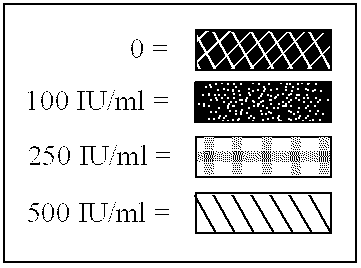
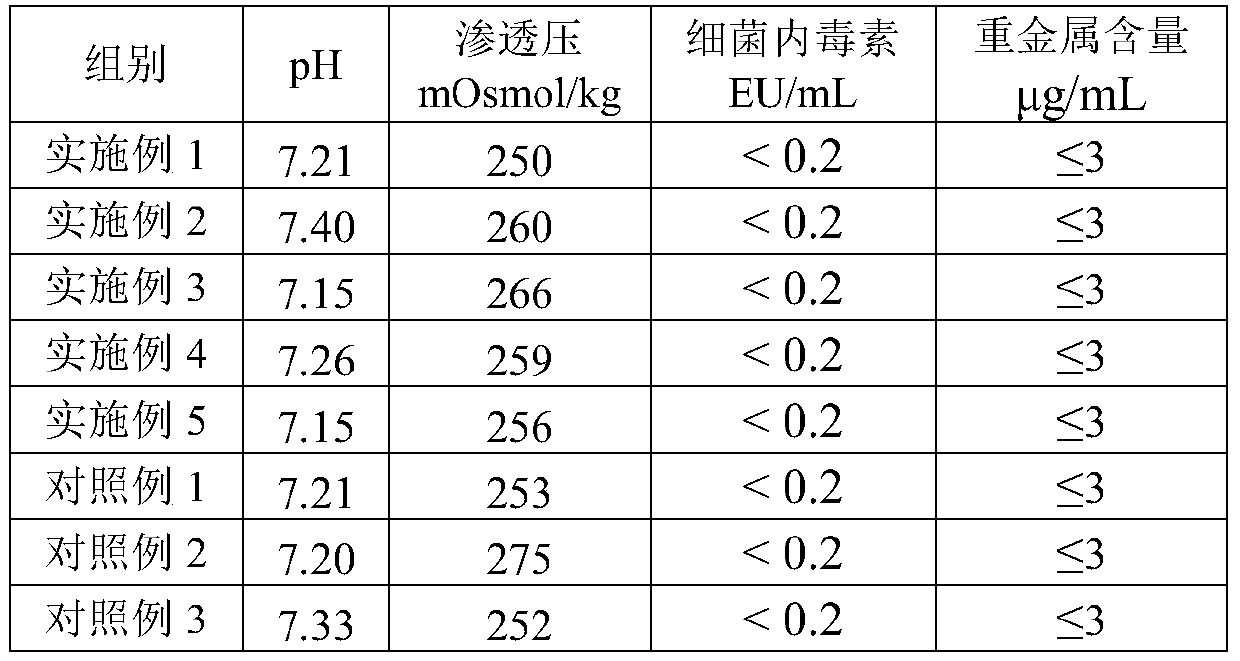
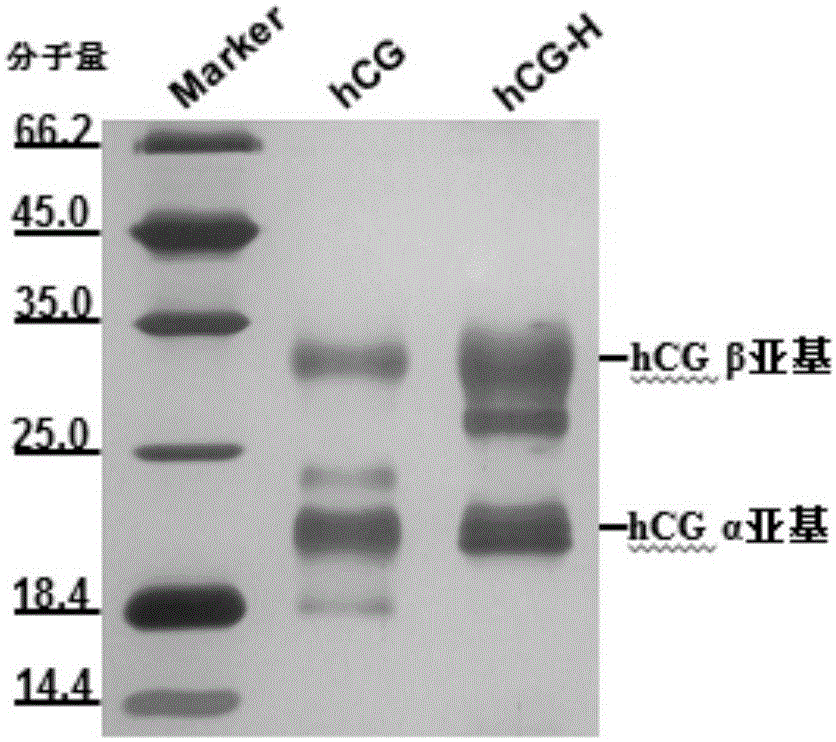
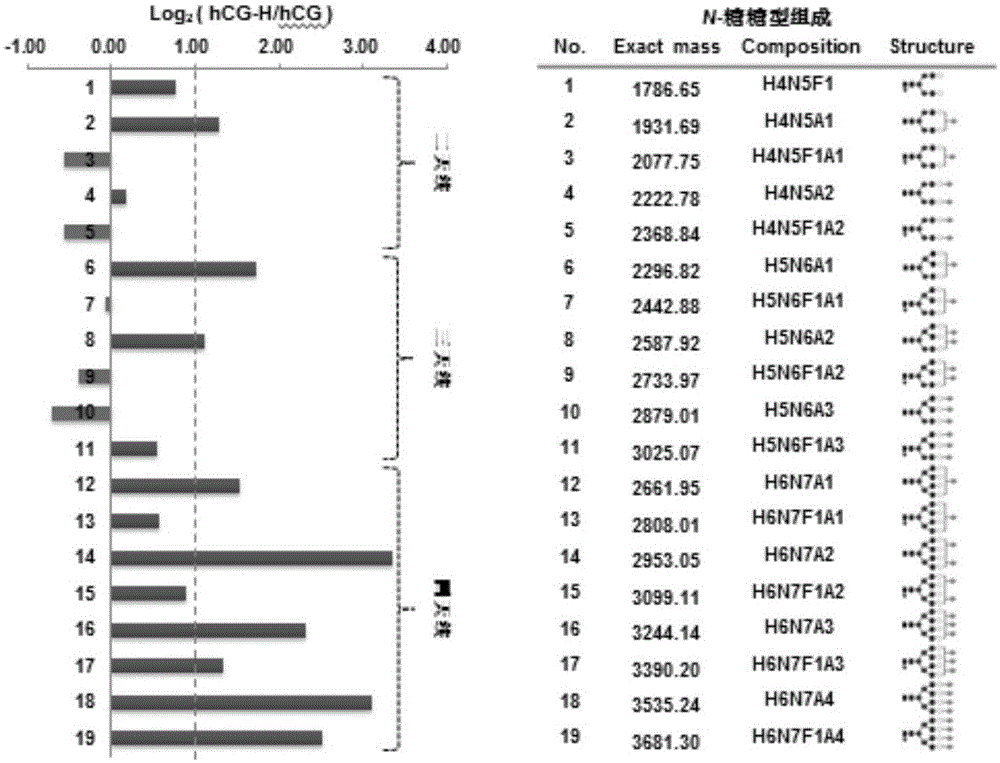
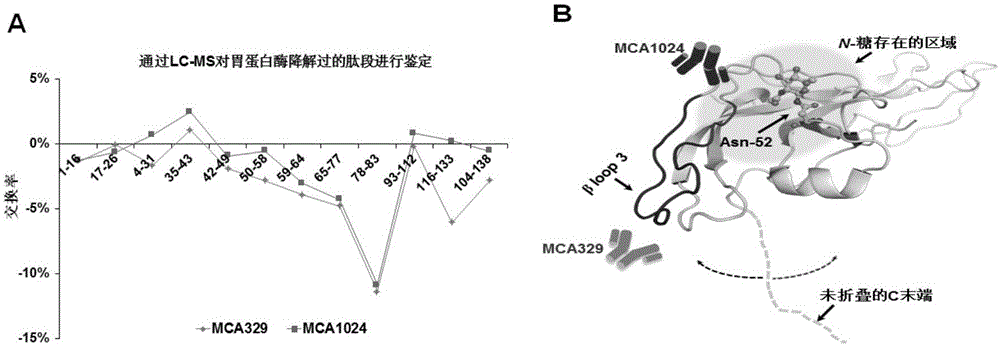
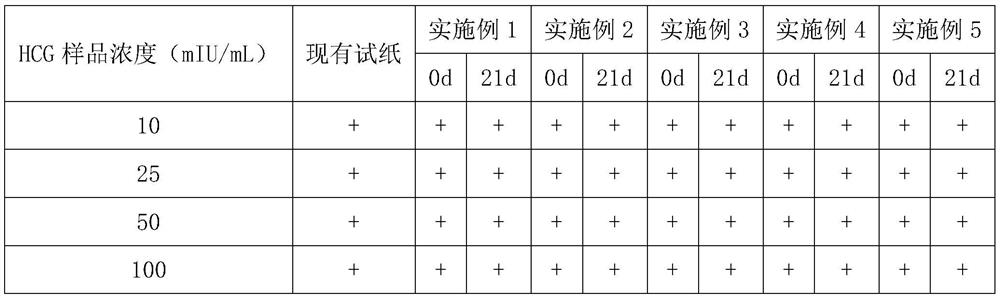
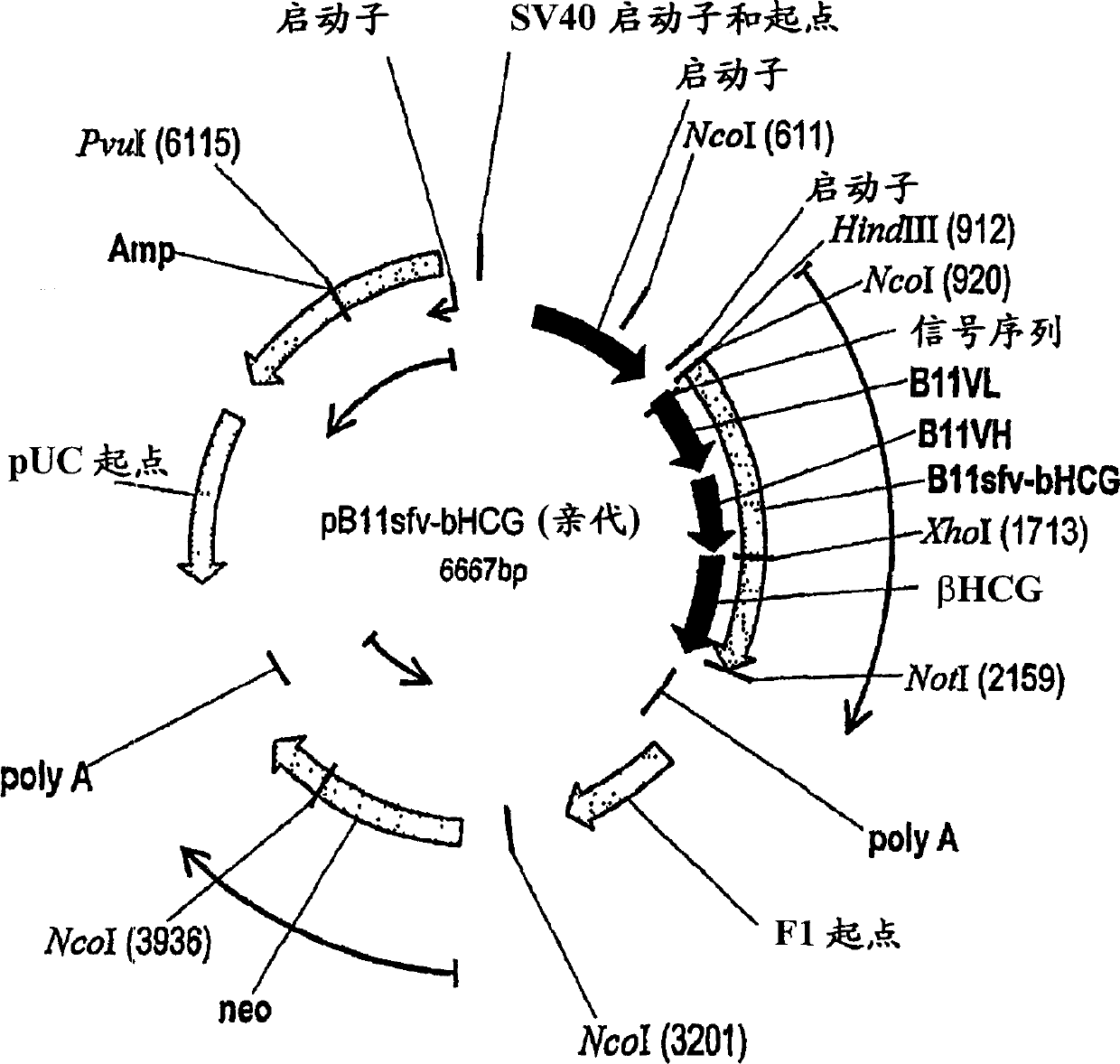
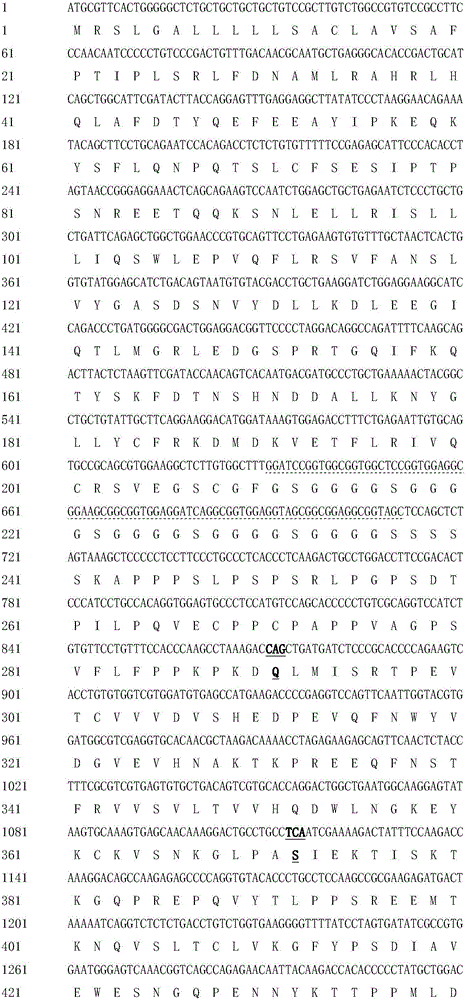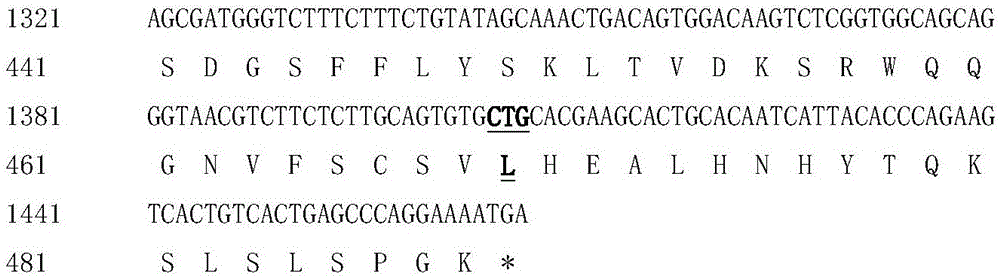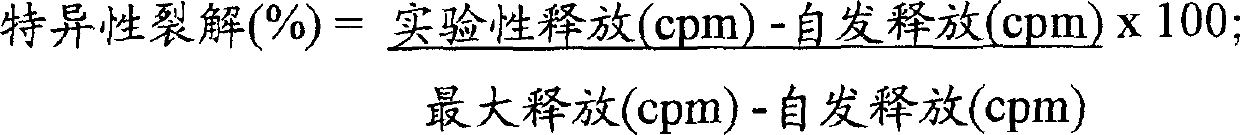Patents
Literature
57 results about "HCG - Human chorionic gonadotropin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Circulating mRNA as diagnostic markers
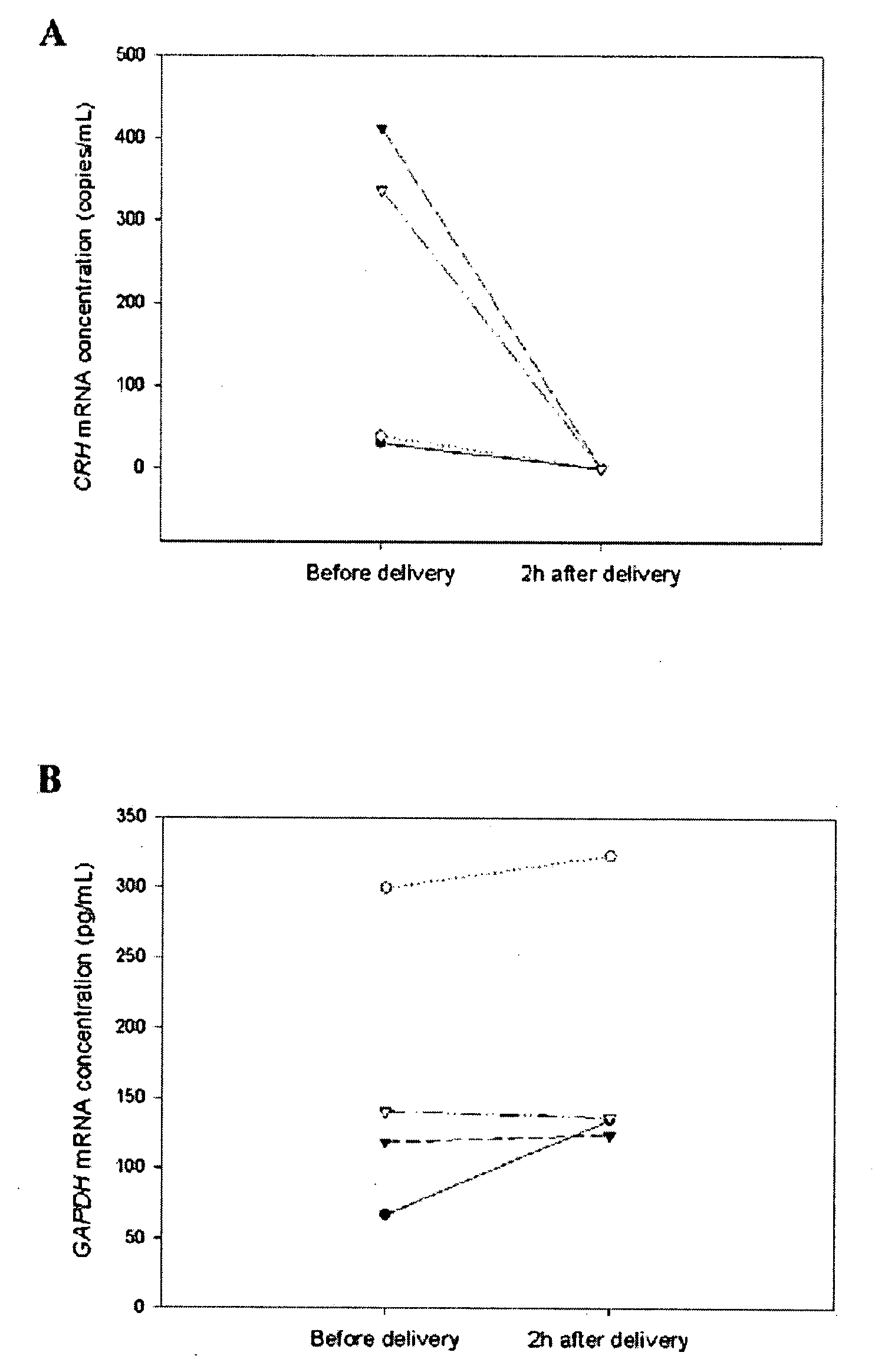
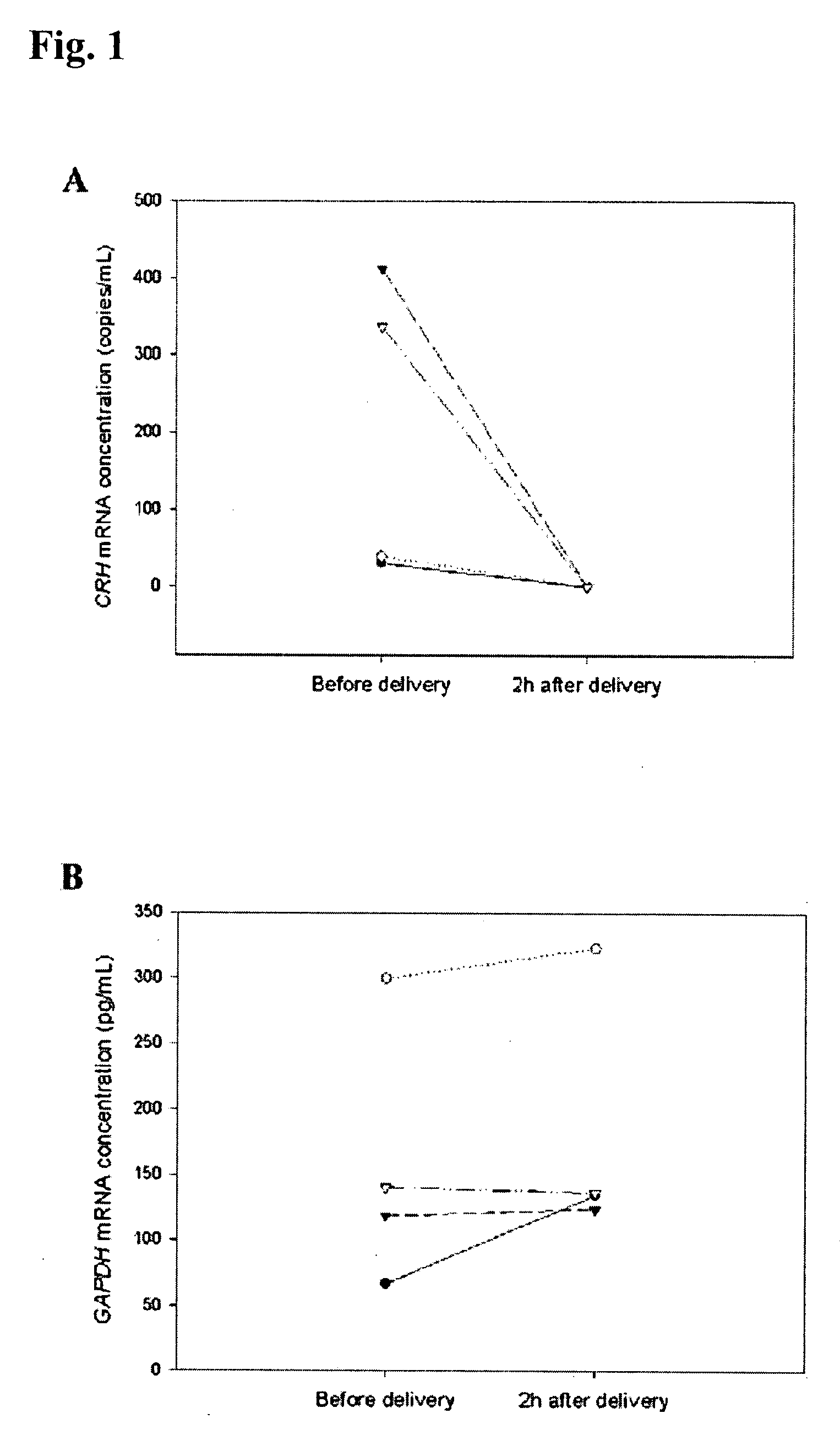
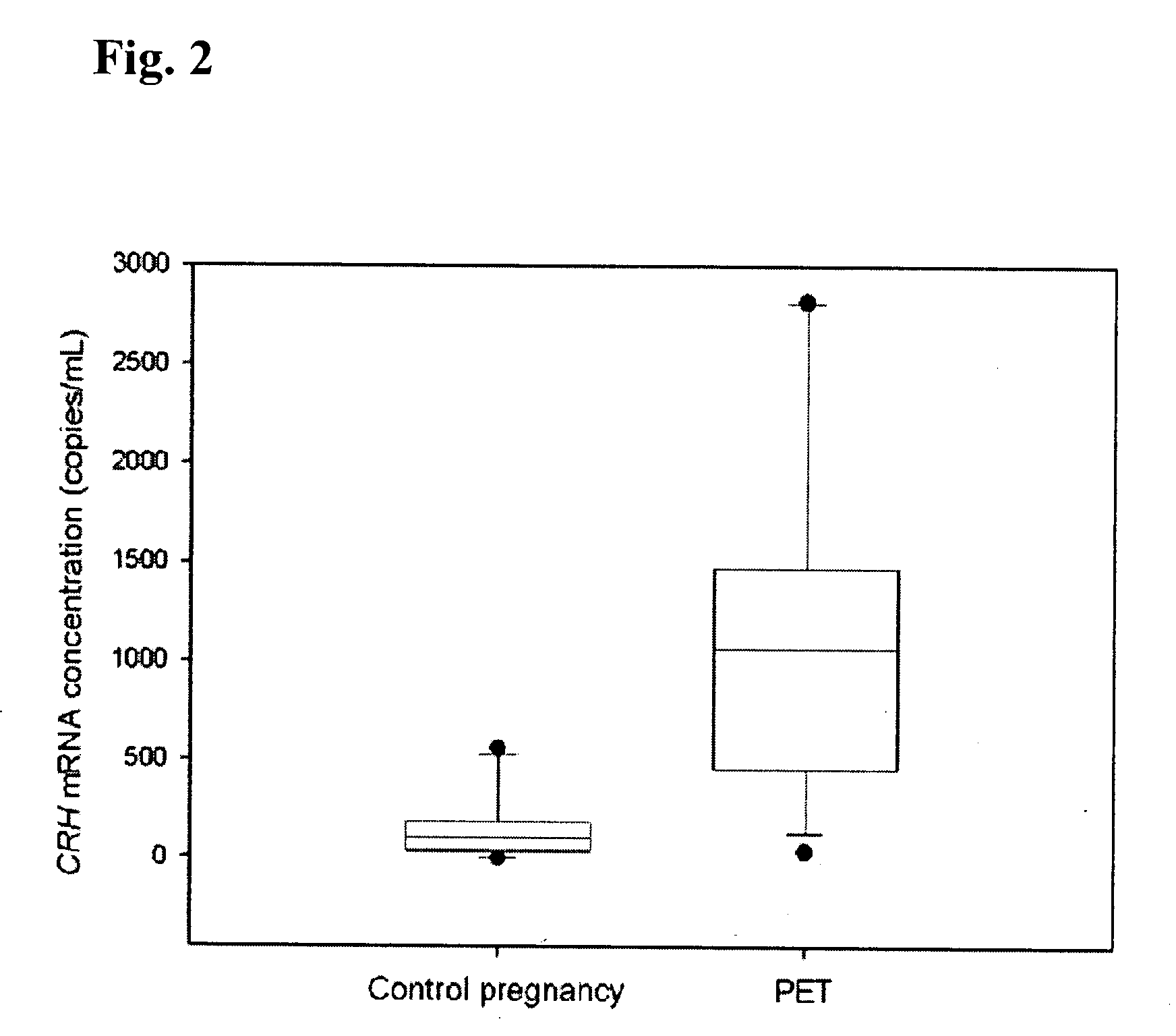
Methods and kits are provided for diagnosing, monitoring, or predicting the conditions of pre-eclaimpsia, fetal chromosomal aneuploidy, and pre-term labor in a pregnant woman, as well as for detecting pregnancy in a woman, by quantitatively measuring in the maternal blood the amount of one or more mRNA species encoding human chorionic gonadotropin β subunit (hCG-β), human placental lactogen (hPL), human corticotropin releasing hormone (hCRH), KiSS-1 metastasis-suppressor (KISS1), tissue factor pathway inhibitor 2 (TPFI2), placenta-specific 1 (PLAC1), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and comparing the amount of the mRNA species with a standard control.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
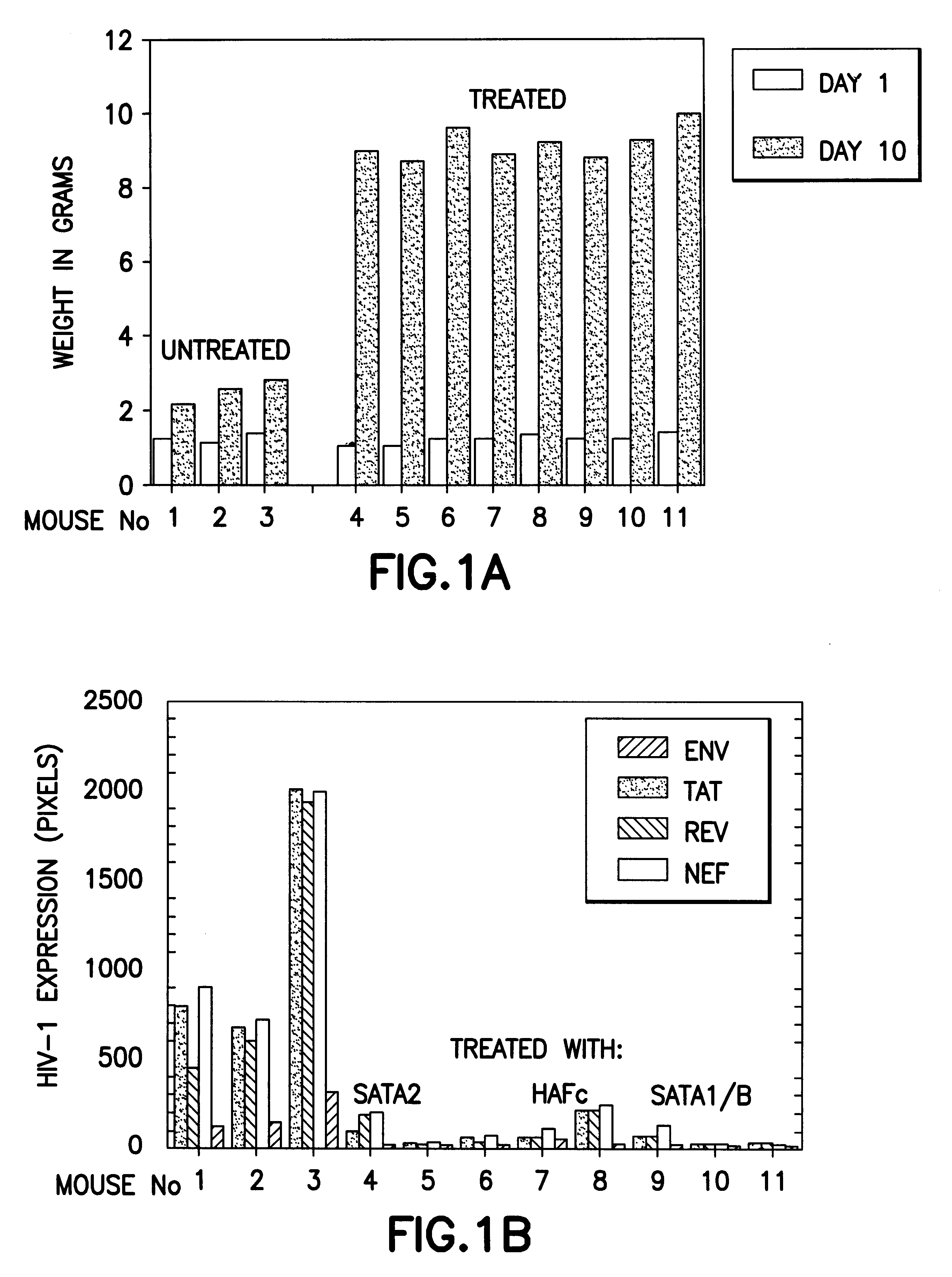
Method for treating HIV
InactiveUS6620416B1Lower Level RequirementsIncrease CD4.sup.+ T cellsBiocidePeptide/protein ingredientsDiseaseHCG - Human chorionic gonadotropin
The present invention relates to peptides of one or more portions of the human chorionic gonadotropin beta-chain as well as methods for treatment and prevention of diseases, including HIV infection, using human chorionic gonadotropin, employing the beta-chain of human chorionic gonadotropin, peptides containing a sequence of one or more portions of the beta-chain of human chorionic gonadotropin and derivatives and analogues thereof. The invention further relates to fractions of sources and or preparations of human chorionic gonadotropin, such as fractions of human early pregnancy urine, which fractions have anti-HIV activity. The present invention further relates to pharmaceutical compositions for treating and / or preventing HIV infection.
