Solid phase synthesis method of leuprorelin
A technology of leuprolide and leucyl, applied in the field of biochemistry, can solve the problems of peptide chain racemization, degradation, low yield of aminolysis, etc., and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
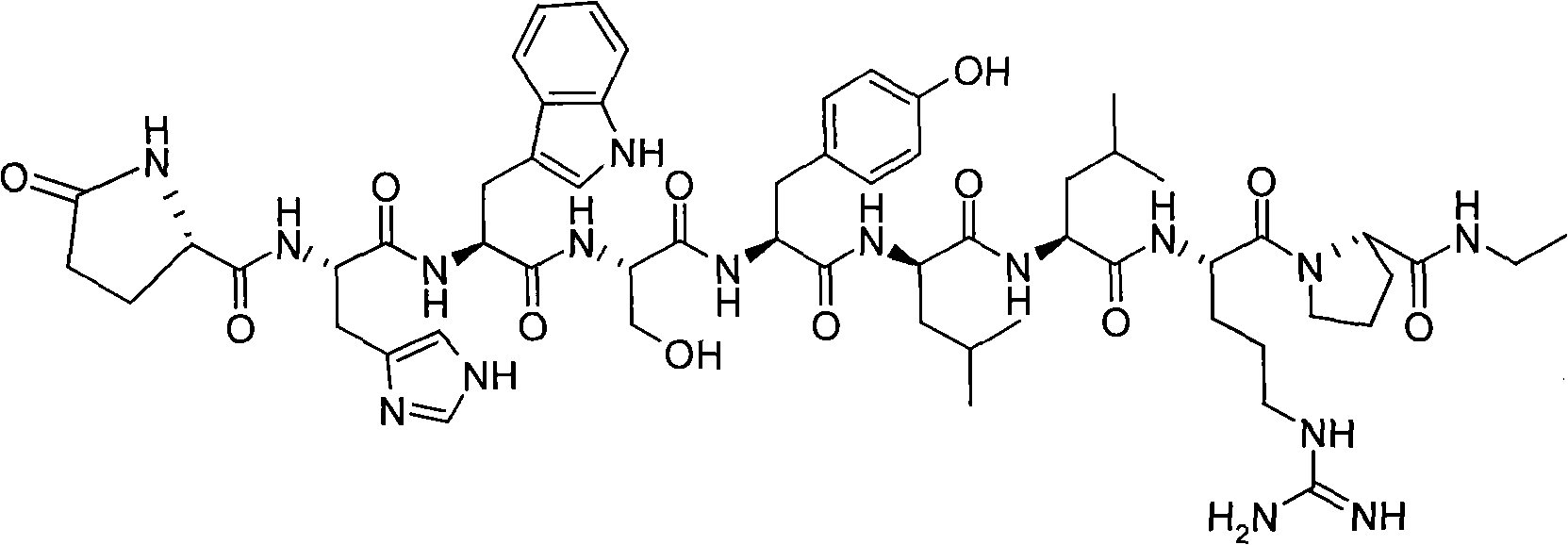
[0029] Example 1: Preparation of leuprorelin peptide resin
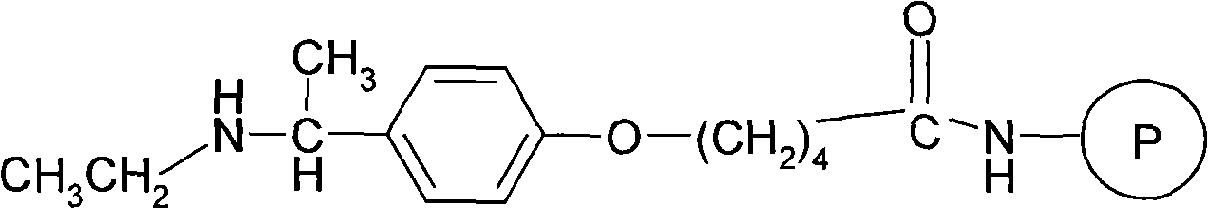
[0030] Operation: (1) 10g (5mmol) of 4-[(1-N-ethylamino)ethyl]phenoxy-butyramide resin (product of sigma-aldrich company) is placed in the peptide synthesis reactor, alternating with 50ml each of dichloromethane and methanol Wash twice, 2 minutes / time, and drain. Add 50 ml of 20% (v / v) piperidine / DMF solution, stir and react at room temperature for 20 minutes, and then drain. Wash the resin alternately with dichloromethane and methanol 50ml 3 times, 2 minutes per time, and drain.
[0031] (2) Dissolve 11.4g of Fmoc protected amino acid and HBTU (0-benzotriazole-N,N,N,N-tetramethylurea hexafluorophosphate, Shanghai Yanchang Biochemical Technology Development Co., Ltd.) in 50ml DMF solution , Then add 13ml of N,N-diisopropylethylamine, mix well and add to the resin, stir at room temperature for 3 hours.
[0032] (3) Drain dry. The resin was washed alternately with dichloromethane and methanol 60ml each for 3 times, 2 minut...
Embodiment 2
[0038] Example 2: Preparation of Leuprolide Crude Peptide
[0039] Operation: Add 18.6g of leuprorelin peptide resin to a 500ml round bottom flask, add 190ml of trifluoroacetic acid: water: 1,2-ethanedithiol (volume ratio 95:2.5:2.5), and stir at room temperature for 2 hours After filtering and washing the resin with 10ml of trifluoroacetic acid, the filtrates were combined, and the filtrate was poured into 2000ml of glacial ether. A large amount of white solid was precipitated. The white solid was collected by filtration and dried to obtain 5.44g of crude leuprolide peptide. The yield was 90%.
Embodiment 3
[0040] Example 3: Purification of crude leuprolide
[0041] Device: C 18 Preparation column (50×300mm)
[0042] Eluent A: 0.1% TFA / H 2 O
[0043] Eluent B: Acetonitrile
[0044] Flow rate: 200ml / min
[0045] UV detection wavelength: 230nm
[0046] Operation: (1) Leuprolide crude peptide is dissolved in 5% (v / v) acetic acid aqueous solution
[0047] (2) Filter with 0.45μm microporous membrane
[0048] (3) Filtrate loading
[0049] (4) 23~25%(v / v) acetonitrile-water mobile phase gradient elution
[0050] (5) Collect the target peptide fraction
[0051] (6) Concentration, freeze drying
[0052] (7) 4.84 g of leuprolide white solid.
[0053] The product was confirmed by MS mass spectrometry, with a total yield of 80.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com