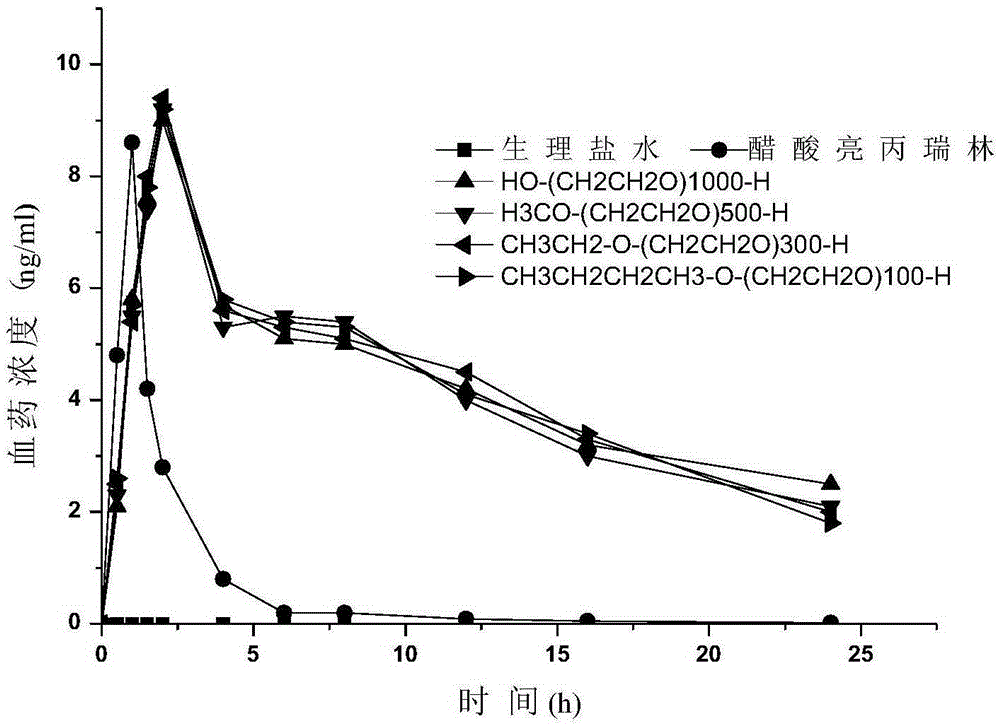
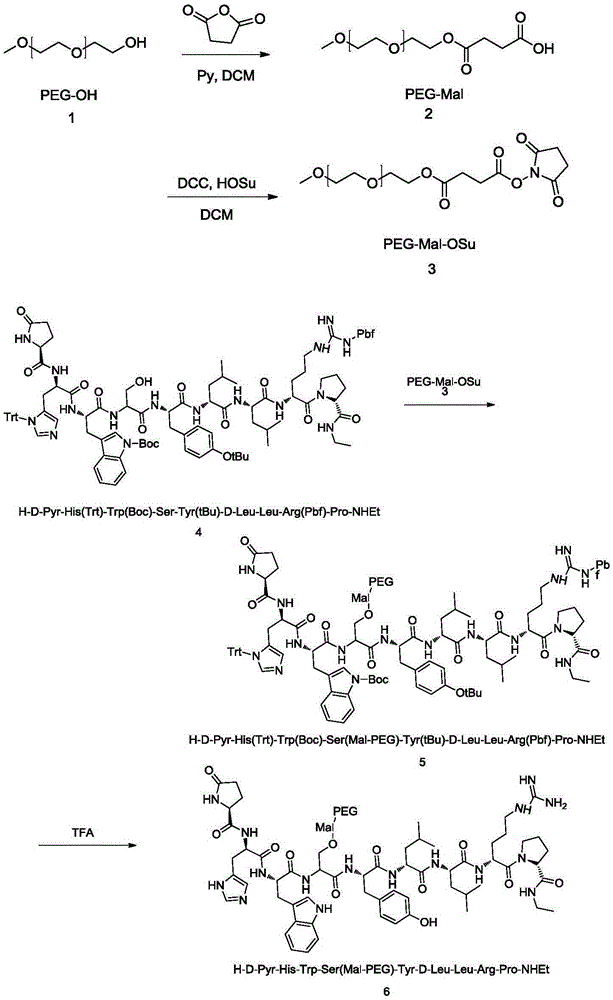
Pegylated leuprorelin
A technology of leuprolide and chemical method, applied in the directions of non-active ingredients medical preparations, peptide/protein ingredients, medical preparations containing active ingredients, etc. , irritation such as induration or redness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 prepares PEG-Mal-leuprolide
[0022] 1.1 Preparation of PEG-MAL sample (2)
[0023] Dissolve monomethoxypolyethylene glycol PEG with different molecular weights in appropriate amount of dichloromethane, add 2 equivalents of pyridine as an acid-binding agent, add 5 equivalents of maleic anhydride, control the temperature at 60°C, react for 8 hours, and concentrate , crystallization of isopropyl ether to obtain monomethoxypolyethylene glycol with a carboxyl group at one end of different molecular weights.
