Fluorescence immunochromatography device for detecting COVID-19 and using method thereof
A fluorescent immunochromatography and coronavirus technology, which is applied in measuring devices, immunoassays, analytical materials, etc., can solve problems such as high technical requirements, inability to apply early preliminary screening, and long detection time for new coronaviruses, and meet personnel requirements low, improve the accuracy of inspection, and avoid false negative results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
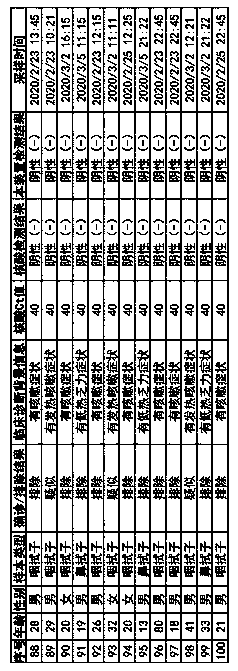
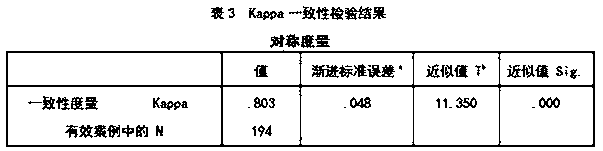
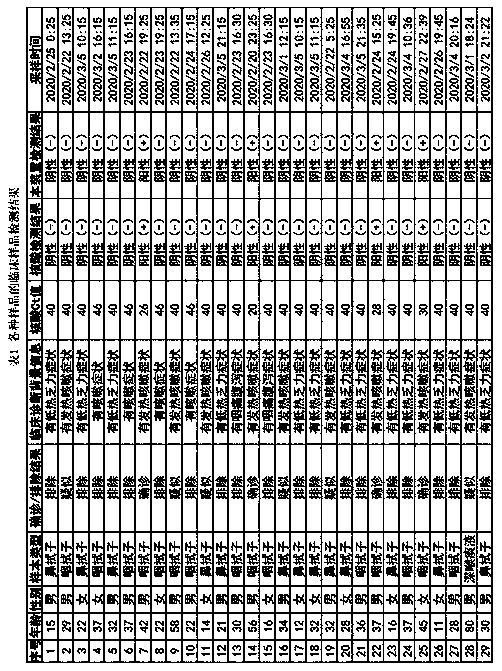
[0080] A fluorescent immunochromatographic device for detecting novel coronavirus COVID-19, including a test strip.
[0081] The test strip includes a bottom plate and a sample pad, a binding pad, a reaction pad and a water-absorbing pad that are connected in sequence above the bottom plate; the new coronavirus NP protein monoclonal antibody labeled with europium microspheres and europium are coated on the binding pad. Microsphere-labeled rabbit IgG antibody, the particle size of the europium microspheres used in the europium microsphere labeling is 610nm; the mass concentration ratio between the novel coronavirus NP protein monoclonal antibody and rabbit IgG antibody is 1.5 : 1; the reaction pad is provided with a detection line and a quality control line successively along the sample flow direction, and the detection line is coated with a new type of coronavirus NP protein monoclonal antibody, and the quality control line is coated with a goat anti-rabbit IgG antibodies.
...
Embodiment 2
[0087] A fluorescent immunochromatographic device for detecting novel coronavirus COVID-19, including a test strip and a sample diluent.
[0088] The test strip includes a bottom plate and a sample pad, a binding pad, a reaction pad and a water-absorbing pad that are connected in sequence above the bottom plate; the new coronavirus NP protein monoclonal antibody labeled with europium microspheres and europium are coated on the binding pad. Microsphere-labeled rabbit IgG antibody, the particle size of the europium microspheres used in the europium microsphere labeling is 600nm, and the mass concentration ratio between the novel coronavirus NP protein monoclonal antibody and the rabbit IgG antibody is 2 : 1; the reaction pad is sequentially provided with a detection line and a quality control line along the flow direction of the sample, the detection line is coated with a new type of coronavirus NP protein monoclonal antibody, and the quality control line is coated with a goat an...
Embodiment 3
[0096] A fluorescent immunochromatographic device for detecting novel coronavirus COVID-19, including a test strip and a sample diluent.
[0097] The test strip includes a bottom plate and a sample pad, a binding pad, a reaction pad and a water-absorbing pad that are connected in sequence above the bottom plate; the new coronavirus NP protein monoclonal antibody labeled with europium microspheres and europium are coated on the binding pad Microsphere-labeled rabbit IgG antibody, the particle size of the europium microspheres used in the europium microsphere labeling is 550nm, and the mass concentration ratio between the novel coronavirus NP protein monoclonal antibody and the rabbit IgG antibody is 1 : 1; the reaction pad is sequentially provided with a detection line and a quality control line along the flow direction of the sample, the detection line is coated with a new type of coronavirus NP protein monoclonal antibody, and the quality control line is coated with a goat ant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com