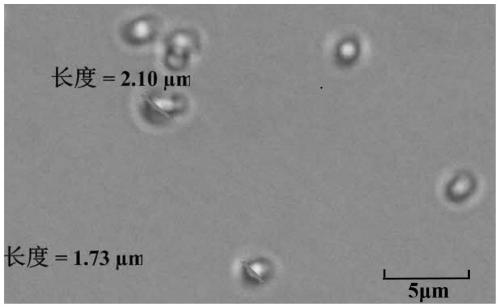
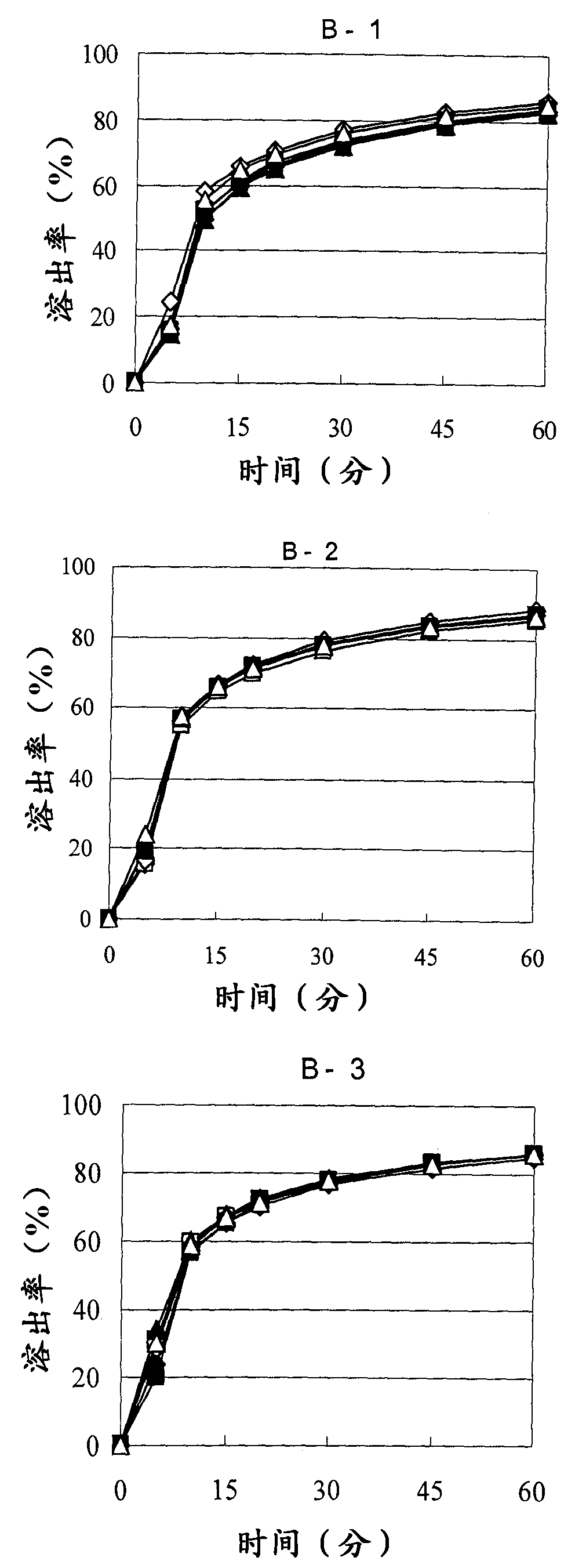