Owner:NOBEL BIOSCI
Linker peptide for constructing fusion protein
ActiveCN106317226AWide applicabilityMeet different requirements of rigidityPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeSide chain
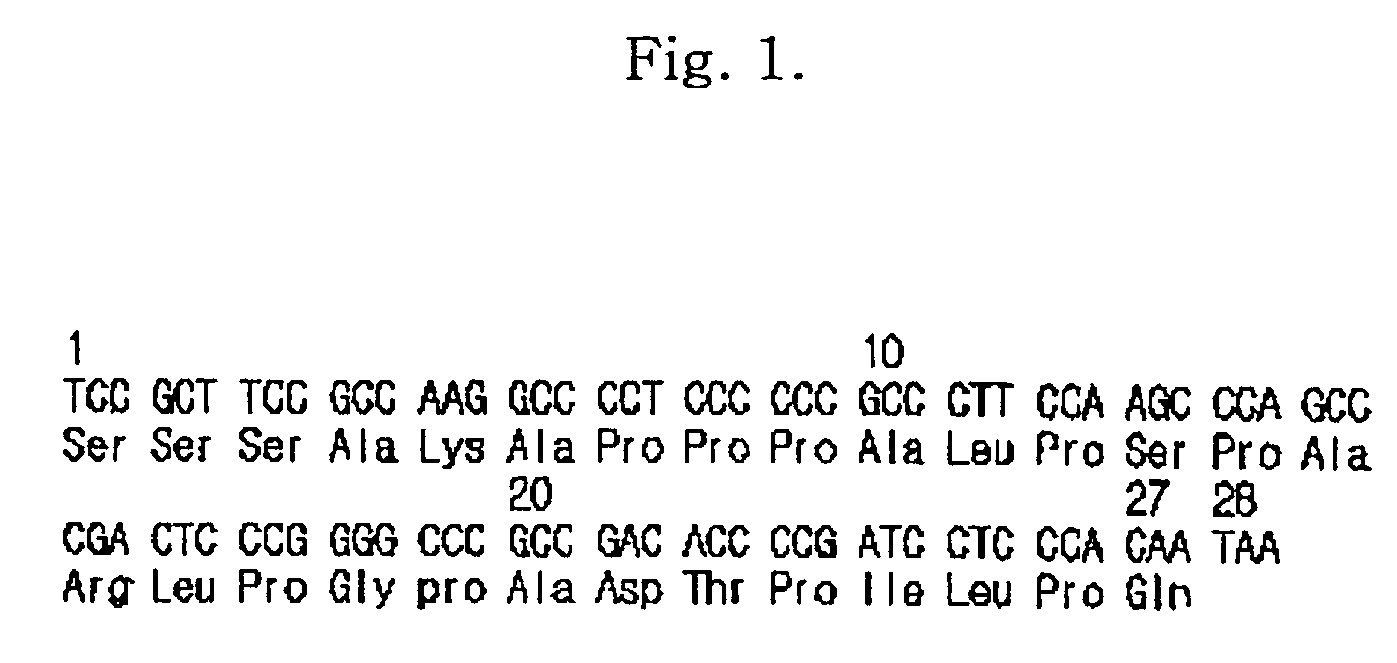
The invention provides a linker peptide for constructing a fusion protein, comprising a flexible peptide and a rigid peptide; the flexible peptide is composed of one or more flexible units, and the rigid peptide is composed of one or more rigid units, wherein each flexible unit comprises two or more amino acid residues selected from Gly, Ser, Ala and Thr, and each ridge unit comprises a plurality of glycosylation-sited carboxyl-terminal peptides (CTP) of human chorionic gonadotrophin beta-subunit. The linker peptide herein is more efficient in eliminating steric-hindrance effect between two fusion molecules, and reducing polymerization or activity decrease or loss due to misfolding or comformational change of active proteins; in addition, negatively-charged high-sialyl CTPs can resist removal by kidney, half-life period of the fusion molecules is further extended, bioavailability of the fusion protein is improved; more additionally, protection from glycosyl side chains of the CTPs enables reduced sensitivity of the linker peptide to protease such that the fusion protein is rarely degraded in a linker region.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Fusion protein having enhanced in vivo erythropoietin activity
ActiveUS7091326B2Enhanced human EPO activityProlong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsRed blood cellHalf-life
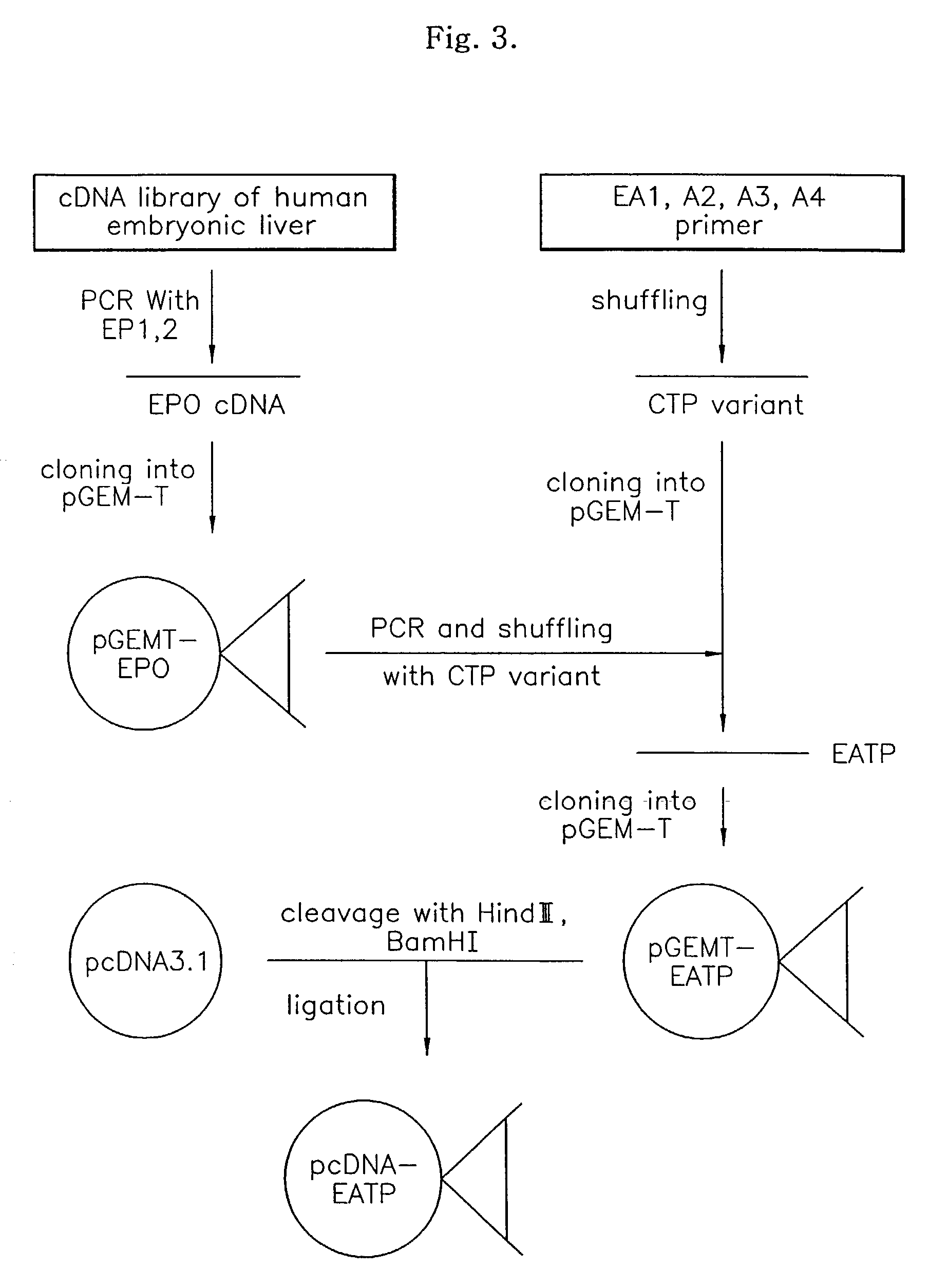
Provided is a fusion protein comprising, at its carboxy terminal of human erythropoietin (EPO), a mutant having one to four amino acid substitutions in the carboxy terminal peptide (CTP) fragment of a human chorionic gonadotropin (HCG) β subunit, for increasing an in vivo half-life activity of EPO. The in vivo half-life can be greatly elongated while retaining the intrinsic activity of the EPO, without increasing the sugar chain content.
