Fluorescent quantitative detection kit for progesterone, estradiol and beta-human chorionic gonadotropin
A technology of chorionic gonadotropin and estradiol, which is applied in biological testing, measuring devices, material inspection products, etc., and can solve the problem of lack of efficient detection kits for progesterone, estradiol and β-human chorionic gonadotropin, etc. problem, achieve the effect of reducing the chance of uterine contractions or infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Progesterone, Estradiol and β-Human Chorionic Gonadotropin Fluorescent Quantitative Detection Kit
[0025] 1. Composition and preparation method of the kit
[0026] Progesterone, estradiol and β-human chorionic gonadotropin fluorescent quantitative detection kit, including: test card, U disk with standard curve.
[0027] (1) Structure of test card
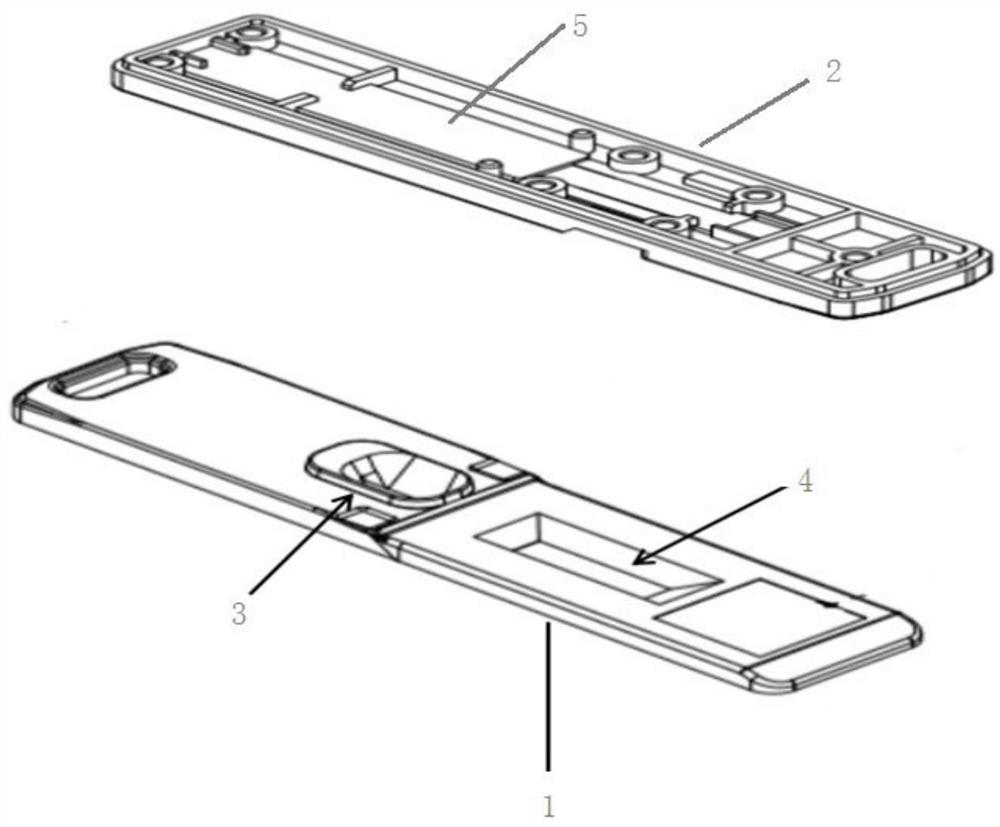
[0028] combined figure 1 and figure 2 , illustrating the structure of the test card.
[0029] The test card includes a card shell and a test strip inside the card shell.
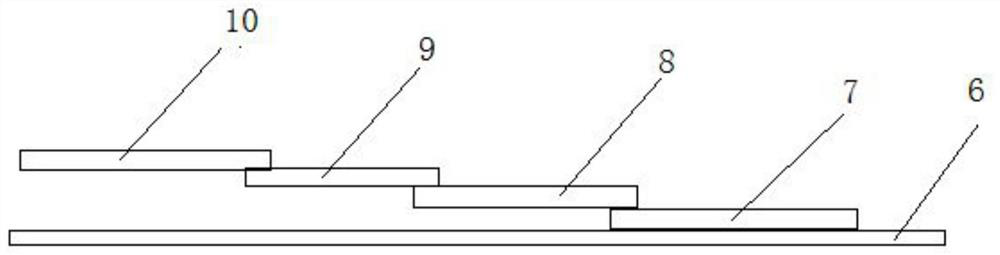
[0030] The test strip includes a substrate 6 and a sample pad 7 , a CP pad 8 , an NC film 9 and an absorbent pad 10 pasted on the substrate in sequence. Adjacent ends of the sample pad, CP pad, NC membrane, and absorbent pad overlap each other.
