Method for synthesizing leuprorelin from polypeptide solid-liquid fragment
A leuprolide and peptide solid-liquid technology, applied in the field of medicine, can solve the problems of difficult management, inconvenient transportation and storage, and high production costs, and achieve the effects of convenient transportation and storage, convenient automatic production, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
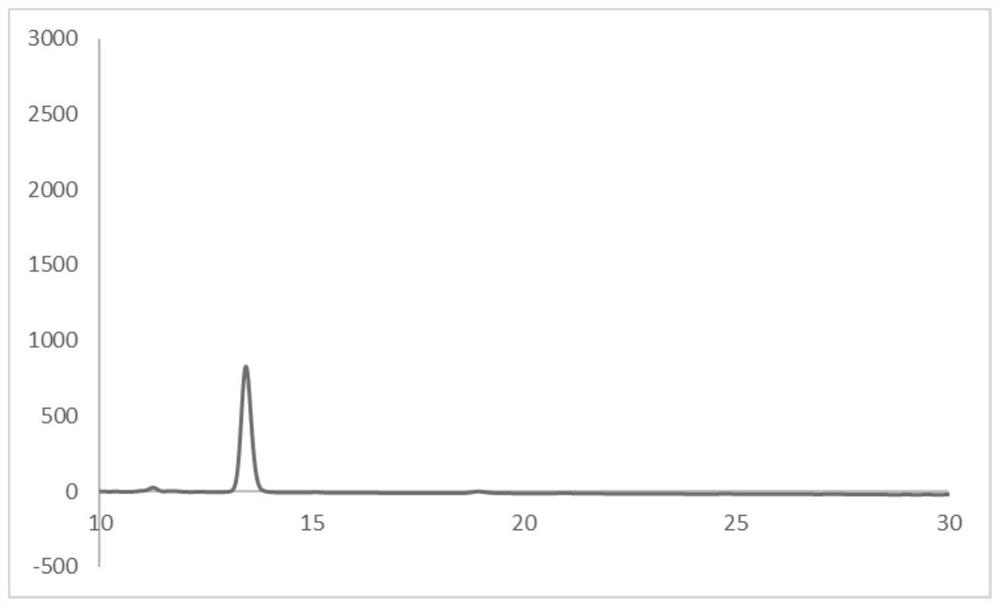
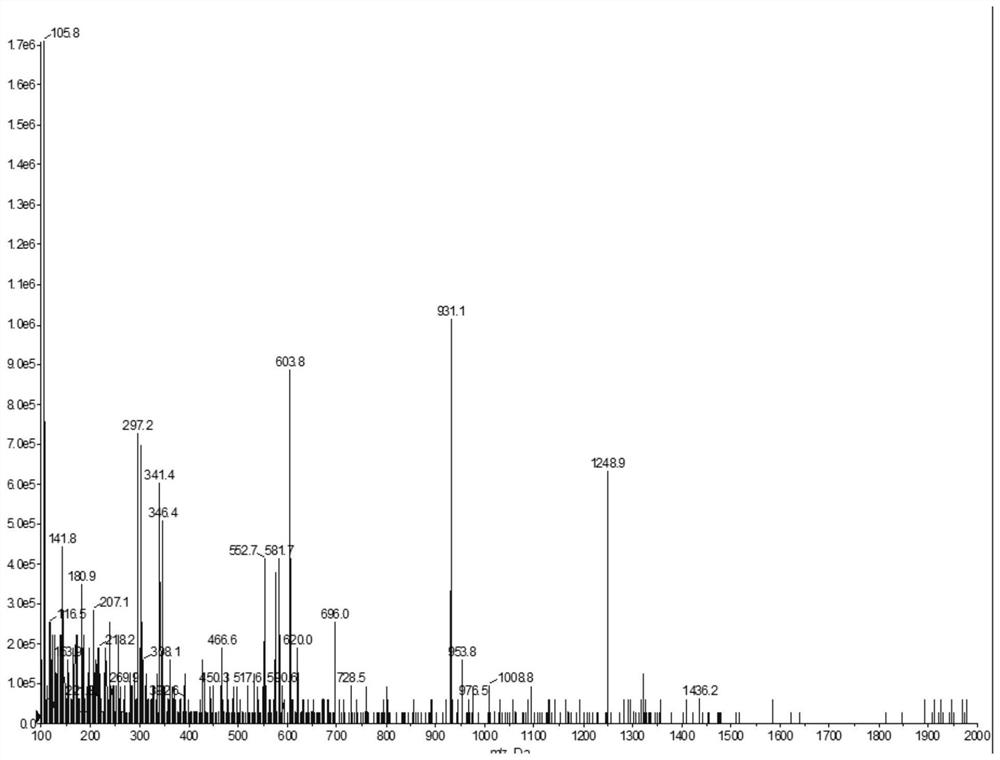
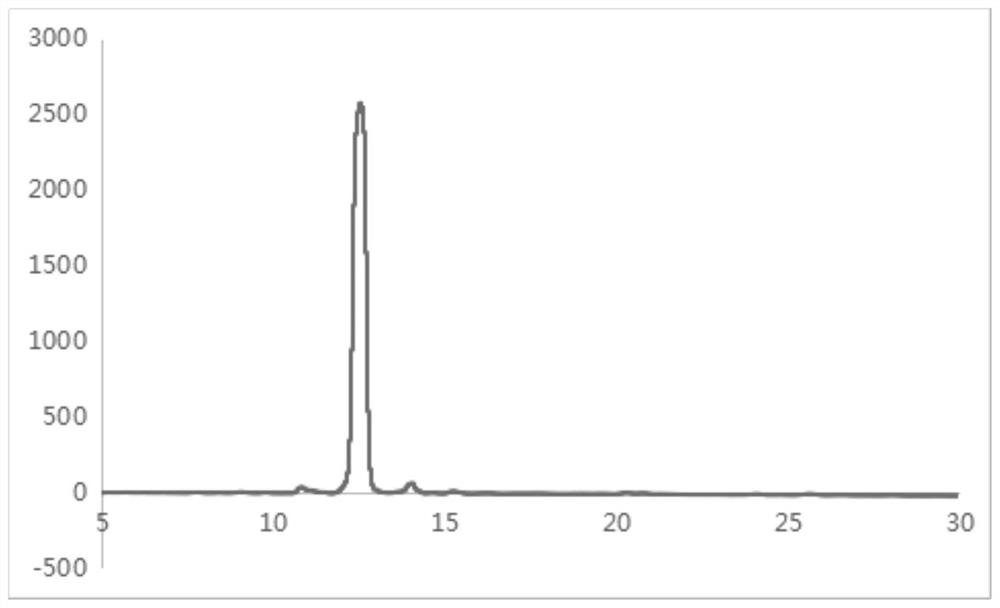
Image
Examples
Embodiment 1
[0077] A method for synthesizing leuprolide from polypeptide solid-liquid fragments, comprising the following steps:
[0078] 1) Compound 1 was synthesized by polypeptide solid-phase synthesis:
[0079] Boc-Pglu-His(Boc)-Trp(Boc)-Ser(tBu)-Tyr(tBu)-D-Leu-Leu-OH;
[0080] 2) Compound 2: H-Arg(pbf)-Pro-NHEt was synthesized by polypeptide liquid phase synthesis;
[0081] 3) Synthesis of compound 3 in liquid phase:
[0082] Boc-Pglu-His(Boc)-Trp(Boc)-Ser(tBu)-Tyr(tBu)-D-Leu-Leu-Arg(pbf)-Pro-NHEt;
[0083] 4) Synthesis of crude leuprolide: Pglu-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-Pro-NHEt.
[0084] Further, in step 1), the compound 1 can be synthesized sequentially from C to N-terminus by a solid-phase method, and the steps include:
[0085] 1) The solid phase carrier is CTC resin;
[0086] Amino acid monomers are Boc-Pglu-OH, Fmoc-His(Boc)-OH, Fmoc-Trp(Boc)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-D- Leu-OH, Fmoc-Leu-OH;
[0087] The condensing agent is one of HBTU, HATU, TATU...
Embodiment 2
[0170] The difference from the above examples is that Compound 1 is synthesized by recycling the resin:
[0171] 1. Resin recycling;
[0172] 1). The resin of Example 1 was washed with DMF, washed with methanol, and the filtrate was sucked out, and dried in vacuum.
[0173] 2). Separately prepare a DCM solution of thionyl chloride (thionyl chloride 10ml, DCM=200ml), add it to a 250ml solid-phase synthesis reactor filled with resin, react for 2 hours, remove the reaction solution, and wash with DCM.
[0174] The feeding table is as follows:
[0175]
[0176]
[0177] Two, the synthesis of Leu-resin;
[0178] 1). Weigh 25g of CTC resin in a 250ml solid-phase synthesis reactor, take Fmoc-Leu-OH in a 250ml Erlenmeyer flask, measure 120ml of DCM in a 100ml graduated cylinder, shake well, add 41ml of DIEA, shake well to dissolve, pour into the reactor to start the reaction. When reacting for 1 hour, remove the reaction solution with a suction filter and wash 4 times with DM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com