Owner:CJ HEALTHCARE CORP
Preparation method of multifunctional test paper for early pregnancy
ActiveCN103777002ASimplify detection stepsThe test result is accurateDisease diagnosisBiological testingPolyesterCellulose
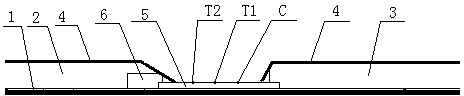
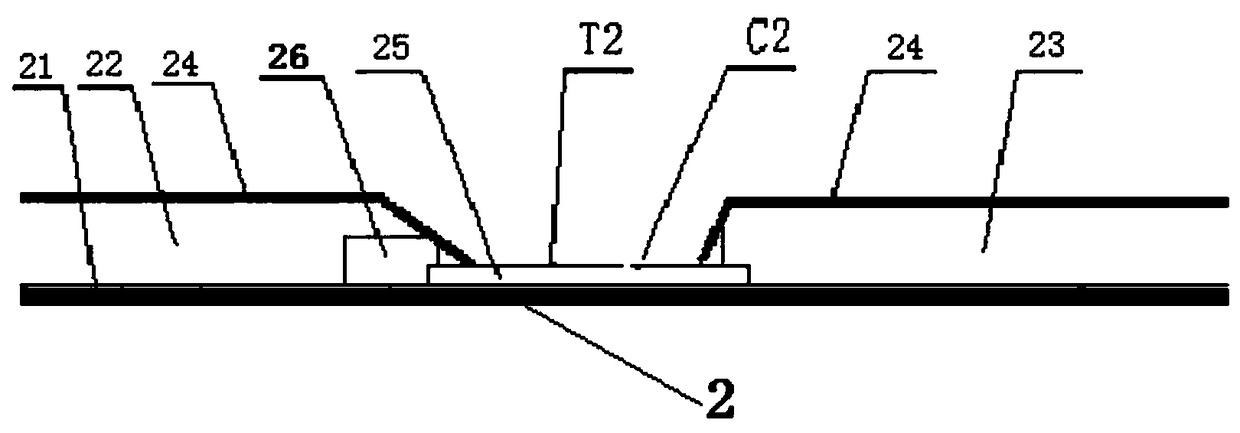
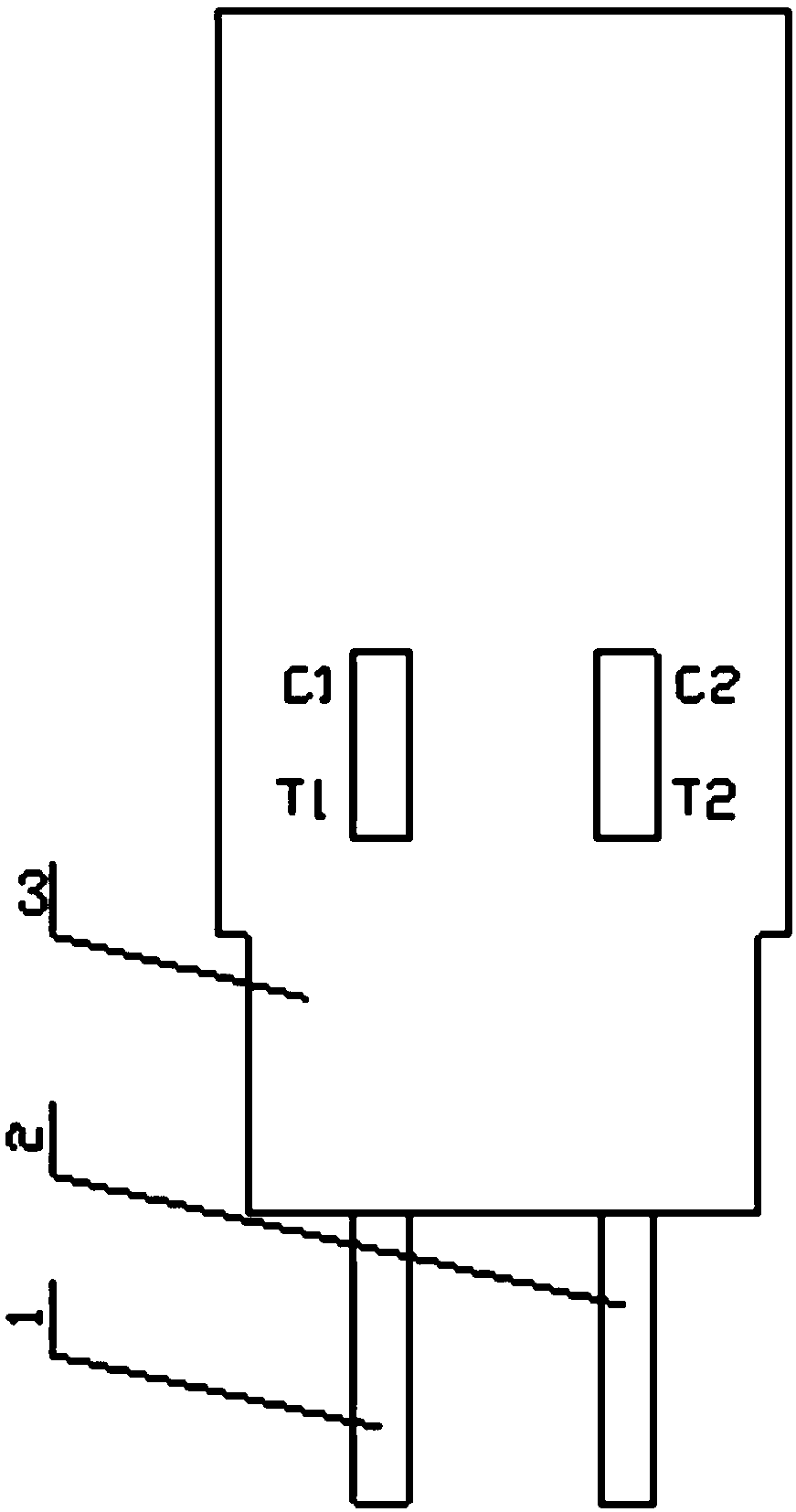
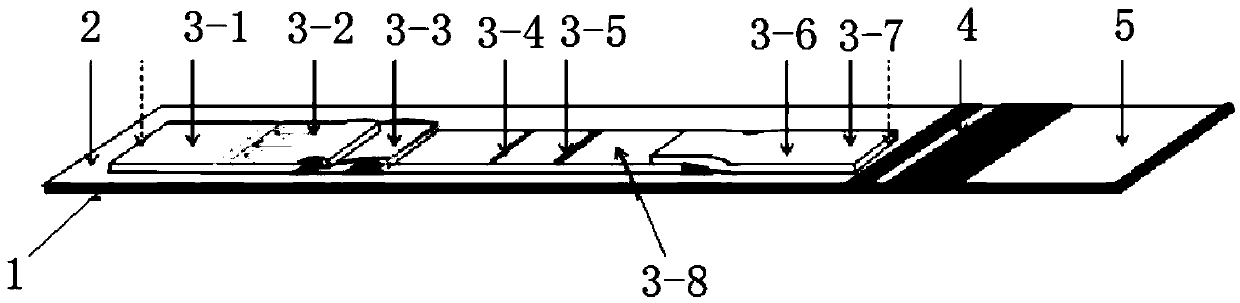
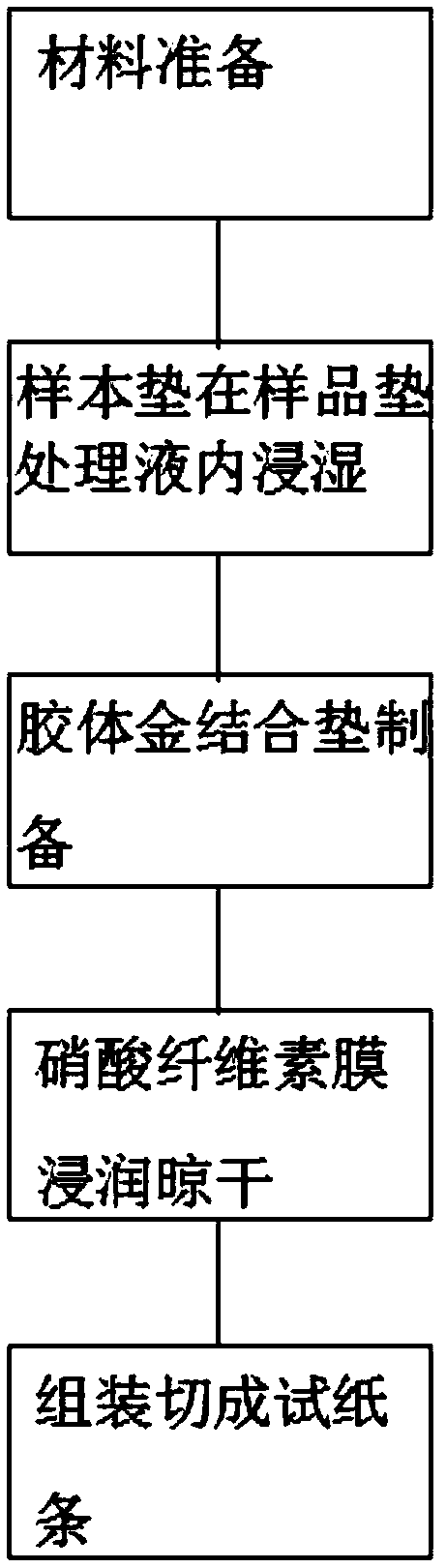
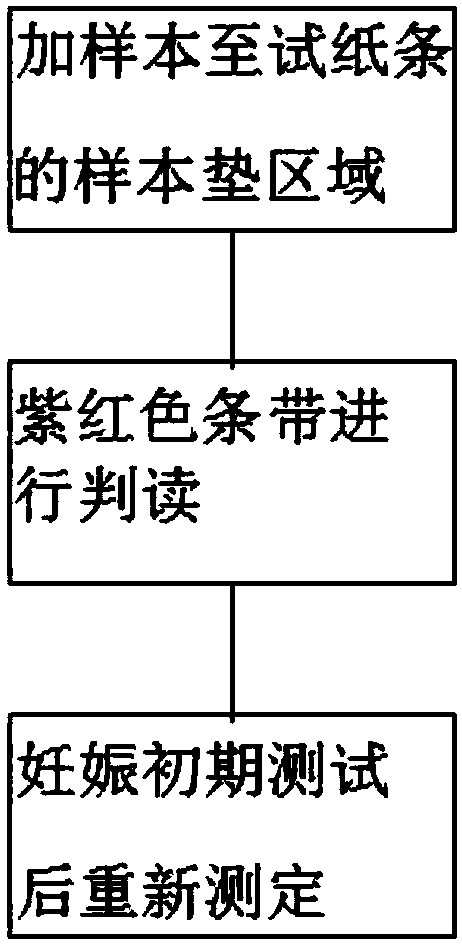
The invention discloses a preparation method of a multifunctional test paper for early pregnancy. The preparation method comprises the steps: preparing a nitrocellulose membrane; labeling and curing a polyester fiber strip; and assembling and cutting. A human chorionic gonadotropin beta core segment and a human chorionic gonadotropin regular molecule in urine are detected by applying a principle of a double-antibody sandwich method and an immunochromatographic method, detection steps are simple, a result can be observed within 5 min, and a common user can detect the result, and the detection result is more accurate. Through comparing color depth of a first detection line and a second detection line, whether to be pregnant and whether to be normally pregnant are determined, and ectopic gestation and threatened abortion are subjected to risk assessment.