[0031] The cartridge includes an upper cartridge 1 and a lower cartridge 2 that are engaged with each other. The inner surface of the lower cartridge 2 is provided with a drawer slot 5 for placing...
Embodiment 2
[0065] Embodiment 2 The effect contrast of test strip of the present invention and other test strips
[0066] Prepare control test strips 1-3.
[0067] According to the preparation method of the test strip of the present invention, prepare the control test strip 1, the difference is that the Prog antibody (purchased from Meridian, product number E82321M) in the ProgmAb labeled conjugate on the CP pad is replaced by Hytest company, the product number is Prog antibody to HPRO-2.
[0068] Prepare the contrast test strip 2 according to the preparation method of the test strip of the present invention, the difference is only that the E2 antigen whose product number is 710050 of the Medix company on the NC membrane is replaced by the E2 antigen which is numbered V56110 by the Biospacific company, and the E2 antigen on the CP pad is replaced. The E2 antibody (Hytest, 2E2) in the mAb-labeled conjugate was replaced with the E2 antibody whose product number is HM177A-1177A from Holmes ...
Embodiment 3
[0078] Embodiment 3 adopts kit of the present invention to detect concrete serum sample
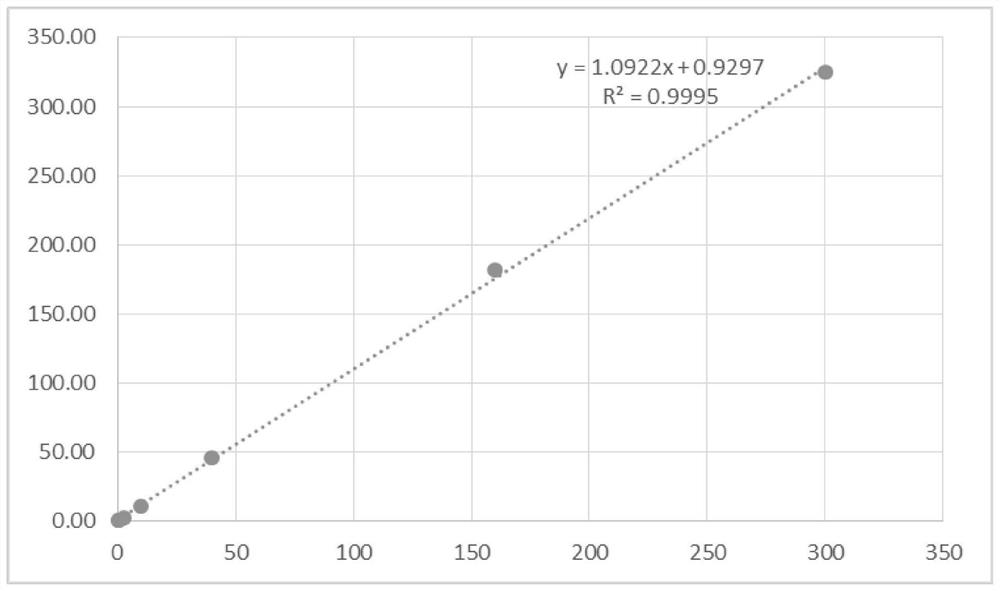
[0079] According to the preparation method of the test strip of the present invention, a batch of detection kits are prepared, and the correlation comparison is carried out with the serum samples assigned by the Roche cobas e411 electrochemiluminescence automatic immunoassay system. Progesterone, estradiol and β-human chorionic gonadotropin each detect 50 samples, take the detection result of Roche cobas e411 electrochemiluminescence automatic immunoassay system as the control group, compare the detection result of the present invention and its correlation, the result is as follows Figure 6-8 . It can be seen that the detection result of the kit of the present invention is extremely accurate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com