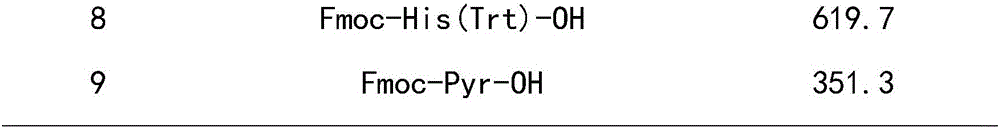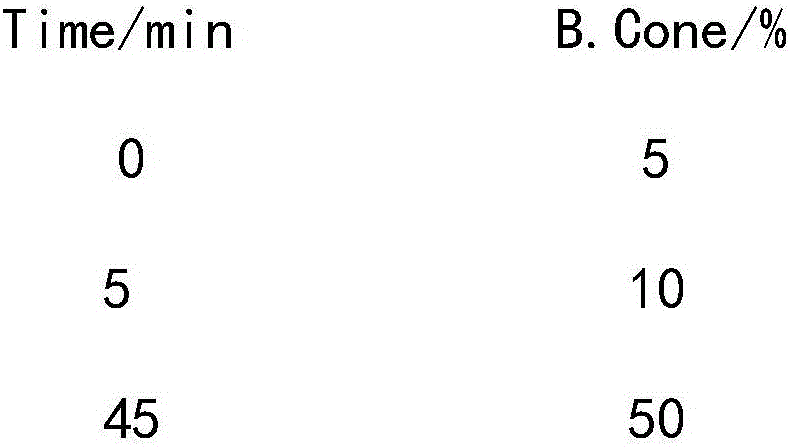Leuprorelin synthesis preparation method
A technology of leuprolide and condensation reaction, which is applied to the preparation method of peptides, chemical instruments and methods, animal/human protein, etc., can solve the problems of high production cost, cumbersome process, and low yield, and achieve improved yield , low-cost mass production, and the effect of simplifying the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Swelling of 2-Cl resin
[0031] Weigh 0.1 g of 2-Cl resin with a degree of substitution of 0.9-1.1 mmol / g into a polypeptide synthesis reactor, add DCM solvent to completely immerse the chlorine resin, and swell for 30 minutes.
[0032] 2. Chlorine resin ethylamination
[0033] Weigh 10 mg of mercaptoethylamine, dissolve it with 1 ml of DCM, add 0.2 ml of DIEA to dissolve it completely, then add it to the swollen chlorine resin, and react overnight at 30°C.
[0034] 3. Head-capping reaction of ethylamine chlorine resin
[0035] Add 0.5ml of methanol and 0.5ml of DIEA solution into the reaction container, so that the resin in the container is in the state of quicksand in the solution, and the reaction container is placed on a shaker and shaken for 20 minutes.
[0036] 4. Synthesis of Fully Protected Leuprolide Peptide-Chloride Resin
[0037] Add 3ml of DMF solution to the reaction vessel, shake and wash for 1min, then drain the liquid in the reaction vessel, then a...
Embodiment 2
[0064] 1. Swelling of 2-Cl resin
[0065] Weigh 0.1 g of 2-Cl resin with a degree of substitution of 0.9-1.1 mmol / g into a polypeptide synthesis reactor, add DCM solvent to completely immerse the chlorine resin, and swell for 30 minutes.
[0066] 2. Chlorine resin ethylamination
[0067] Weigh 10 mg of mercaptoethylamine, dissolve it with 1 ml of DCM, add 0.2 ml of DIEA to dissolve it completely, then add it to the swollen chlorine resin, and react overnight at 30°C.
[0068] 3. Head-capping reaction of ethylamine chlorine resin
[0069] Add 0.5ml of methanol and 0.5ml of DIEA solution into the reaction container, so that the resin in the container is in the state of quicksand in the solution, and the reaction container is placed on a shaker and shaken for 20 minutes.
[0070] 4. Synthesis of Fully Protected Leuprolide Peptide-Chloride Resin
[0071] Add 3ml of DMF solution to the reaction vessel, shake and wash for 1min, then drain the liquid in the reaction vessel, then a...
Embodiment 3
[0085] 1. Swelling of 2-Cl resin
[0086] Weigh 1 g of 2-Cl resin with a degree of substitution of 0.9-1.1 mmol / g in a polypeptide synthesis reactor, add DCM solvent to completely immerse the chlorine resin, and swell for 40 minutes.
[0087] 2. Chlorine resin ethylamination
[0088] Weigh 100mg of mercaptoethylamine, dissolve it with 12ml of DCM, add 4ml of DIEA to dissolve it completely, then add it to the swollen chlorine resin, and react overnight at 30°C.
[0089] 3. Head-capping reaction of ethylamine chlorine resin
[0090] Drain the resin in the reaction container, then add 10ml of methanol and 10ml of DIEA solution to make the resin in the container in the state of quicksand in the solution, and place the reaction container on a shaker for 20 minutes to react.
[0091] 4. Synthesis of Fully Protected Leuprolide Peptide-Chloride Resin
[0092] Add 30ml of DMF solution to the reaction vessel, shake and wash for 1min, then drain the liquid in the reaction vessel, then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com