Owner:NANTONG EGENS BIOTECH
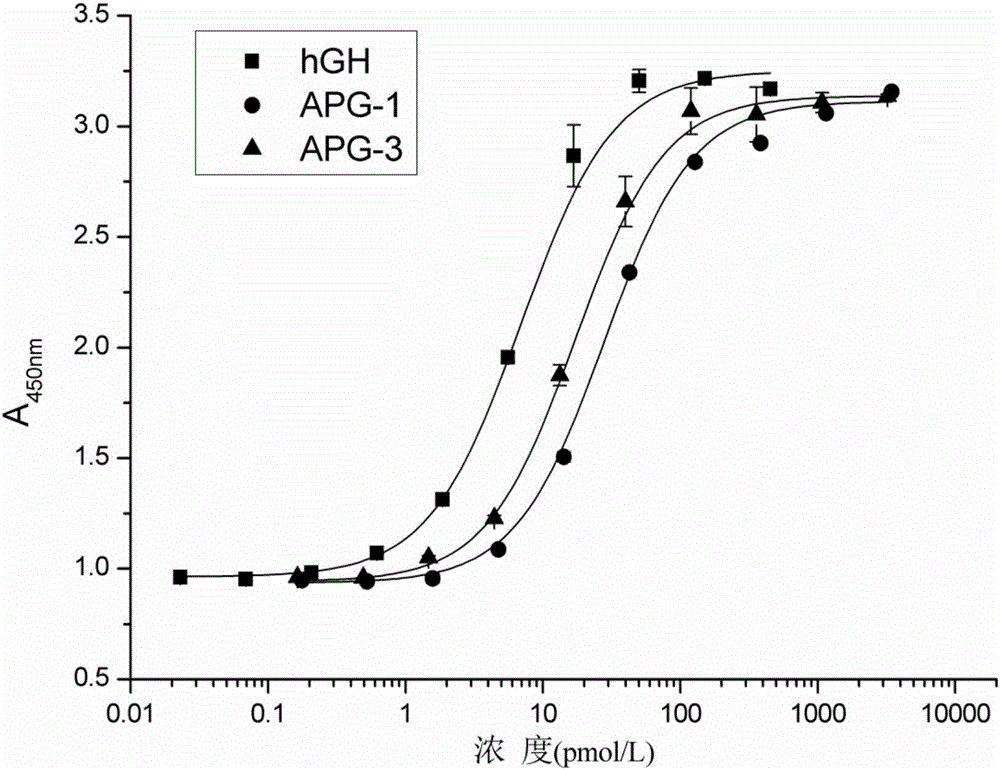
Hyperglycosylated human growth hormone fusion protein and preparation method and application thereof
InactiveCN106256835AImprove stabilityLow immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeImmunoglobulin Fc Fragments
The invention discloses hyperglycosylated human growth hormone fusion protein. The human growth hormone fusion protein sequentially contains a human growth hormone (hGH), a flexible peptide joint (L), human chorionic gonadotropin beta-carboxyl terminal rigid peptide (CTP) and a human immunoglobulin Fc fragment from the N terminal to the C terminal. The invention further discloses a method for efficiently preparing the fusion protein. Compared with a recombinant hGH, the built fusion protein has more excellent in-vivo drug efficacy, the in-vivo circulation half-life period is prolonged, the administration frequency is greatly decreased, and the bioavailability is improved; meanwhile, the production process is simpler and more efficient.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
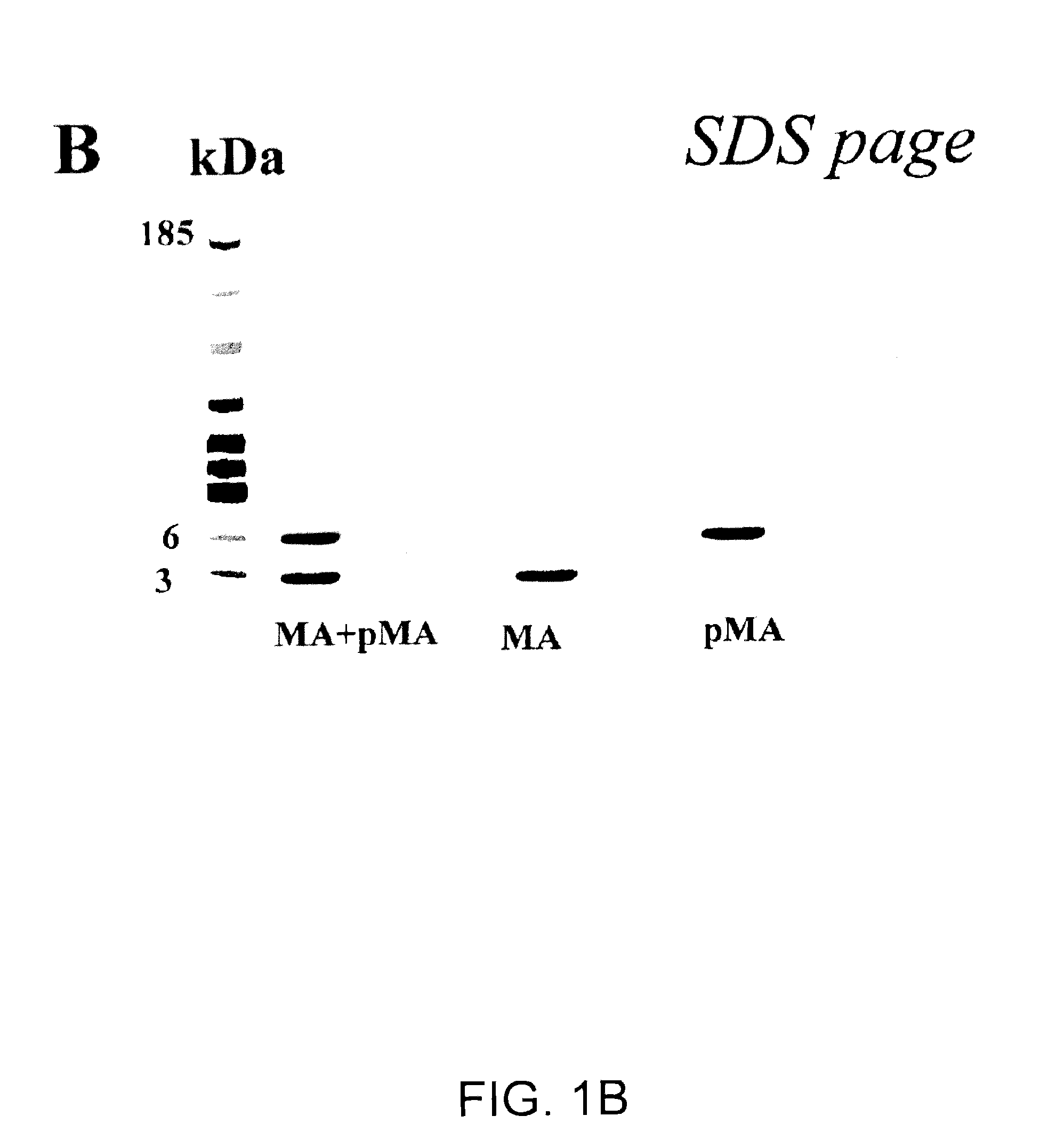
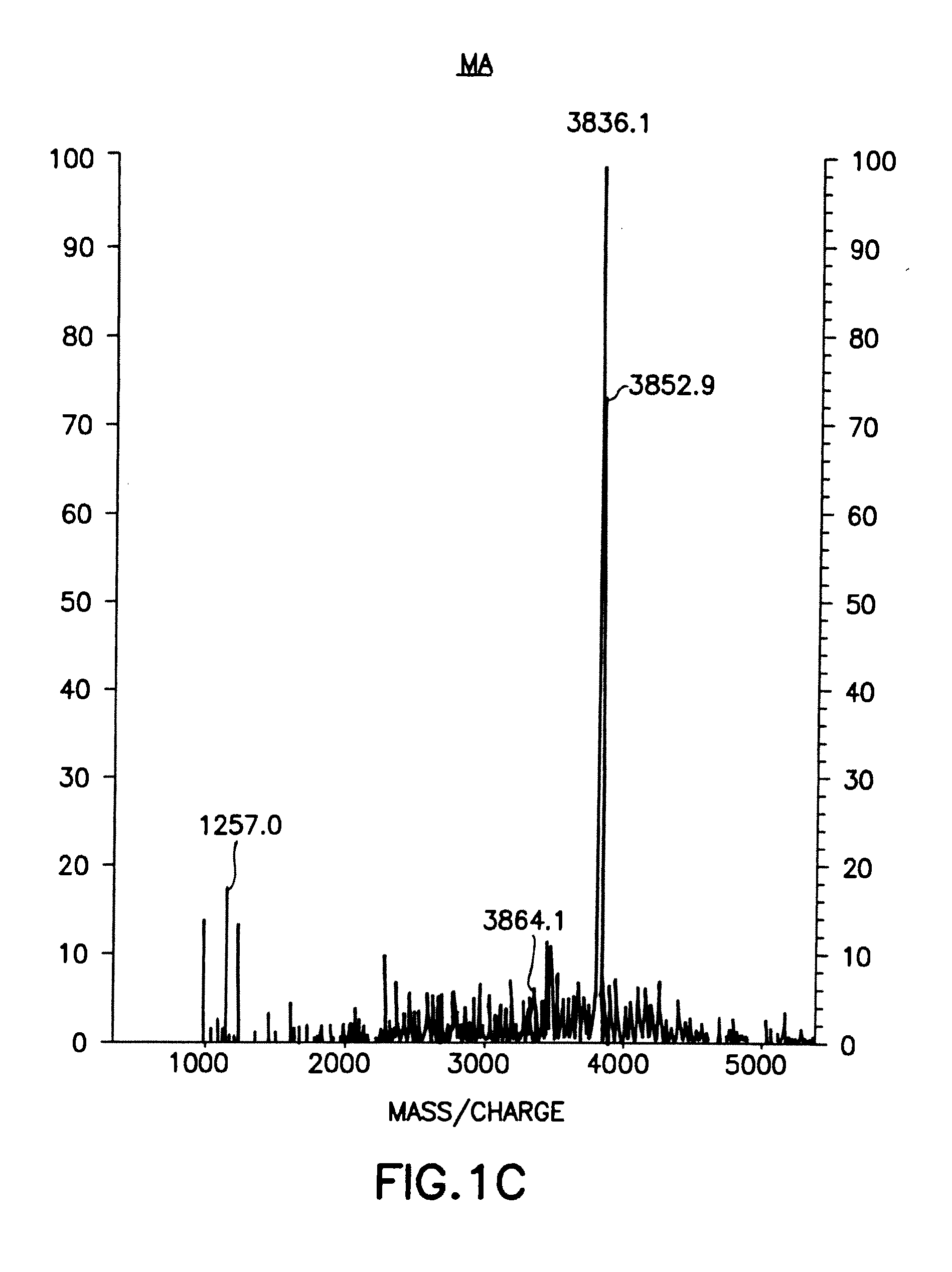
Biologically active polypeptides derived from a novel early stage pregnancy factor designated maternin (MA)
The invention relates to therapeutic polypeptides isolated from beta-human chorionic gonadotropin (β-hCG) found in human early pregnancy urine, now synthetically produced and designated Maternin. The therapeutic polypeptides and their functional equivalents are useful in treating and / or preventing various medical conditions. Examples of therapeutic effects of the therapeutic polypeptides include anti-HIV, anti-cancer, anti-wasting, prohematopoietic (e.g., anemias, radiation-mediated bone marrow damage, and trauma-mediated blood loss), and anti-angiogenic effects. The invention also provides pharmaceutical compositions comprising the therapeutic polypeptides, as well as methods for using the therapeutic polypeptides, functional equivalents and / or pharmaceutical compositions in the treatment and / or prevention of such medical conditions.
Owner:NOBEL BIOSCI
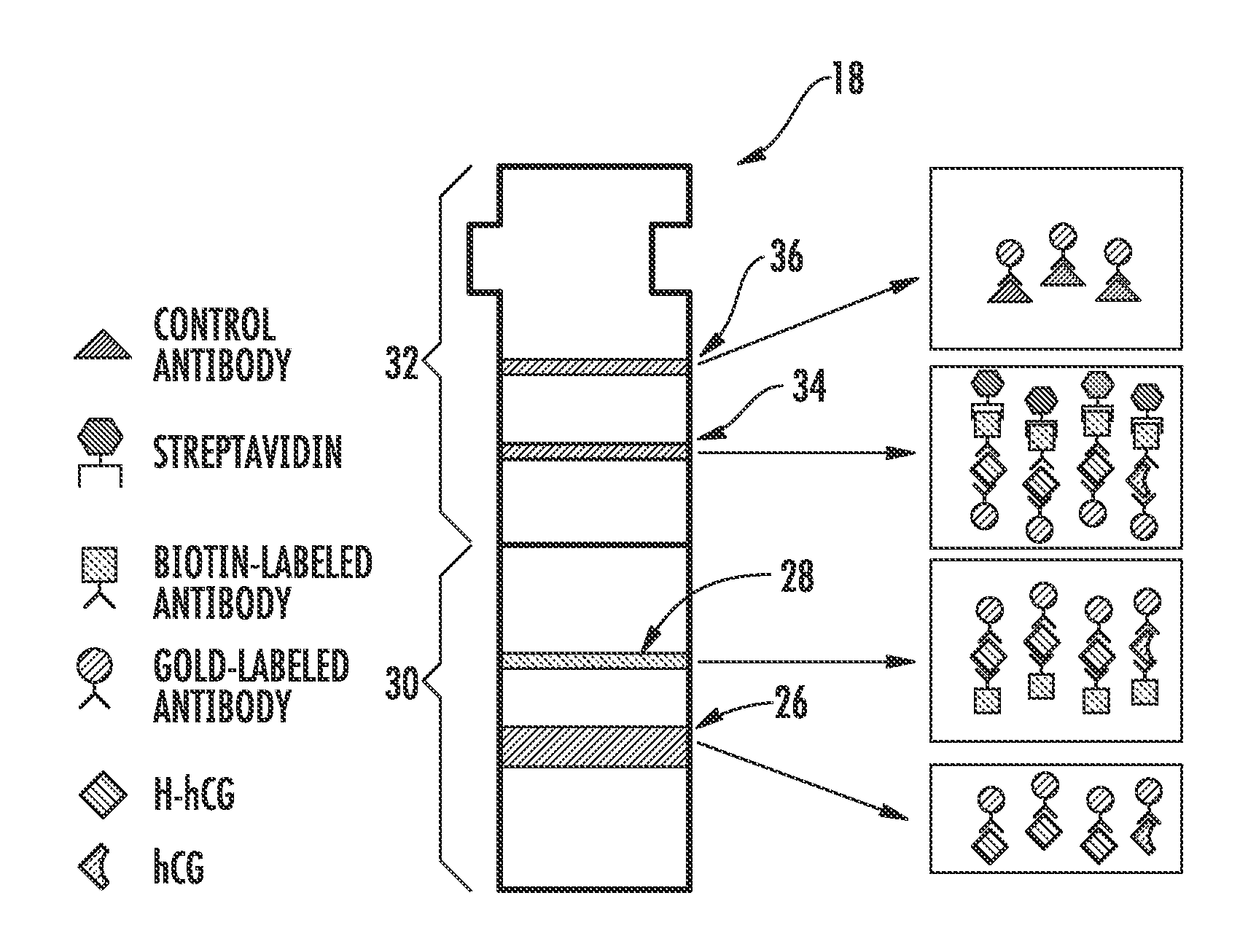
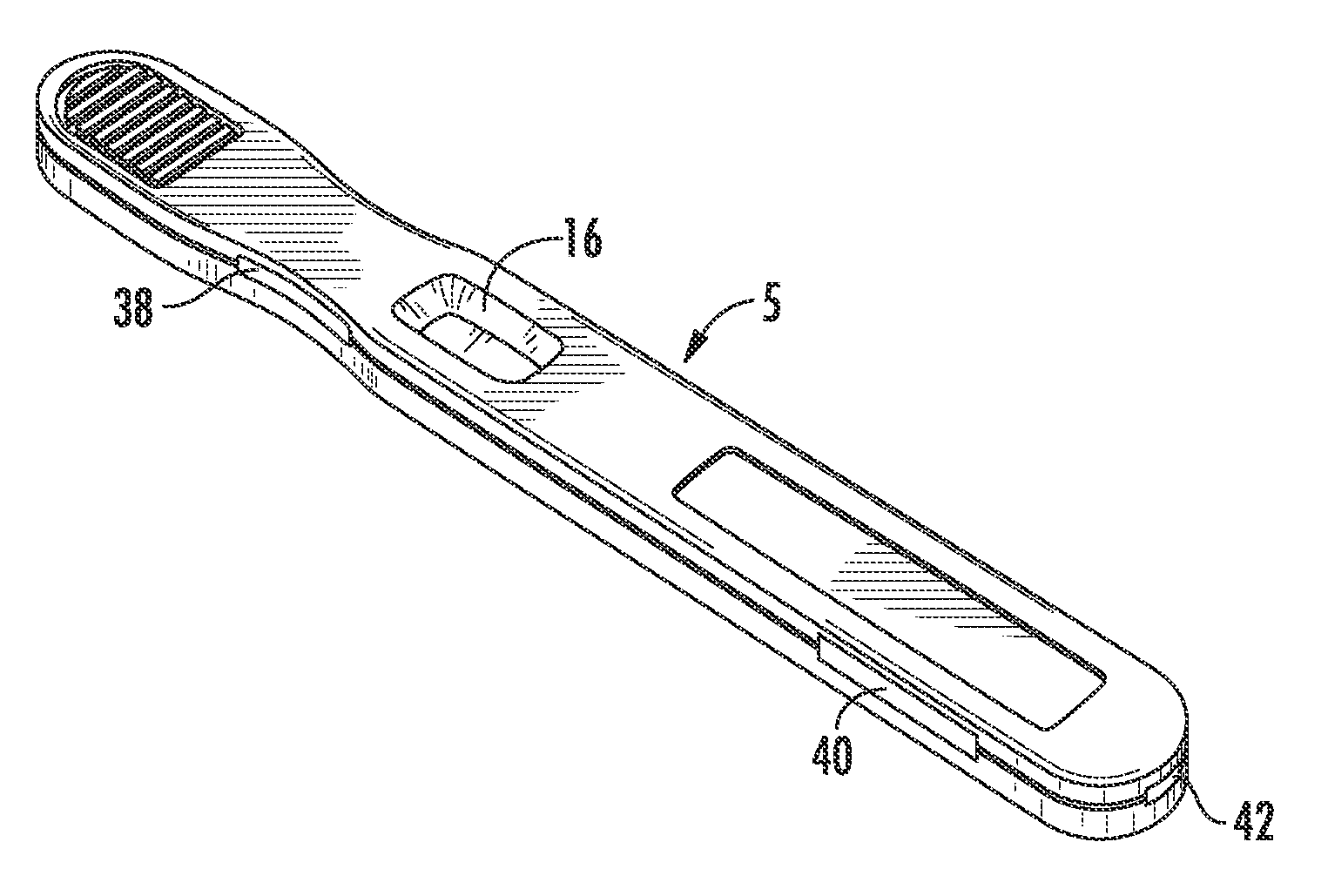
HYPERGLYCOSYLATED hCG DETECTION DEVICE
ActiveUS20110201122A1High affinityRaise the possibilityComponent separationBiological testingPregnancy testsPregnancy test
The present invention related to a pregnancy test device that can selectively detect hyperglycosylated human chorionic gonadotropin (hCG-H) in a liquid sample. The sample can be deposited on a proximal portion of the device for transport to a distal portion of the device. The device can include a release medium formed of a first material and including a detectable label thereon and a capture medium, including a capture site, in fluid communication with the release medium and formed of a second, different material. At least one of the release medium and the capture medium includes a binding member that exhibits a moderate to high affinity for hCG-H and is selectively or preferentially reactive with hCG-H.
