepared in Example 1 against pneumonia caused by Streptococcus pneumoniae was tested.
[0122] Experimental method: Pneumonia model caused by Streptococcus pneumoniae in vivo: SPF male SD male rats (weight about 200g) were randomly divided into several groups, including blank control group, model group, positive control group of cefuroxime axetil tablets, and the like Caffeine group, caffeine analog group (the substances used in the caffeine analog group are: 1-propyl 3,7-dimethylxanthine (caffeine analog A), 1-isopropyl 3,7- Dimethylxanthine (caffeine analog B), 3-ethyl 3,7-dimethylxanthine (caffeine analog C)) and compound 1 to 24 groups, 8 in each group, under standard environment Raised for 7 days. Before the experiment, the rats were lightly anesthetized with ether, and then by nasal inhalation, in addition to the nasal instillation of normal saline in the blank control group, the model group, the positive control grou...
Embodiment 3
[0128] In this example, the activity of compounds 1-24 prepared in Example 1 against influenza A virus pneumonia was tested.
[0129] C57 mice (22-25g) were randomly divided into several groups, namely the blank control group (Normal), model group (Model), oseltamivir positive control group, analog caffeine group, and caffeine analog group ( The substances used in the caffeine analog group are: 1-propyl 3,7-dimethylxanthine (caffeine analog A), 1-isopropyl 3,7-dimethylxanthine (caffeine analog B), 3-ethyl 3,7-dimethylxanthine (caffeine analog C)), compound 1-24 group. On the first day, the mice were inoculated with influenza A virus. Except for mice in the blank control group who were instilled with normal saline through the nose, the mice in all other groups were infected with influenza A H1N1 strain FM1 (30 μL) through the nose. After 24 hours, the mice in the blank control group and the model group were given the same dose of normal saline in the drug group, and the mice in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com