Human chorionic gonadotropin detection kit, preparation method and application method thereof
A chorionic gonadotropin and detection kit technology, which is applied in the direction of biological testing, measuring devices, material inspection products, etc., can solve the problems of unfavorable automatic detection, low technical sensitivity, unfavorable popularization, etc., and achieve favorable signal detection, The effect of high signal sensitivity and long platform stability period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
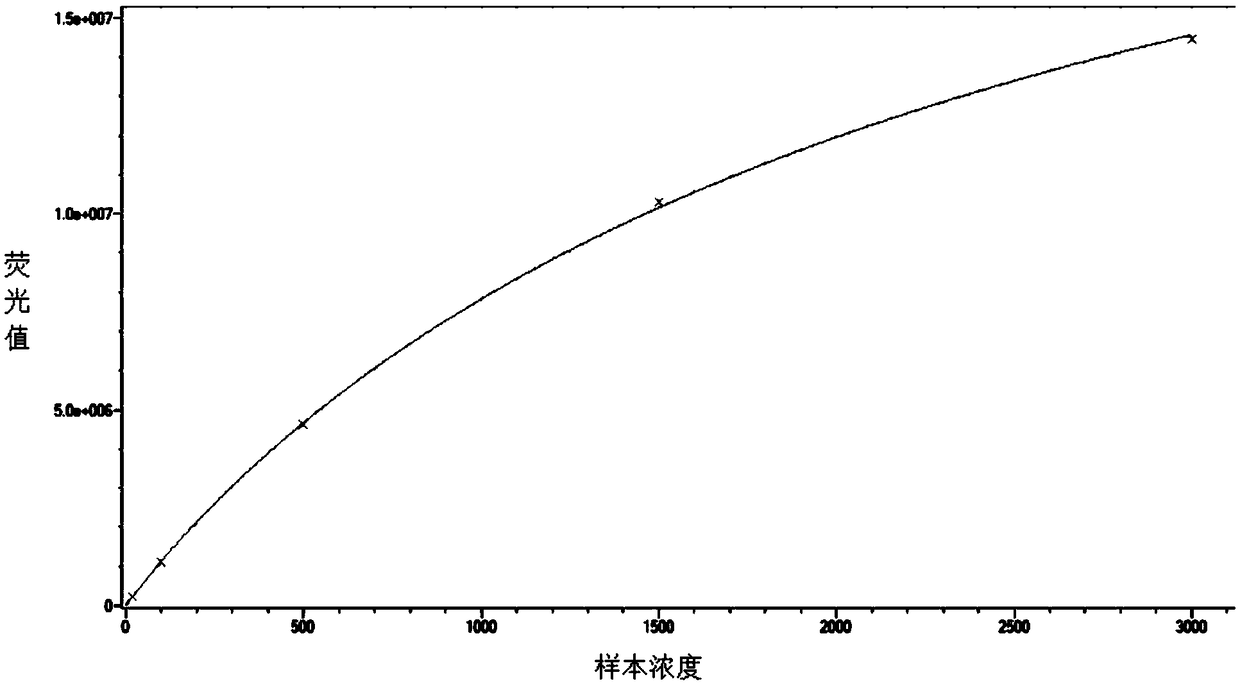
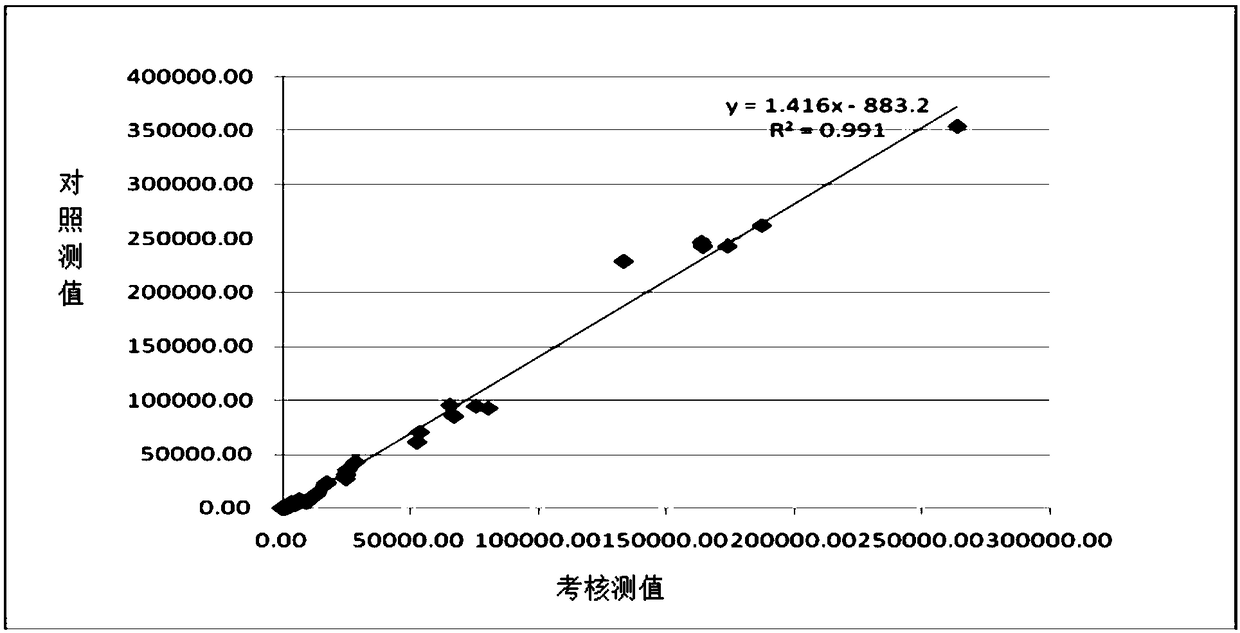
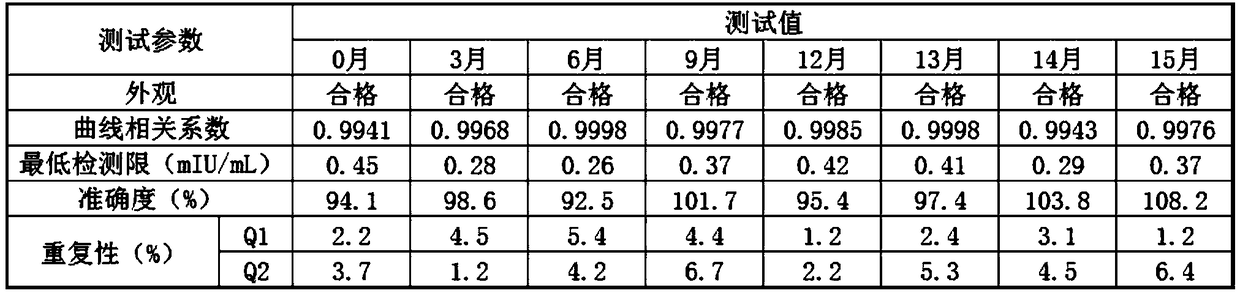
Image
Examples
Embodiment 1
[0048] The coating antibody described in the present invention is a monoclonal antibody that can specifically bind to the human chorionic gonadotropin (Total-βhCG) antigen in the human body, and the labeled antibody is a monoclonal antibody that can specifically bind to the human chorionic gonadotropin (Total-βhCG) antigen. βhCG) antigen-specific binding monoclonal antibody. In addition to being capable of specifically binding to the human chorionic gonadotropin (Total-βhCG) antigen, the coating antibody and the labeled antibody can form a "sandwich" sandwich structure with the antigen when paired.
[0049] The human chorionic gonadotropin (Total-βhCG) antigen used in the present invention can adopt but not limited to the human chorionic gonadotropin (Total-βhCG) antigen of Fitzgerald company; The human chorionic gonadotropin (Total-βhCG) antigen used in the present invention ( Total-βhCG) coating antibody and labeled antibody can be used but not limited to human chorionic gon...
Embodiment 2
[0077] A human chorionic gonadotropin detection kit, the detection kit includes the following reagent components:
[0078] Calibrators, quality controls, anti-reagent A, anti-reagent B, magnetic particle reagents and luminescent substrates;
[0079] The calibrators and quality controls are prepared by dissolving the Total-βhCG antigen in a calibrator buffer solution to a constant volume of 0.3 mg / mL, and diluting it with a calibrator buffer solution after 20 min; the calibrator buffer solution includes the following components: bovine serum, tetracycline accounting for 0.01% by mass of the newborn bovine serum and neomycin sulfate accounting for 0.1% by mass of the newborn bovine serum, obtained by filtering through a 0.22 μm filter membrane;
[0080] Calibrators and quality controls were calibrated to different concentrations with calibrator buffer (see the table below for specific concentrations):
[0081] Reagent name
Target Concentration (mIU / mL)
Calib...
Embodiment 3
[0104] A human chorionic gonadotropin detection kit, the detection kit includes the following reagent components:
[0105] Calibrators, quality controls, anti-reagent A, anti-reagent B, magnetic particle reagents and luminescent substrates;
[0106] The calibrators and quality controls are prepared by dissolving the Total-βhCG antigen in a calibrator buffer to a volume of 0.8 mg / mL, and diluting it with a calibrator buffer after 40 min; the calibrator buffer includes the following components: bovine serum, tetracycline accounting for 0.05% by mass of the newborn bovine serum and neomycin sulfate accounting for 0.5% by mass of the newborn bovine serum, obtained by filtering through a 0.22 μm filter membrane;
[0107] The anti-reagent A is prepared by adding fluorescein isothiocyanate-labeled Total-βhCG coating antibody to Tris salt buffer; the Tris salt buffer includes the following components: Tris salt 0.5mM, accounting for the Tris Tetracycline at 0.05% by volume of the sal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com