Owner:CHURCH & DWIGHT CO INC
Hyperglycosylated hCG detection device
ActiveUS8278109B2Confirmation of the viability of a pregnancyHigh affinityAnalysis using chemical indicatorsComponent separationPregnancy testsPregnancy test
Owner:CHURCH & DWIGHT CO INC
hCG cycle detection test paper, hCG cycle detection kit, and preparation method and applications of hCG cycle detection test paper
InactiveCN108761099AEasy to operateThe detection method is simpleBiological material analysisBiological testingAnimal scienceAntiendomysial antibodies
Owner:NANTONG EGENS BIOTECH CO LTD
In-vitro culture solution of immature oocytes, and preparation method and application of in-vitro culture solution
ActiveCN111518749ASingle ingredientClear efficacyCulture processCell culture active agentsAnimal scienceCorpus luteum graviditatis
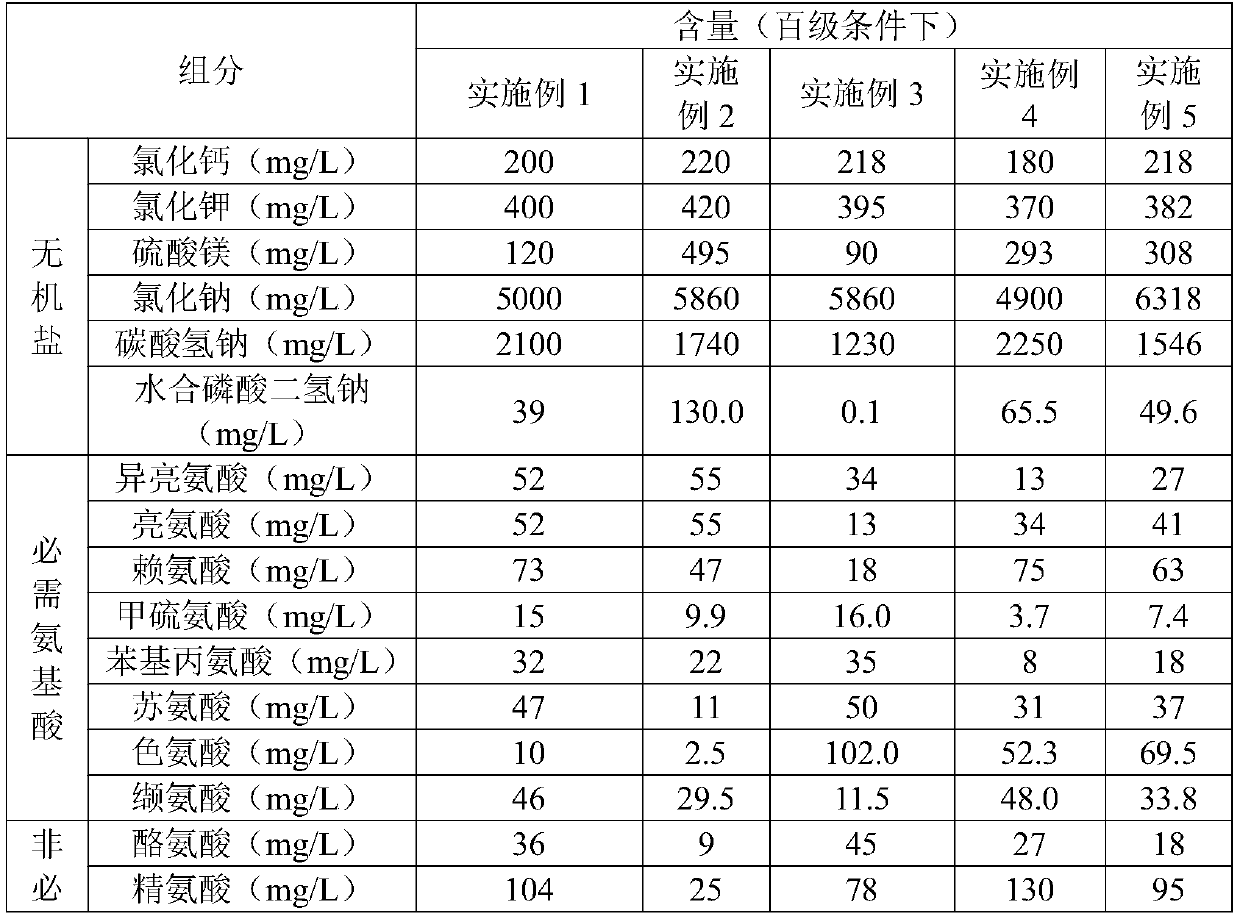
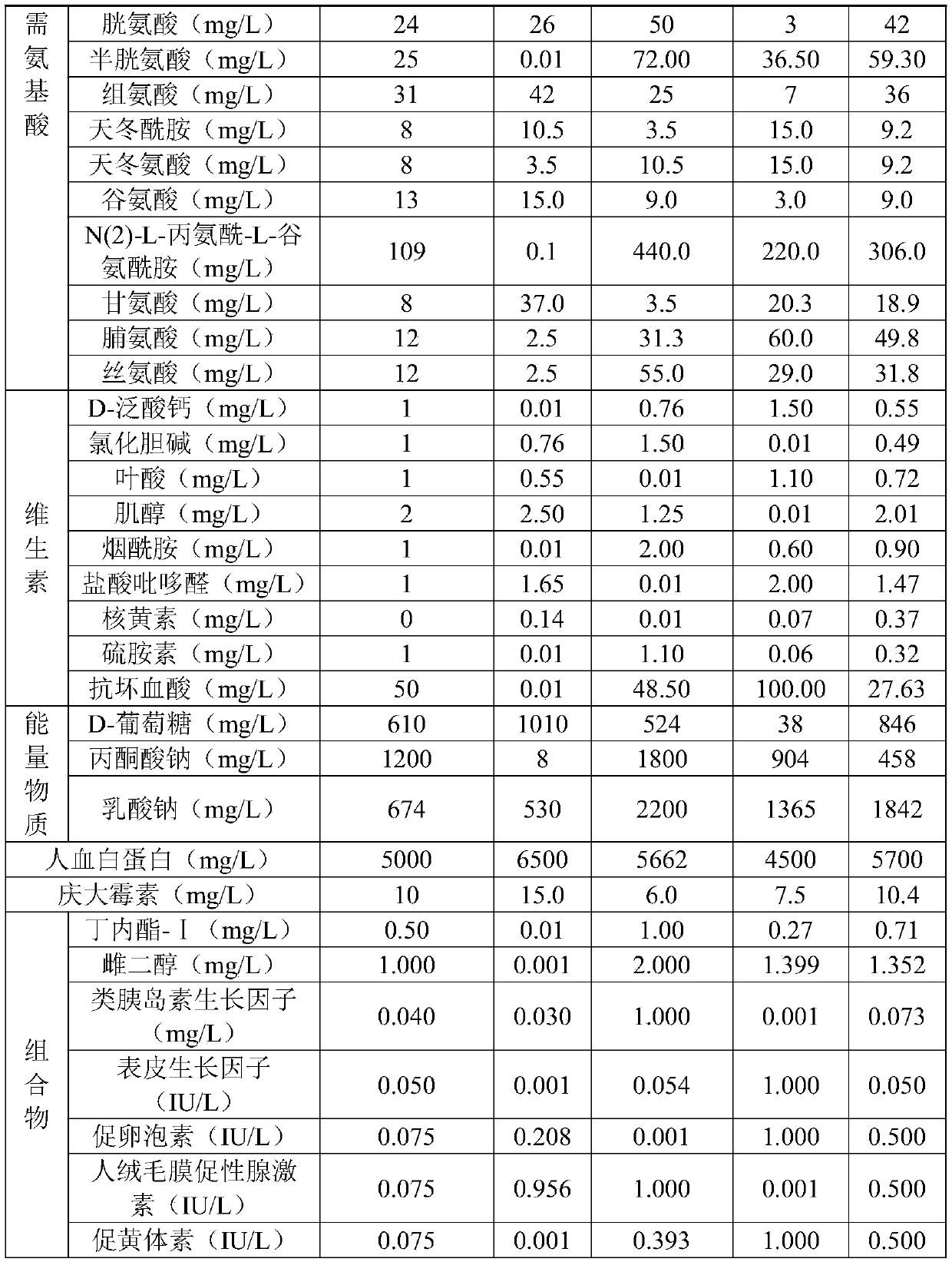
The present invention relates to the technical field of assisted reproduction and discloses an in-vitro culture solution of immature oocytes. The in-vitro culture solution comprises a basic culture solution and a composition. The basic culture solution comprises a solvent and a solute, the solvent comprises water, and the solute comprises vitamins, energy substances, inorganic salts and amino acids; and the composition comprises 0.01-1 mg / L of butyrolactone-I, 0.001-2 mg / L of estradiol, 0.001-1 IU / L of follicle-stimulating hormone, 0.001-1 IU / L of luteinizing hormone, 0.001-1 IU / L of epidermalgrowth factor, 0.001-1 mg / L of insulin-like growth factor and 0.001-1 IU / L of human chorionic gonadotropin. The present invention also discloses a preparation method and an application of the in-vitro culture solution. In addition to adding basic substances, by adding the energy substances, composition and vitamins, nucleus and cytoplasm of the immature oocytes are synchronously mature and besides, a fertilization rate and a high-quality embryo rate are improved.
Owner:成都艾伟孚生物科技有限公司
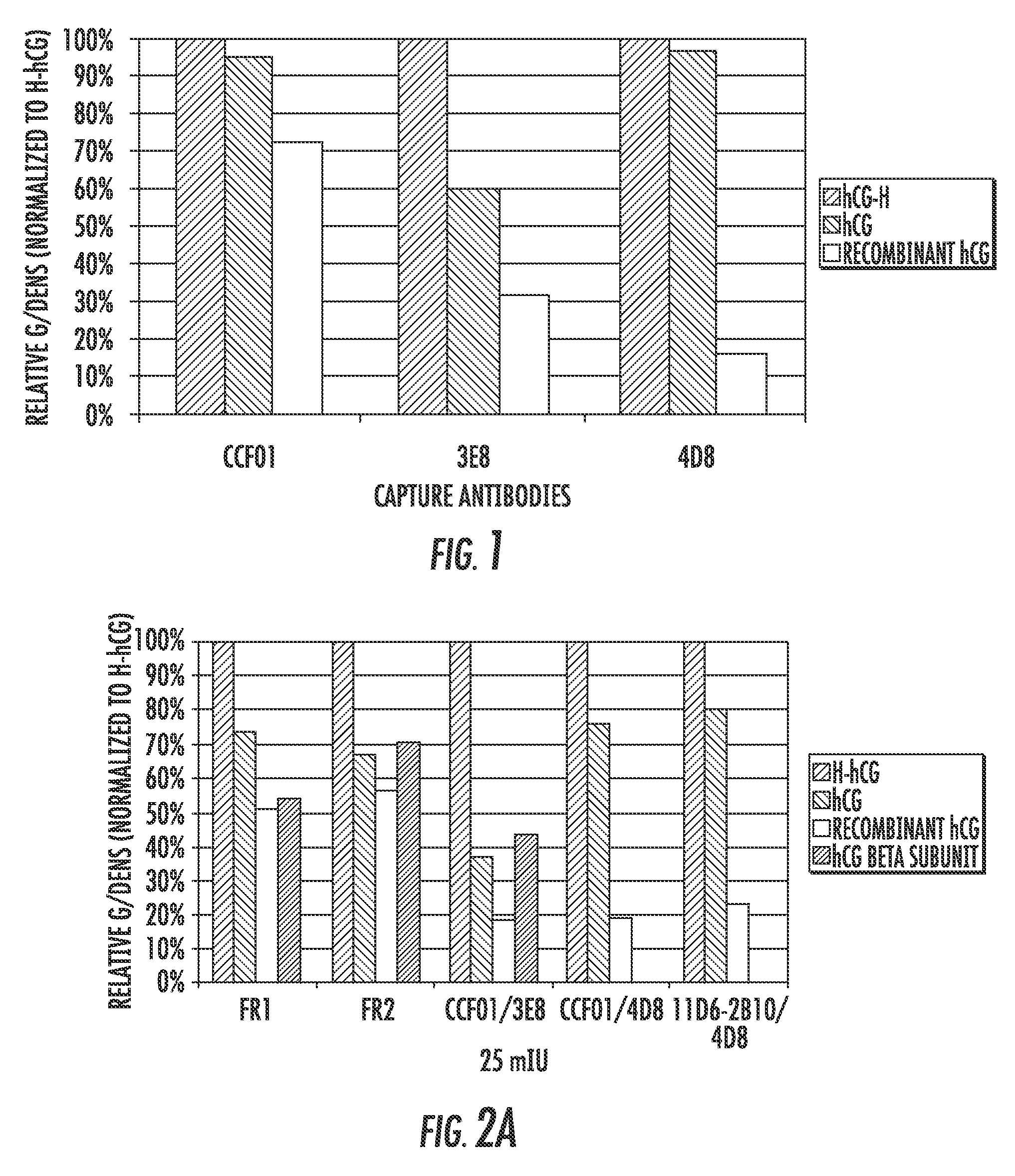
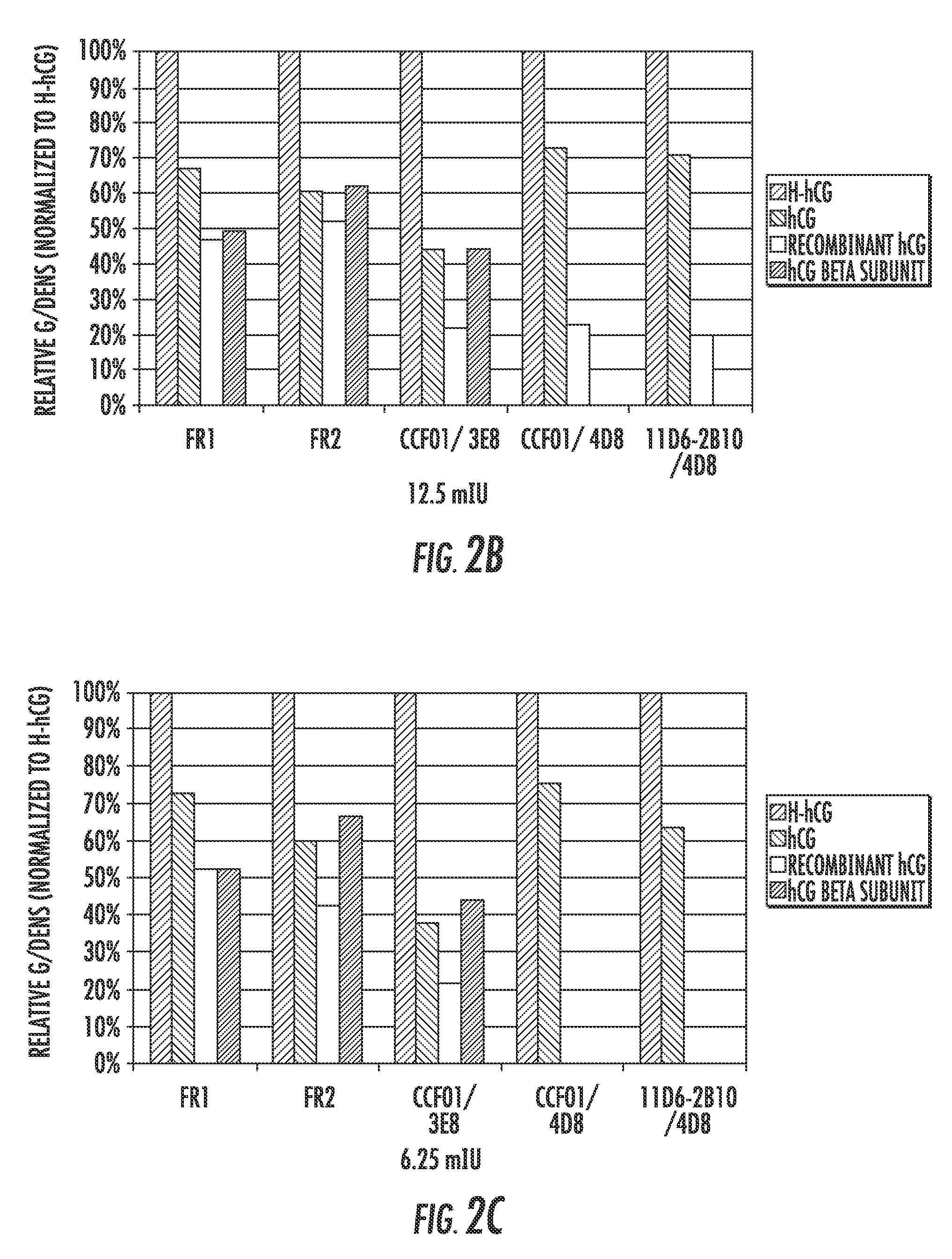
Detection kit for hyperglycosylated modification of hCG (human chorionic gonadotropin) tumor marker
InactiveCN105158485AHigh sensitivityImprove featuresDisease diagnosisBiological testingTumor BiomarkersImmunodiagnostics
The invention relates to a detection kit for hyperglycosylated modification of an hCG (human chorionic gonadotropin) tumor marker. According to the detection kit, magnetic beads are combined with an antibody (MCA-A) to serve as a capture antibody to capture total hCG in a to-be-detected sample, wherein the affinity of the antibody (MCA-A) is least influenced by glycosylation change; an antibody (MCA-B) most influenced by glycosylation change and another antibody (MCA-C) less influenced by glycosylation are used for detecting the total protein content and the N-glycosylation modification degree of hCG in the sample simultaneously, so that a tumor biomarker is identified. The detection kit improves the accuracy, the sensitivity and the specificity of an immunodiagnosis method greatly and has great practical value for detection of hyperglycosylated modification of the tumor marker in the actual sample.
Owner:SHANDONG UNIV
Hormone kit for scatophagus argus spawning induction and artificial spawning induction and insemination method for obtaining scatophagus argus fertilized eggs in batches
ActiveCN111000987ASolve difficultySolve the problem of gonadal degenerationOrganic active ingredientsPeptide/protein ingredientsAnimal scienceHCG - Human chorionic gonadotropin
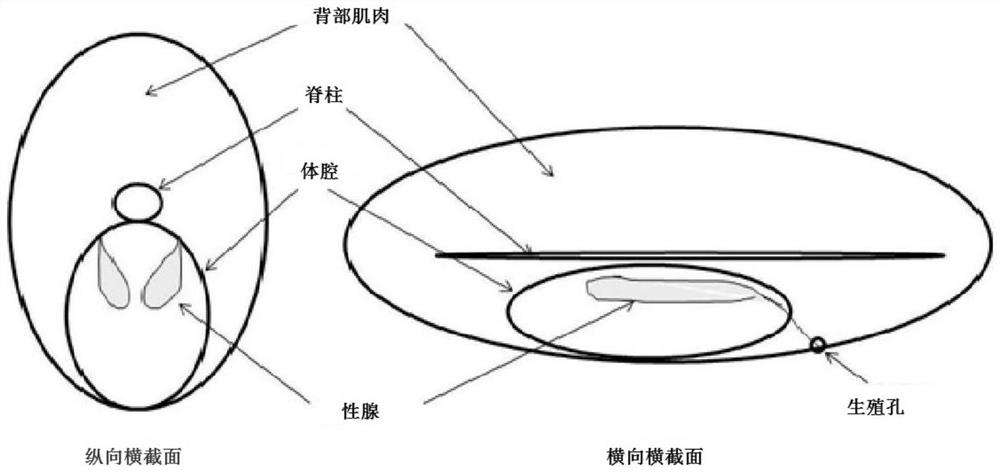
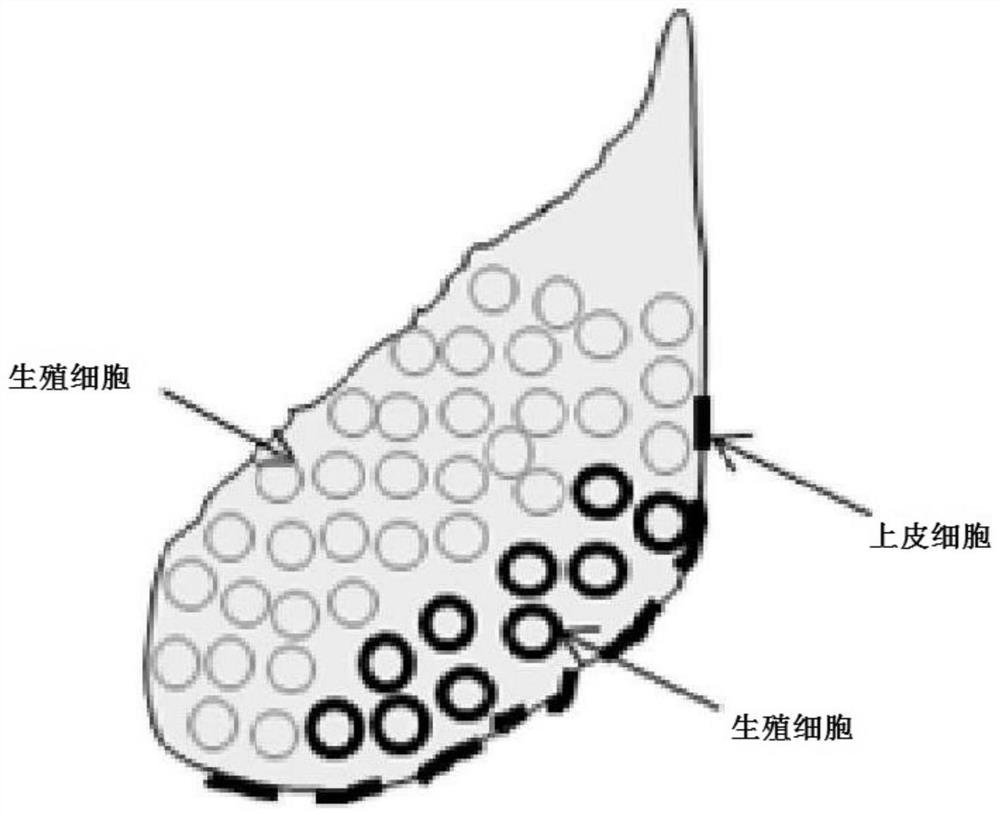
The invention provides a hormone kit for scatophagus argus spawning induction and an artificial spawning induction and insemination method for obtaining scatophagus argus fertilized eggs in batches, which belong to the technical field of aquaculture. The hormone kit for scatophagus argus spawning induction comprises gonadotropin releasing element A4, doxolone maleate and human chorionic gonadotropin which are independently subpackaged. Based on the hormone kit, scatophagus argus is subjected to artificial spawning induction by adopting a three-needle method, so that the problems of long effecttime, female scatophagus argus stagnation, high difficulty in artificial spawn extrusion after spawning induction, egg mass and gonad degeneration are effectively solved. The artificial inseminationmethod is adopted, and the problems that natural spawning eggs of scatophagus argus are over-mature or collapse in vivo, the influence of the environment is large, the spawning induction success rate,the fertilization rate and the hatching rate are extremely low, and fertilized eggs are difficult to obtain in batches are effectively solved.