[0024] 1.2 Preparation of PEG-MAL-OSu sample (3)
[0025] Dissolve monomethoxypolyethylene glycol PEG-MAL with carboxyl group at one end of different molecular weights in appropriate amount of THF, add HOSu, DCC, stir, a large amount of white solid precipitates, filter, crystallize with isopropyl ether, filter, use Wash with appropriate amount of water and isopropyl ether, and dry in vacuum to obtain PEG-MAL-OSu with different molecular weights.
[0026...
Embodiment 2
[0030] The modification condition of embodiment 2PEG-Mal-leuprolide
[0031] 1. The influence of reaction pH value on the modified product
[0032] Take the protected leuprolide and prepare 2mg / mL with 100mmol / LPB buffer solution (pH4.0, pH5.0, pH5.5 and pH6.0) respectively. Weigh PEG-MAL-OSu-20KD at a molar ratio of 1:3 (ProLeuprorelin:PEG-MAL-OSu-20KD) and add it to the leuprolide reaction solution, stir and react at 100 rpm at 4°C for 24 hours, then add 1M glycine to terminate the reaction , purified by Sephadex Superdex200 column chromatography, concentrated, added to TFA:Tis:H 2 O (95:2.5:2.5) solution, stirred at room temperature for 3 to 5 hours, settled with isopropyl ether, filtered, purified by Sephadex Superdex200 column chromatography, concentrated, and freeze-dried. Samples were taken by enzyme-linked immunosorbent assay to determine the modification rate of PEG-Mal-leuprolide.
[0033] The experimental results showed that the proportion of PEG-Mal-leuprolide w...
Embodiment 3
[0043] Example 3HO-(CH 2 CH 2 O) 1000 - Preparation of Mal-Leuprorelin
[0044] HO-(CH 2 CH 2 O) 1000 -H is dissolved in an appropriate amount of dichloromethane, 2 equivalents of pyridine is added as an acid-binding agent, 5 equivalents of maleic anhydride is added, the temperature is controlled at 60°C, the reaction is for 8 hours, concentrated, crystallized from isopropyl ether, and one end is a carboxyl group. Monomethoxypolyethylene glycol, dissolve it in an appropriate amount of THF, add HOSu, DCC, stir, a large amount of white solid precipitates, filter, crystallize with isopropyl ether, filter, wash with appropriate amount of water and isopropyl ether, vacuum Dry to get HO-(CH 2 CH 2 O) 1000 -MAL-OSu; NaH with 100 mM pH 5.5 2 PO 4 -H 3 PO 4 The buffer solution dissolves the protected leuprolide to configure a 3mg / mL solution, and HO-(CH 2 CH 2 O) 1000 -MAL-OSu was reacted at a molar ratio of 1:5. After reacting at 4°C for 24h, 1M glycine was added to ter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com