Combined device and detection method for synchronously detecting influenza A virus, influenza B virus, chlamydia pneumoniae IgM antibody and mycoplasma IgM antibody
A technology of Chlamydia pneumoniae and Mycoplasma pneumoniae, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of inability to fully analyze and understand the disease, procrastination of the disease, and medication errors, so as to simplify the clinical detection process and improve the efficiency of diagnosis. The effect of rational drug use and convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
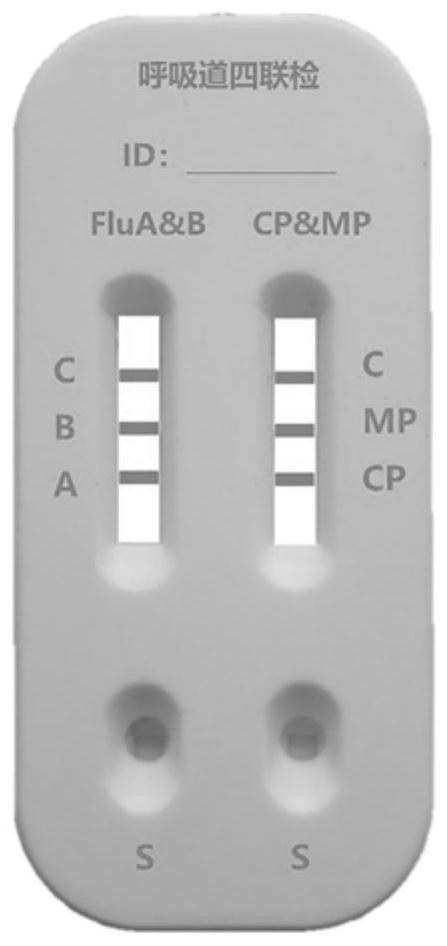
[0042] A combined device for synchronous detection of influenza virus type A, type B and IgM antibodies of chlamydia pneumoniae and mycoplasma, its structure is as follows figure 2 As shown, it includes a dual-channel jam; the test strip FluA & B and the test strip CP & MP arranged side by side in the dual-channel jam; the first one independently connected to the test strip FluA & B and the test strip CP & MP that is arranged on the jam Sample hole S1 and the second sample hole S2.
[0043] The specific design of each part is as follows:
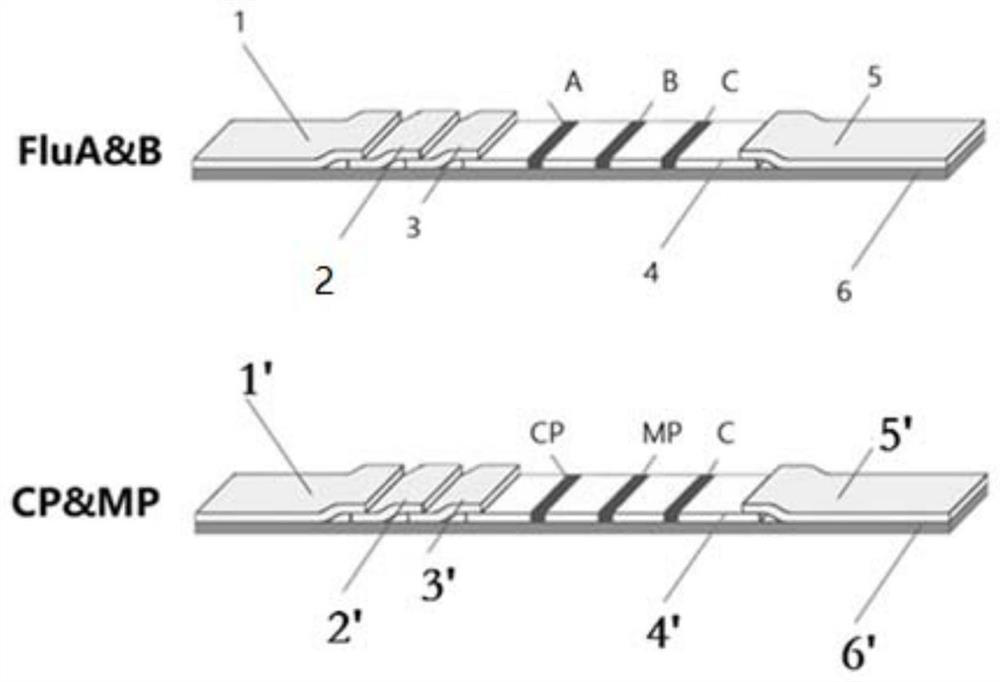
[0044] The structure of the test strip FluA&B is as follows image 3 As shown, including the first sample pad 1 pretreated with anti-erythrocyte antibody, the first filter blood pad 2, the FluA & B tracer sprayed glass fiber pad 3, the FluA & B antigen-coated nitrocellulose membrane 4, and the first absorbent paper 5 and the first plastic bottom plate 6; the first sample pad 1, the first blood filter pad 2, the FluA & B tracer sprayed gla...
Embodiment 2
[0051] The preparation method of the combined device of synchronously detecting influenza virus type A, type B and chlamydia pneumoniae and mycoplasma described in embodiment 1 may further comprise the steps:
[0052] a) Prepare tracers: tracers include but are not limited to colloidal gold, colloidal selenium, colored latex microspheres, fluorescent dyes, fluorescent microspheres, quantum dots and carbon black particles, etc.;
[0053] b) using the tracer prepared in step a) to label the anti-human IgM μ chain antibody and the quality control line to label the ligand to obtain the labeled tracer;
[0054] c), using the tracer buffer to dilute the marked tracer in step b) to obtain a tracer solution, and spray the tracer solution on the glass fiber to obtain a tracer sprayed glass fiber mat;
[0055] d) Spray a piece of nitrocellulose membrane with pre-diluted highly specific influenza virus A and B as the influenza virus A detection line and influenza virus B detection line, ...
Embodiment 3
[0060] Detection method and result interpretation
[0061] A method for synchronous detection of influenza virus type A, type B and chlamydia pneumoniae CP, mycoplasma pneumoniae MP, it uses the synchronous detection of influenza virus type A, type B and chlamydia pneumoniae CP, mycoplasma pneumoniae MP in embodiment 1 The combination means of IgM antibody comprises the following steps:
[0062] The first step is to obtain test specimens in accordance with clinical sample collection specifications;
[0063] The second step is to use the supporting sample diluent in combination at the same time, that is, while adding the test specimen to the first sample injection hole S1 and the second sample injection hole S2, add the sample diluent to each sample injection hole, and the sample dilution The amount of solution used is 70-95ul / sample well;
[0064] The third step, after adding the sample, start timing for 10-15 minutes;
[0065] The fourth step, when the timing is over, imme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com