Owner:GUANGDONG OCEAN UNIVERSITY
Micro-fluidic chemiluminescence detecting system for magnetic particles for detecting gonad series
ActiveCN107655879AAccurate quantitative detectionHigh sensitivityChemiluminescene/bioluminescenceEngineeringMicroparticle
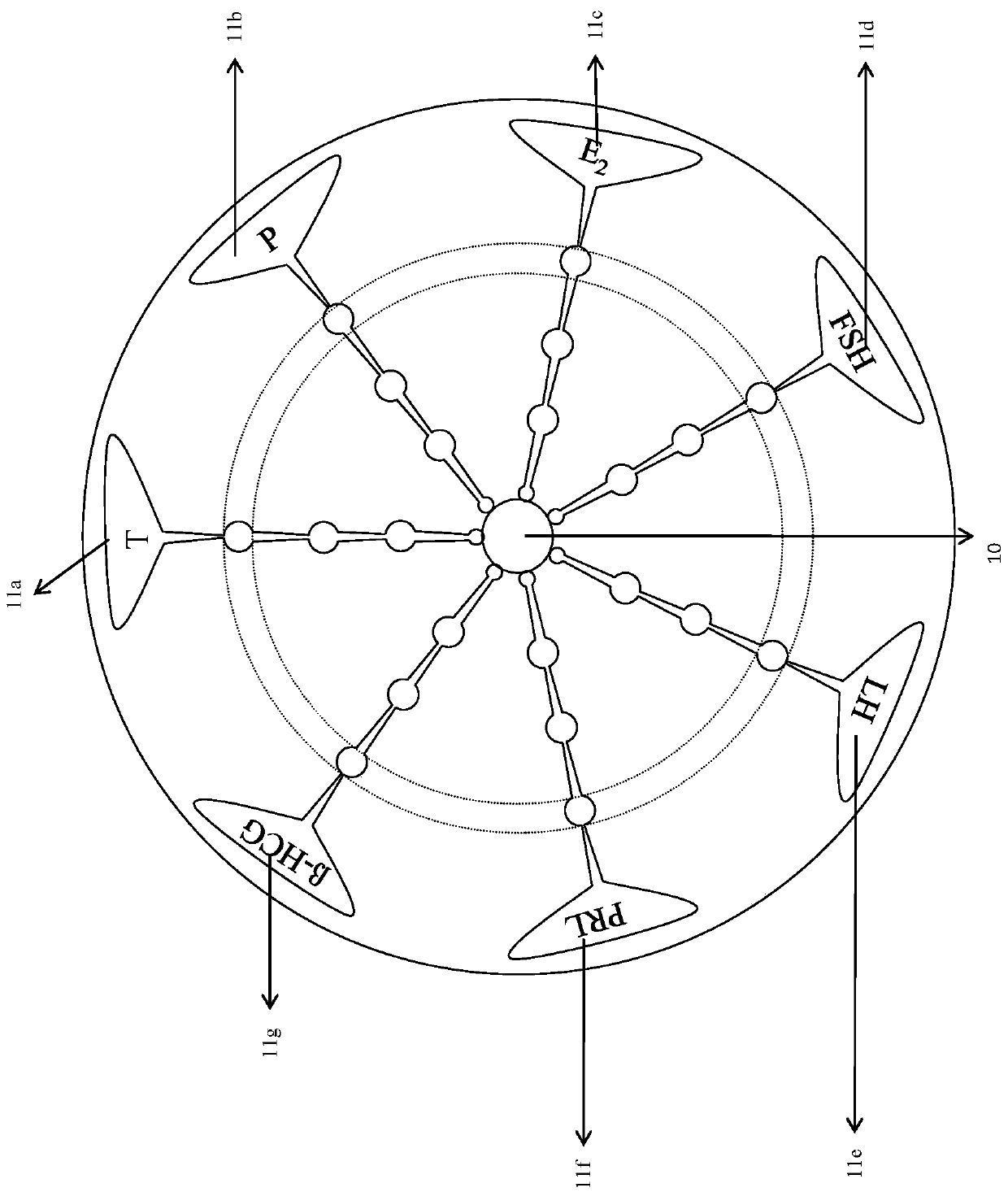
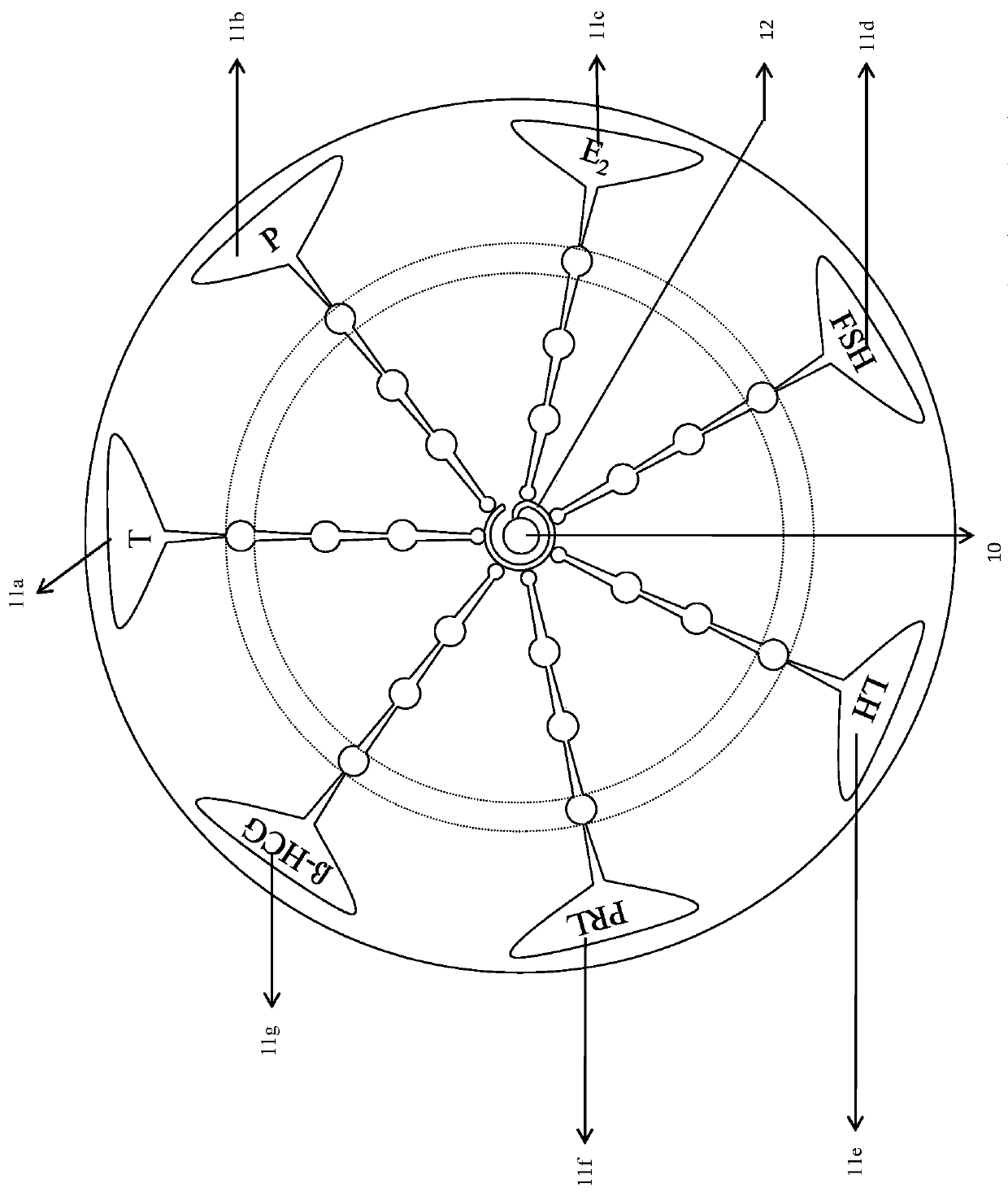
The invention discloses a micro-fluidic chemiluminescence detecting system for magnetic particles for detecting gonad series. The system comprises a bottom plate and an upper chip, and the upper chipcomprises a sample adding area in the center of the upper chip and seven micro-fluidic reaction detecting channels communicated with the sample adding area, wherein the seven micro-fluidic reaction detecting channels include a testosterone micro-fluidic reaction detecting channel, a progesterone micro-fluidic reaction detecting channel, an estradiol micro-fluidic reaction detecting channel, a follicle-stimulating hormone micro-fluidic reaction detecting channel, a luteinizing hormone micro-fluidic reaction detecting channel, a prolactin micro-fluidic reaction detecting channel and a beta-humanchorionic gonadotropin micro-fluidic reaction detecting channel. In application of the system, the bottom plate is arranged under the upper chip, and magnets which are permanent magnets or electromagnets are arranged at positions, corresponding to magnetic particle coating areas of the micro-fluidic reaction detecting channels, of the bottom plate.
Owner:BEIJING ELCOTEQ BIO TECH
Systhesizing of chimeric peptide from anteron promoted by human's fine hair
InactiveCN1438242AHigh affinityOvercoming the problem of "vaccines with different names"DepsipeptidesFermentationAntigenEscherichia coli
The invention refers to human chorion gonadotropic hormone (hCG) chimera peptide CP1, ramification CP10, and the making method by gene engineering. They have 6 amino (N) ends or 5 continuous broad-spectrum or single strong T-cell table, and 3 B-cell tables of hCG beta-antigen at carboxylic end: beta5 45-52, beta9 113-116 and beta8 137-144. The genes coding CP1 and CP10 can all be expressed in colibacillus, and the expressed product can be obtained by the SDS-polyacrylamide gelatin electrophoresis (SDS-PAGE).
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +1
Human chorionic gonadotropin detection kit, preparation method and application method thereof
InactiveCN109061203AHigh sensitivityImprove featuresBiological testingAntigenFluorescein isothiocyanate
The invention discloses a human chorionic gonadotropin detection kit, a preparation and an application method thereof. The kit comprises a calibrator, a quality control serum, an anti-reagent A, an anti-reagent B, a magnetic particle reagent and a luminescent substrate. The calibrator and the quality control serum are prepared by first dissolving a Total-[beta]hCG antigen with a protein-containingbuffer solution, and then diluting the protein-containing buffer solution to a fresh bovine serum-containing buffer solution; the anti-reagent A is a Total-[beta]hCG coating antibody labeled by a fluorescein isothiocyanate, and the anti-reagent B is a Total-[beta]hCG labeled antibody labeled by a alkaline phosphatase; the magnetic particle reagent is prepared by coupling the fluorescein isothiocyanate antibody with magnetic particles. The invention also relates to a preparation method of the kit and a method for quantitatively detecting the content of human chorionic gonadotropin (Total-[beta]hCG) in human serum by using the kit. According to the human chorionic gonadotropin detection kit, the preparation and the application method thereof, the kit is reliable in performance, high in sensitivity, wide in linear range, and can be used in conjunction with semi-automatic and full-automatic instruments.
Owner:TAIZHOU ZECEN BIOTECH CO LTD
Novel method for determining human chorionic gonadotropin
ActiveCN110702754AHigh sensitivityImprove stabilityChemiluminescene/bioluminescenceBiological testingHCG - Human chorionic gonadotropinCoordination polymerization
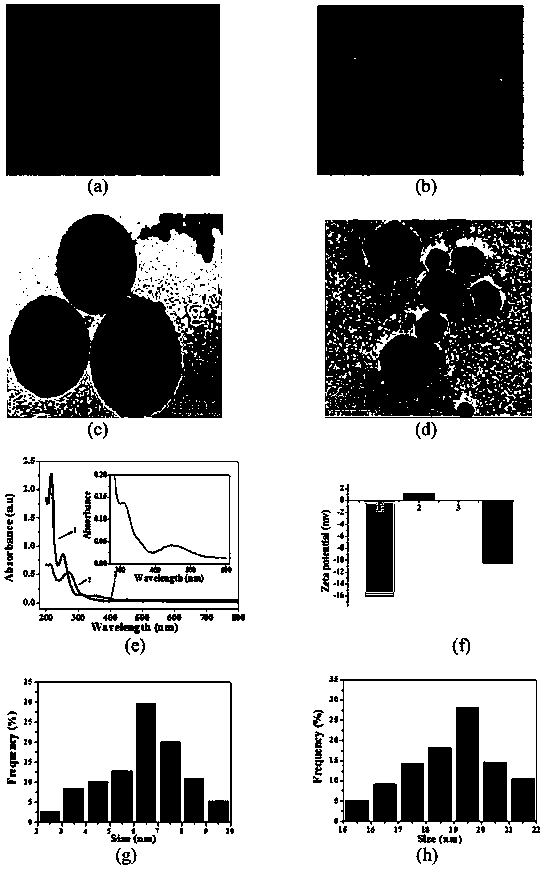
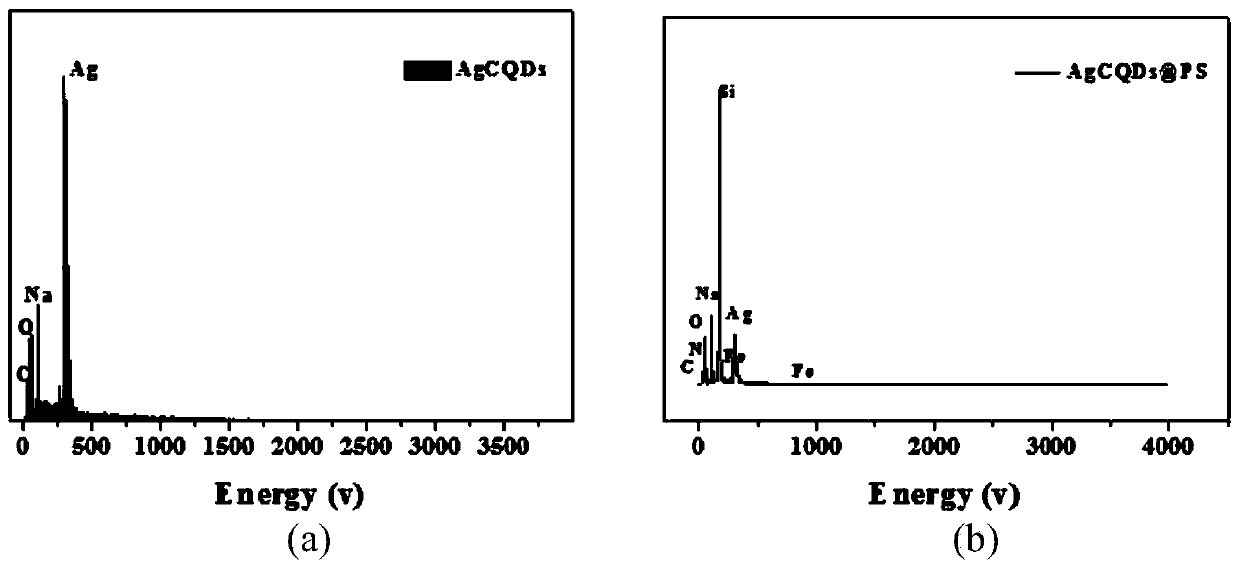
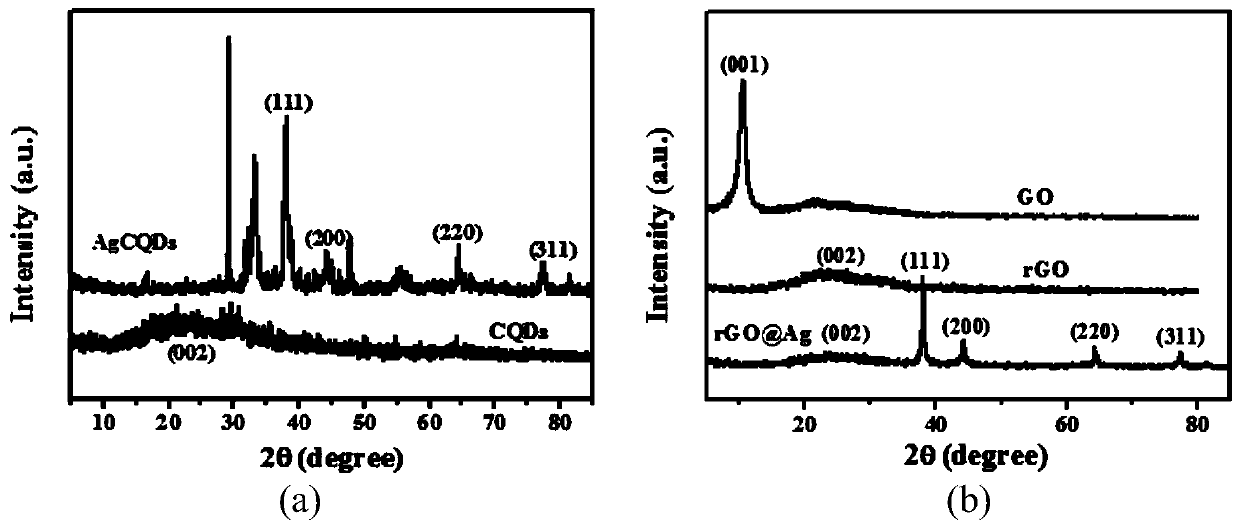
The invention discloses a novel method for determining human chorionic gonadotropin. The method comprises the following steps of: by taking waste biomass papaya peel as a raw material, synthesizing blue high-fluorescence carbon quantum dots in one step by adopting a hydrothermal method, and combining the blue high-fluorescence carbon quantum dots with silver ions to form AgCQDs; and synthesizing PS through photolysis and coordination polymerization of ferrocene dicarboxylic acid molecules in methyl alcohol, and combining AgCQDs with negative electricity and PS modified by PEI to be changed into PS with positive electricity through electrostatic attraction, wherein the obtained composite material and an rGO@Ag composite material jointly prepare a sandwich type immunosensor for HCG detection. The method is high in sensitivity and good in stability.
Owner:GUANGXI NORMAL UNIV
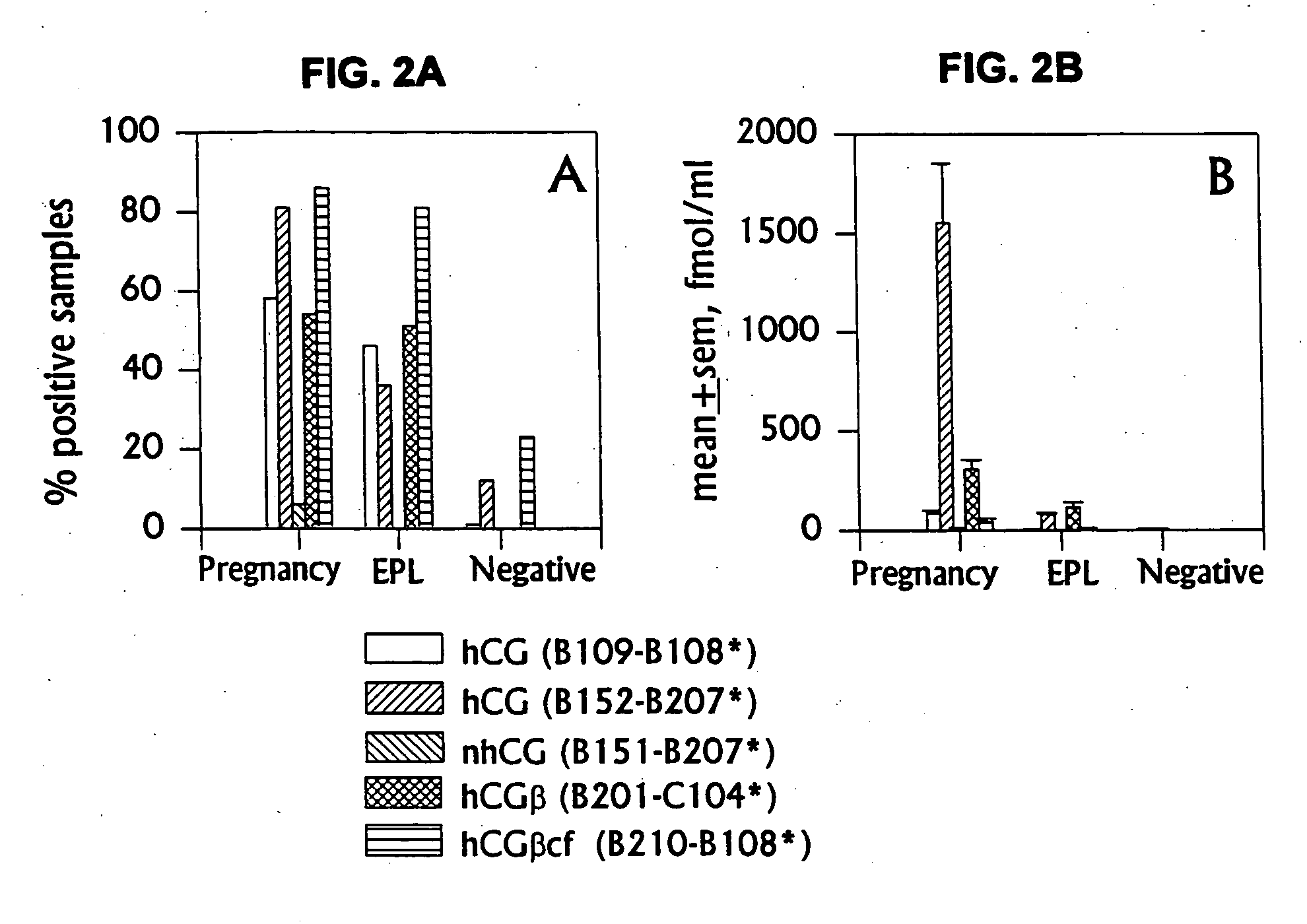
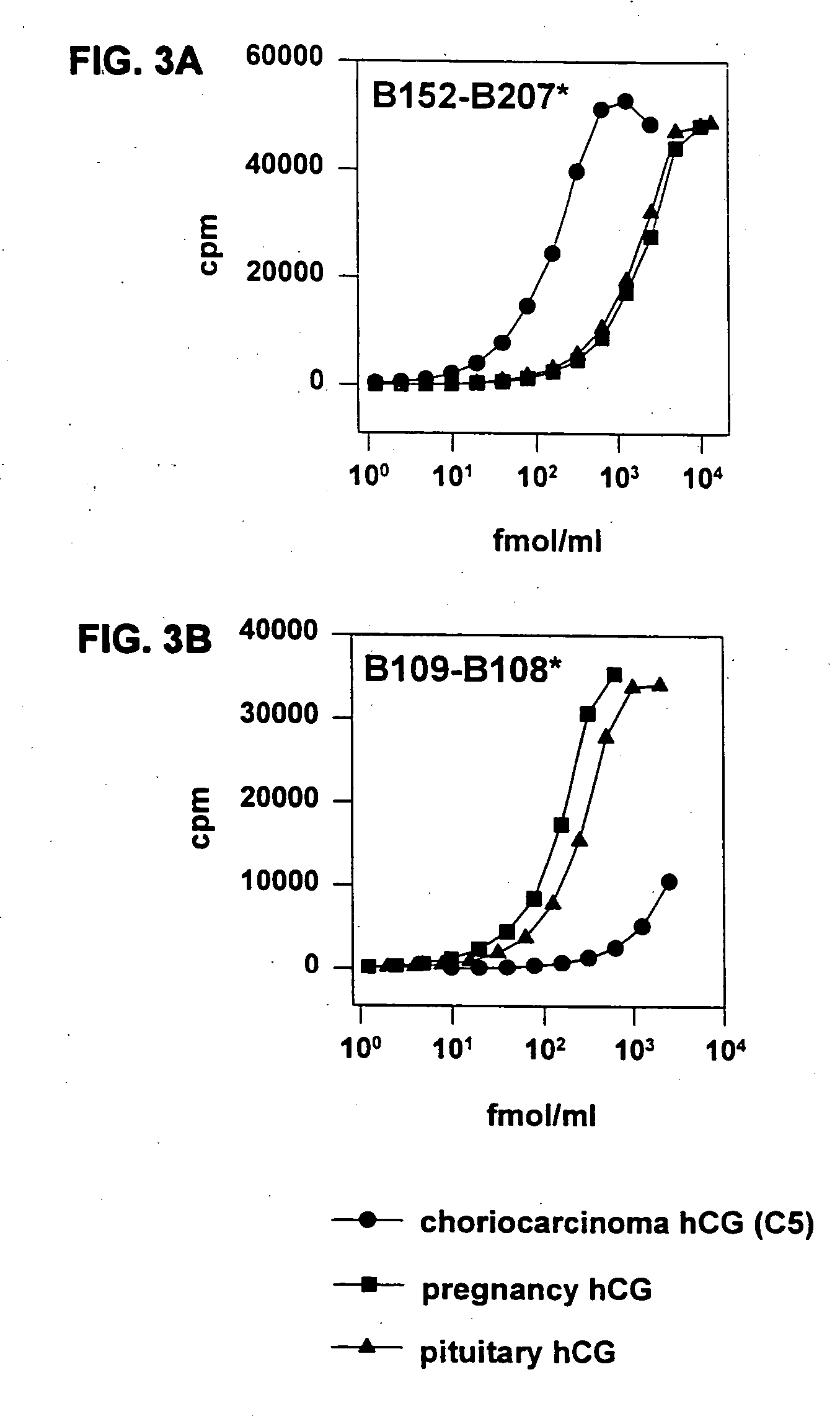
Methods for predicting pregnancy outcome in a subject by hCG assay
The present invention provides a method of predicting pregnancy outcome in a subject by determining the amount of an early pregnancy associated molecular isoform of hCG in a sample. The present invention further provides a method for determining the amount of early pregnancy associated molecular isoforms of human chorionic gonadotropin (hCG) in a sample. The present invention also provides a diagnostic kit for determining the amount of early pregnancy associated hCG in a sample. The present invention additionally provides an antibody which specifically binds to an early pregnancy associated molecular isoform of human chorionic gonadotropin. Finally, the present invention provides methods for detecting trophoblast or non-trophoblast malignancy in a sample.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Drying device for extracting human chorionic gonadotropin from human urine and extraction method
PendingCN114812108AIncrease temperatureIncrease drying speedDrying solid materials without heatDrying machines with local agitationPharmacyHCG - Human chorionic gonadotropin
The embodiment of the invention provides a drying device for extracting human chorionic gonadotropin from human urine and an extraction method, and relates to the technical field of biological pharmacy. The drying device for extracting human chorionic gonadotropin from human urine comprises a heating storage mechanism and a centrifugal drying mechanism. The heating storage mechanism comprises an outer tank body, a bottom plate, a top cover, a first partition plate, a second partition plate and an electric heater, the top cover is hinged to the top of the outer tank body, the bottom plate is fixedly arranged at the bottom of the outer tank body, and the first partition plate and the second partition plate are fixedly connected to the lower portion of the interior of the outer tank body; and the first partition plate is positioned above the second partition plate. When the electric heater heats and dries the interior of the outer tank body, the driving assembly drives the shaft rod to rotate, and the rotating shaft rod drives the supporting frame piece and the filter element cylinder on the rotating shaft rod to quickly rotate, so that residual water in precipitates is quickly thrown out; and the device is matched with an electric heater to quickly dry the precipitate.
Owner:JIANGSU YOULIKA BIOTECHNOLOYG CO LTD
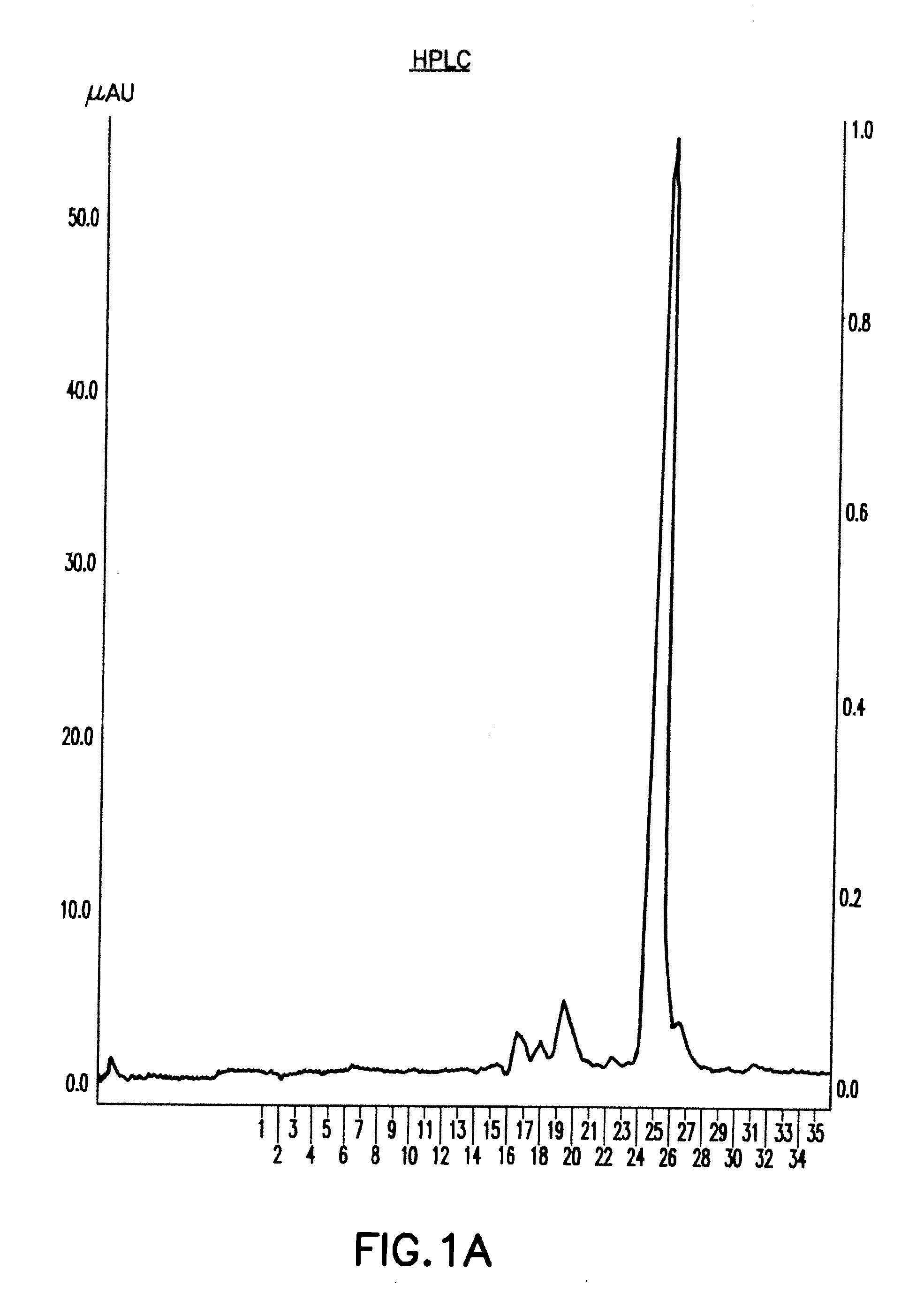
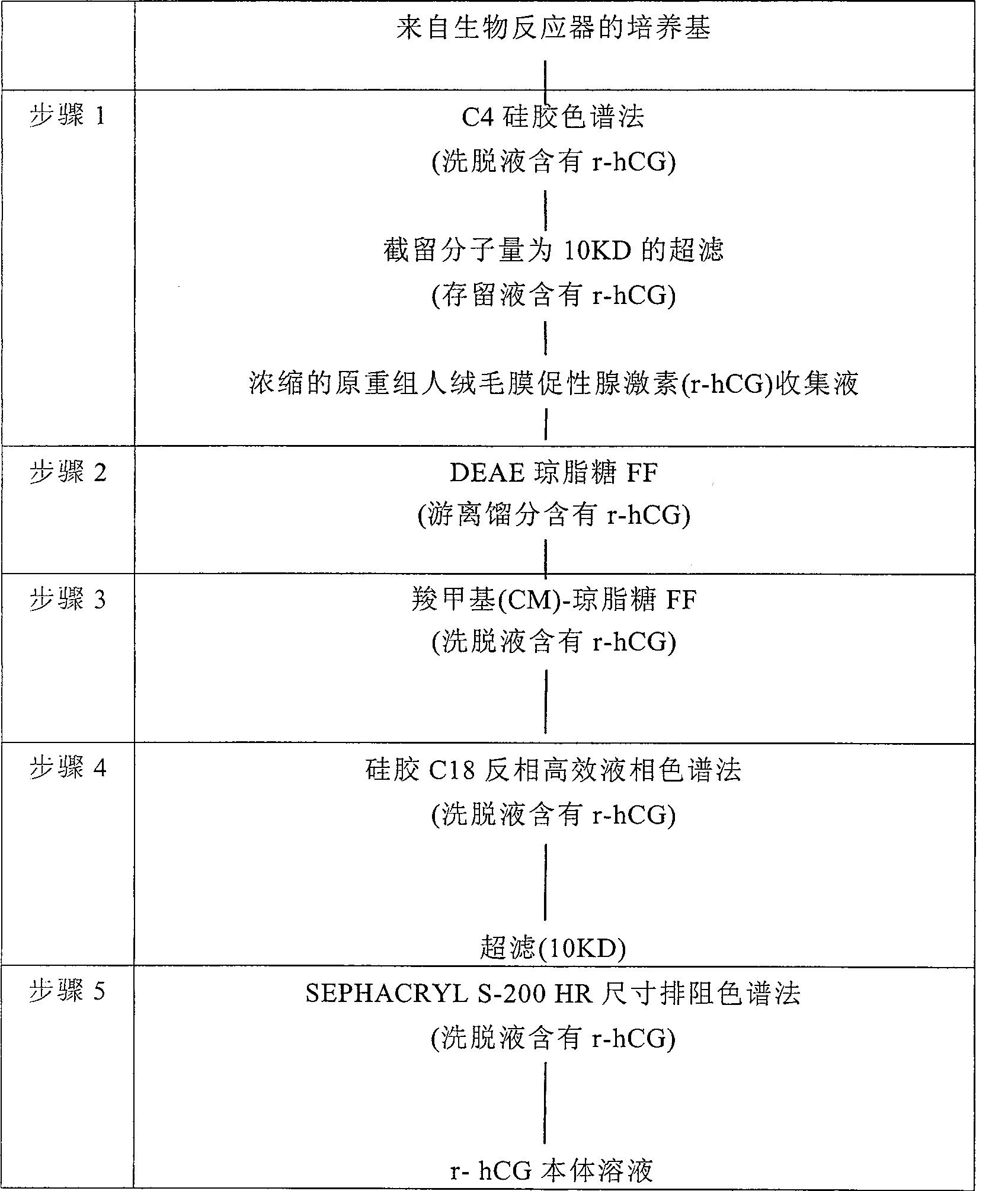
Process of purification of hCG and recombinant hCG purified by that method
InactiveCN100482679CPeptide/protein ingredientsOther chemical processesRecombinant Human Chorionic GonadotropinHCG - Human chorionic gonadotropin
A method for purifying recombinant human chorionic gonadotropin (hCG) from a raw recombinant human chorionic gonadotropin (hCG) sample of Chinese hamster ovary (CHO) cell supernatant, which includes the combined use of ion exchange chromatography and reversed-phase high-performance liquid Chromatography. Human chorionic gonadotropin is purified completely free of any contaminants by elution using ion exchange chromatography twice and finally by particle size exclusion chromatography. Highly pure human chorionic gonadotropin can be produced by this method, with a very high specific biological activity of approximately 25,000IU / mg.
Owner:MERCK SERONO SA
Method for inducing estrus synchronization and superovulation of female cat
InactiveCN111956366AWide variety of sourcesEasy to useAnimal reproductionAnimal husbandryObstetricsHCG - Human chorionic gonadotropin
The invention discloses a method for inducing estrus and ovulation of a female cat, and belongs to the technical field of animal artificial propagation. The invention provides the method for inducingestrus synchronization and superovulation of the female cat in order to solve the problem that estrus and ovulation are difficult to synchronize in artificial propagation of the female cat. The methodspecifically comprises the following steps: continuously feeding adult healthy female cats with altrenogest with a dosage of 0.01-20mg / cat / day for 10-20 days, stopping feeding the altrenogest for 2 to 3 days, injecting 10-1000IU of pregnant mare serum hormone into the muscle of the female cat, and intramuscularly injecting 10-1000 IU of human chorionic gonadotropin after 72-96 hours, so that thefemale cat can perform estrus synchronization and superovulation within a preset time. The key technical difficulty of artificial breeding of the domestic cat or the feline is solved.
Owner:NORTHWEST A & F UNIV
Sperm cryopreservation method of scatophagus argus
InactiveCN110786321AAffect the preservation effectAffect the survival rateDead animal preservationObstetricsHCG - Human chorionic gonadotropin
The invention discloses a sperm cryopreservation method of scatophagus argus. The sperm cryopreservation method comprises four steps of sperm collection, sperm dilution, sperm cryopreservation and sperm thawing, firstly male scatophagus argus is selected to inject human chorionic gonadotropin into dorsal muscle, then the sperm of the scatophagus argus is collected by an artificial abdominal extrusion method, then the sperm and antifreezing liquid are mixed to obtain preservation liquid of the scatophagus argus sperm, a diluent is prepared by mixing any one of a TS-2 diluent, a TS-19 diluent, aHank's solution, a Cortland diluent and sterilized seawater with dimethylsulfoxide; the sperm preservation liquid is injected into a cryopreservation tube, then freezing is conducted by adopting a step-by-step cooling method, and then the cryopreservation tube is immersed in liquid nitrogen for long-term preservation; when taking for use, the cryopreservation tube containing the sperm preservation liquid is taken out from the liquid nitrogen, and then is placed in a water bath with the temperature of 42 DEG C. According to the sperm cryopreservation method, the sperm of the scatophagus arguscan be preserved for a long time, and the sperm survival rate and insemination rate after thawing and resuscitation are high.
Owner:GUANGXI ACADEMY OF FISHERY SCI
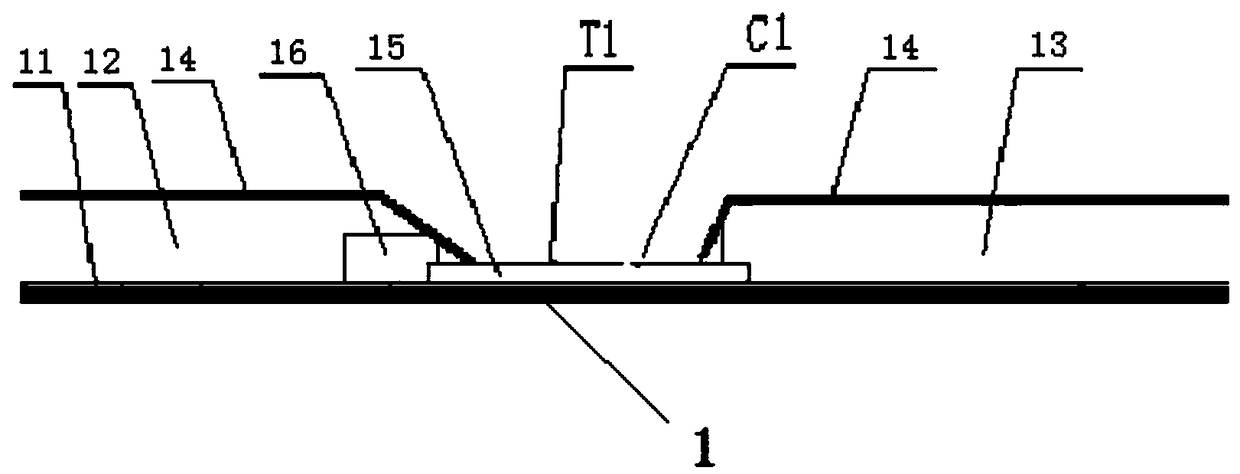
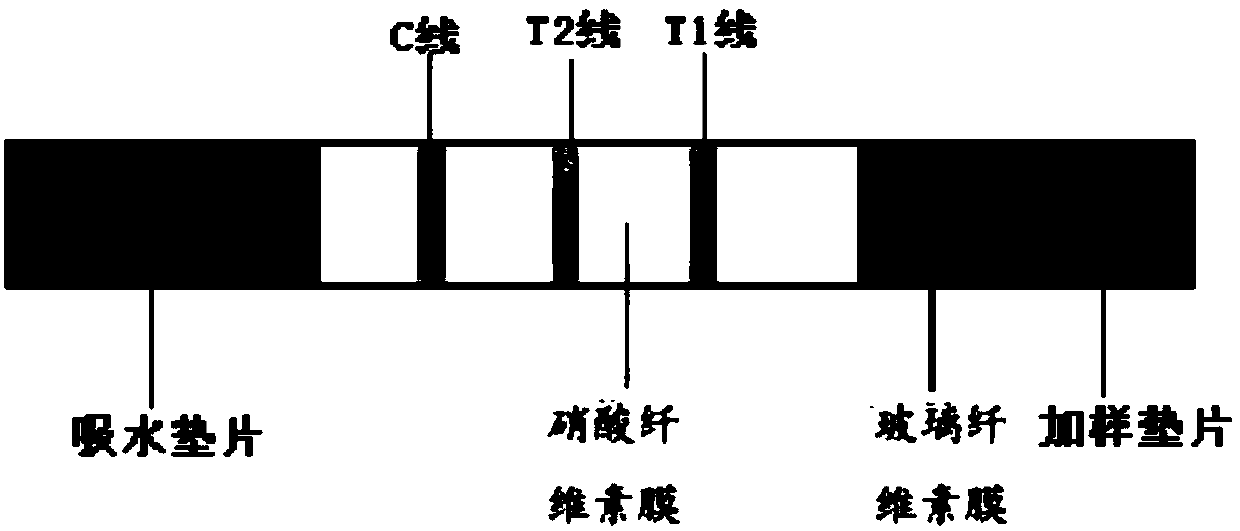
Beta-human chorionic gonadotrophin near-infrared fluorescence detection reagent card, kit and purpose thereof
InactiveCN107941742AImprove accuracyAvoid mutual interferenceFluorescence/phosphorescenceAntigenCellulose
The invention relates to the field of field of fluorescence immunochromatography, and concretely relates to a beta-human chorionic gonadotrophin near-infrared fluorescence detection reagent card, a kit and a purpose thereof. The beta-human chorionic gonadotrophin near-infrared fluorescence detection reagent card comprises a sampling gasket, a glass cellulose membrane, a cellulose nitrate membrane,and a water-absorption gasket arranged in order; wherein, the glass cellulose membrane contains a fluorescence probe-first anti-beta human chorionic gonadotrophin antibody and a fluorescence probe-test antigen; and a first test line, a second test line and a control line are arranged on the cellulose nitrate membrane in order; the first test line is coated with a second anti-beta human chorionicgonadotrophin antibody; the second test line is coated with the beta-human chorionic gonadotrophin antigen; and the control line is coated with the antibody of the test antigen.
Owner:SHANGHAI CHEMTRON BIOTECH
Method for extracting high-purity polydeoxyribonucleotide from salmon testis
PendingCN114149963AHigh yieldCell dissociation methodsOrganic active ingredientsAnimal scienceHCG - Human chorionic gonadotropin
The present disclosure relates to a method of extracting high purity polydeoxyribonucleotide (PDRN) from a salmon testis, the method comprising: 1) separating seminal fluid and an immature testis region from the salmon testis, 2) mildly grinding the immature testis region and then diluting with artificial seminal plasma, 3) treating the diluent with a predetermined concentration of human chorionic gonadotropin (hCG), and 4) extracting the high purity polydeoxyribonucleotide (PDRN) from the salmon testis. The method comprises the following steps: (1) extracting sperm from a sperm diluent to induce artificial maturation of the sperm cells into sperms, (4) centrifuging the sperm diluent and collecting potential active sperms after hCG treatment for a preset time, and (5) extracting PDRN from the collected sperms. According to the method, 100-200 times of PDRN raw materials with higher purity can be extracted from 20 mL (maximum 50 mL) of sperms (seminal fluid) collected by salmons at a time. In addition, the method enables the extraction of PDRN in high yield in a cost-effective and economically feasible manner.
Owner:BIOMEDICS INC
Synthetic chimeric peptide of human chorionic gonadotrophin genetic engineering and its preparation method
InactiveCN1446825AHigh affinityOvercoming the problem of "vaccines with different names"DepsipeptidesFermentationEscherichia coliAntigen
A kind of human chorionic gonadotropic homone (hCG) chimeric peptides CP7 (and its derivative) and CP11 is disclosed, whose coding gene can be expressed in colibacillus. Its expression resultant can be obtained by preparing SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Then can be used to prepare hCG peptide vaccine source for the purpose of treating tumor and contraception.
Owner:FUDAN UNIV +1
Preparation method of early pregnancy test paper
PendingCN113376385AHigh detection sensitivityImprove detection accuracyDisease diagnosisBiological testingPregnancy testsObstetrics
The invention relates to the technical field of medical test reagents, and discloses a preparation method of early pregnancy test paper, wherein the preparation method comprises the following steps: preparation of a sample loading pad, preparation of a water absorption pad, preparation of a detection pad, and assembly and cutting of the test paper to obtain the early pregnancy test paper. The preparation method has the following advantages and effects that a human chorionic gonadotropin beta core fragment and human chorionic gonadotropin regular molecules in urine are qualitatively detected by optimizing the preparation processes of the sample loading pad, the water absorption pad and the detection pad and applying the principles of a double-antibody sandwich method and an immunochromatography method, so that while the detection efficiency is considered, the method has the advantages of high detection sensitivity and accuracy.
Owner:HUBEI MEIBAO BIOTECH
Devices for pregnancy detection and corresponding methods thereof
There is disclosed a device for pregnancy detection is provided, the device comprising: a central main body comprising a first end and a second end; a pregnancy detection element that is mounted at an edge of the second opening, and an upper longitudinal crease and a lower longitudinal crease, wherein the device is opened by applying simultaneous pressure to the upper longitudinal crease and the lower longitudinal crease, wherein, when in operation, the first opening of the first end is configured to receive urine from a female user while the female user is in a standing position and enables the urine to pass through the pregnancy detection element, and wherein, when the urine comes in contact with the pregnancy detection element, the pregnancy detection element immunologically detects human chorionic gonadotropin (hCG) present in the urine of the female user and indicates positive pregnancy result if the female user is pregnant.
Owner:INSTLOO PTE LTD
Antibody vaccine conjugates and uses therefor
InactiveCN1767852AAntibody mimetics/scaffoldsVaccinesAntigenHuman Chorionic Gonadotropin Beta Subunit
The present invention provides novel antibody vaccine conjugates and methods of using them to induce cytotoxic T cell (CTL) responses. In a specific embodiment, the vaccine conjugate includes human chorionic gonadotropin beta subunit (βhCG) antigen linked to an anti-mannose receptor (MR) antibody.
Owner:CELLDEX THERAPEUTICS INC
Using and preparation methods of human chorionic gonadotropin test strip for full-automatic analyzer
PendingCN110850106AEasy to interpret the resultsRelatively fastBiological testingBiotechnologyHigh concentration
The invention discloses a using and preparation methods of a human chorionic gonadotropin test strip for a full-automatic analyzer. The preparation method comprises the following steps: adhering and fixing a coating film to a PVC rubber plate; sequentially assembling a sample pad, a colloidal gold combination pad and a water absorption material which are configured according to the requirements onto the PVC rubber plate with the coating film; adhering a protective film adhesive tape containing a warning line on the sample pad according to the requirements; adhering a protective film adhesive tape containing a warning line to the water absorption paper strip; carrying out cutting to obtain a test strip; testing the lowest detection limit of the test strip; and carrying out positive / negativespecificity analysis with high-concentration hLH, hFSH, hLH and hTSH samples. According to the using method, about 60[mu]L of a sample urine, serum or plasma sample is added to a sample pad area of the test strip by using a full-automatic analyzer sample adding needle or manually using a dropper or a dropper / pipette. Positions of the detection line and the quality control line are relatively fixed, so that full-automatic instrument result interpretation is facilitated, the clinical application value is achieved, and the inspection efficiency can be greatly improved.
Owner:深圳市坪山区妇幼保健院 +1
Anti-interference photoelectrochemical biosensor as well as preparation method and application thereof
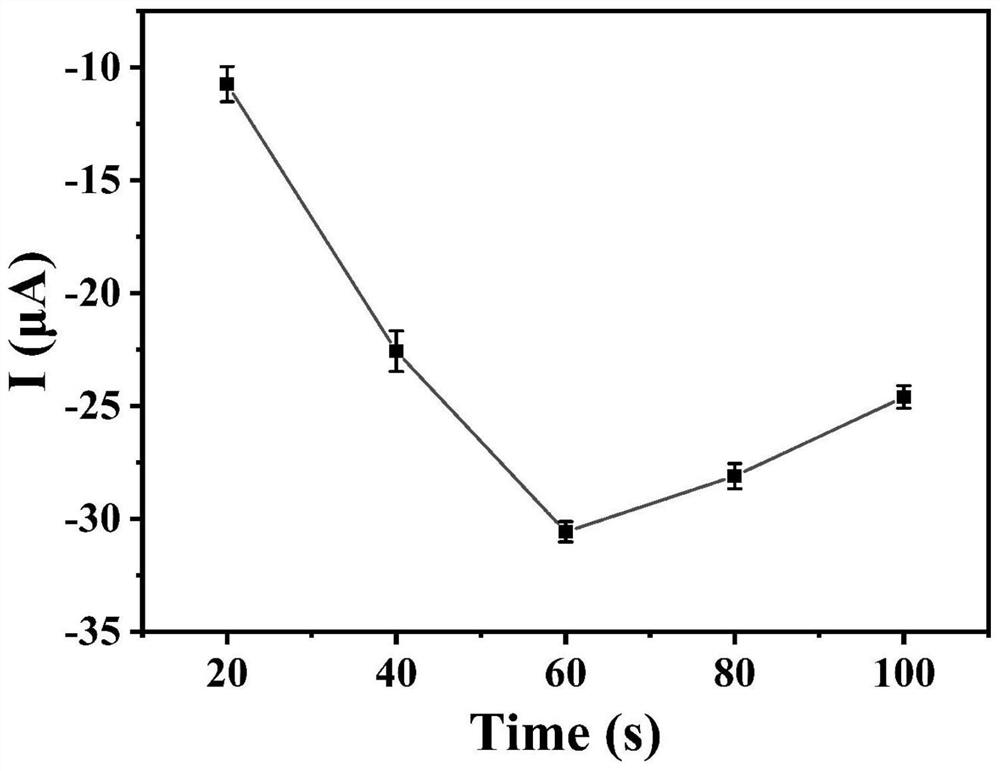
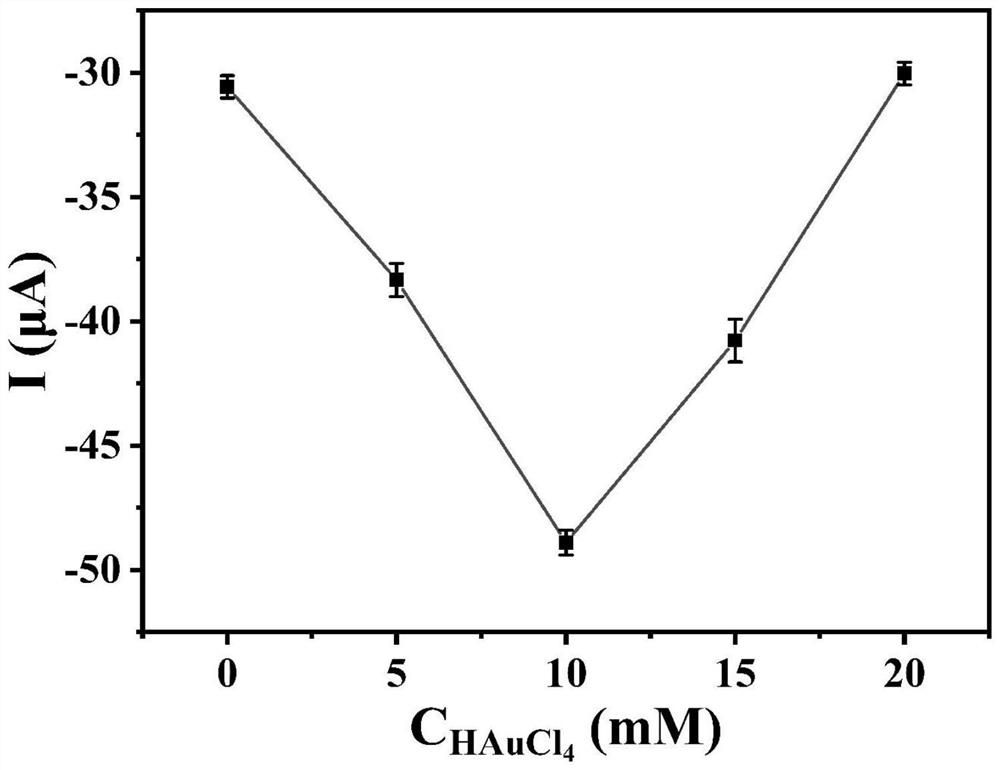
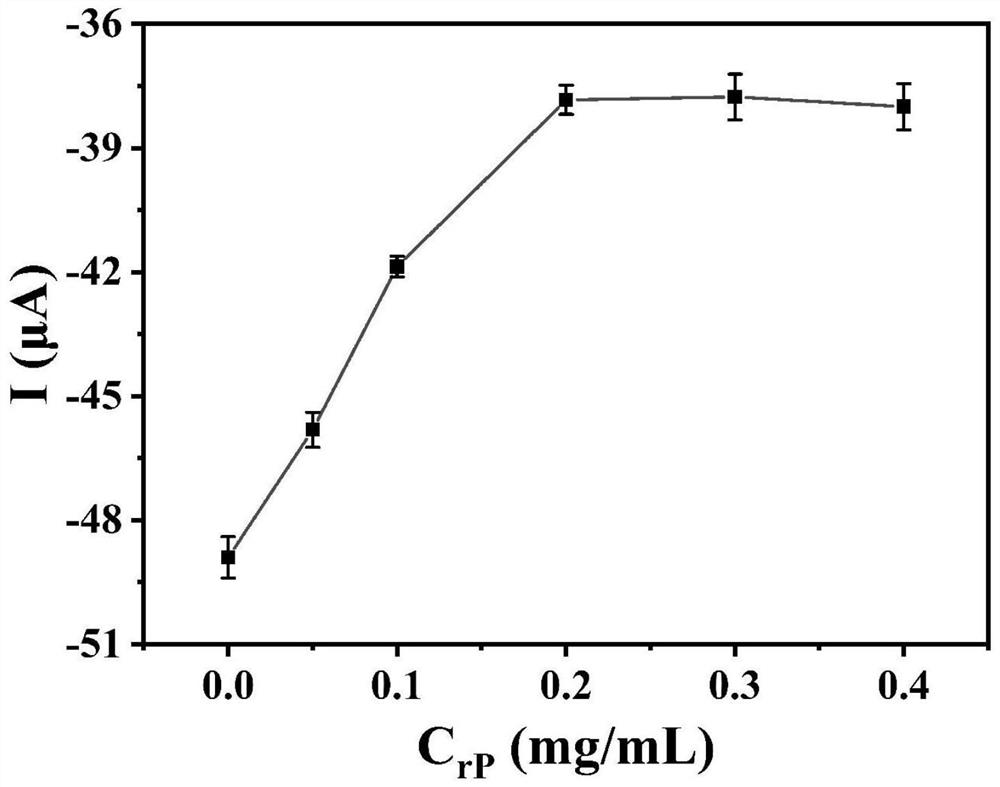
ActiveCN112114012AEasy to prepareEasy to operateMaterial electrochemical variablesElectrochemical biosensorHCG - Human chorionic gonadotropin
The invention discloses an anti-interference photoelectrochemical biosensor as well as a preparation method and application thereof, and belongs to the technical field of biosensors. The photoelectrochemical biosensor is used for detecting human chorionic gonadotropin hCG, and the prepared photoelectrochemical biosensor has excellent performance of resisting reducing small molecules and biomacromolecule interference detection signals at the same time. The anti-interference photoelectrochemical biosensor is constructed by sequentially modifying hCG recognition polypeptide and antifouling polypeptide on a photocathode, and photocurrent signal detection is realized by utilizing the hindering effect of the obvious steric hindrance effect of the hCG on charge transfer of the sensor. The anti-interference photoelectrochemical biosensor constructed on the basis of the polypeptide is simple and convenient in preparation process, has the application potential of accurately and sensitively detecting the hCG in an actual biological sample, and is suitable for market popularization and application.
Owner:QINGDAO UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com