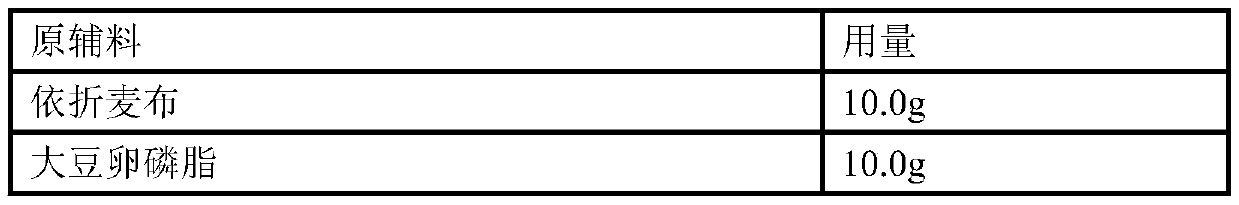
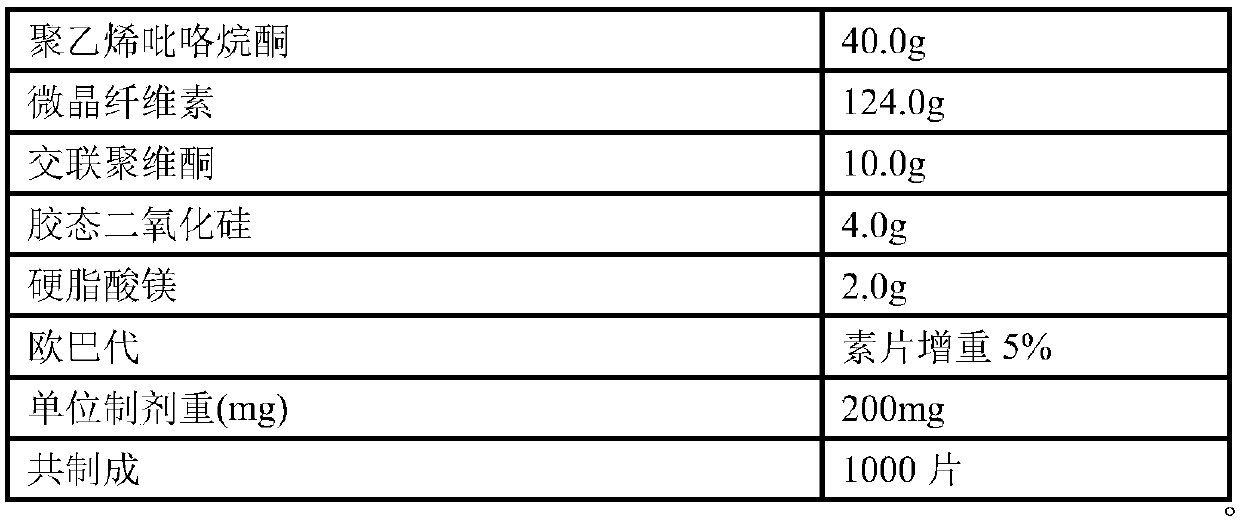
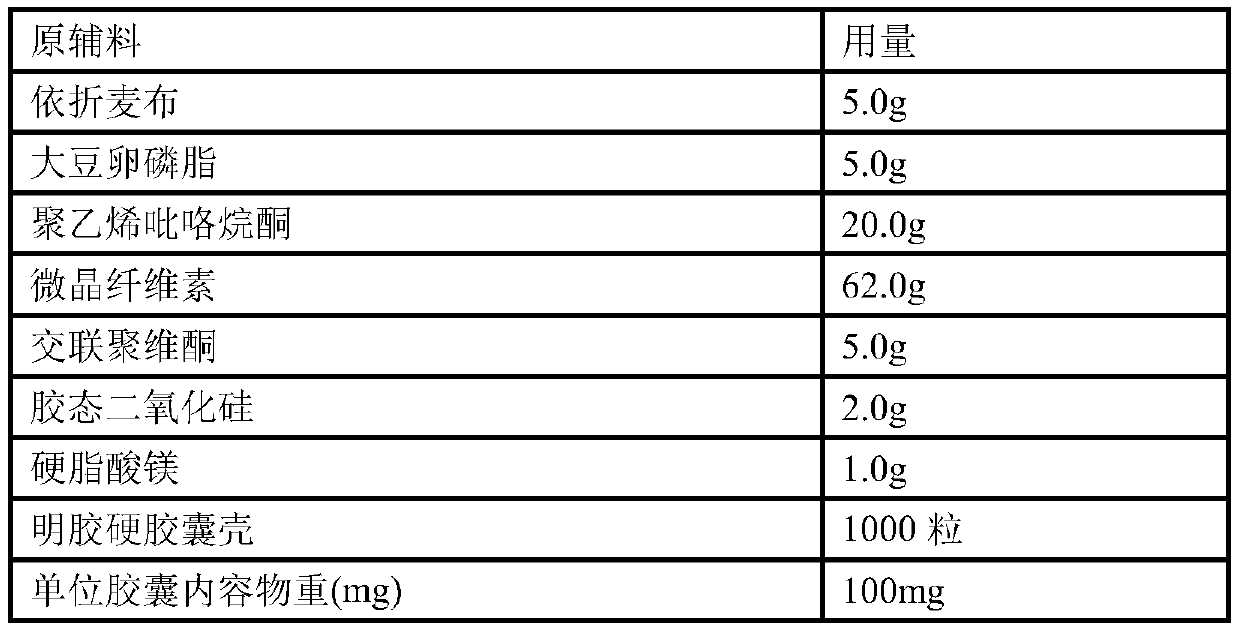
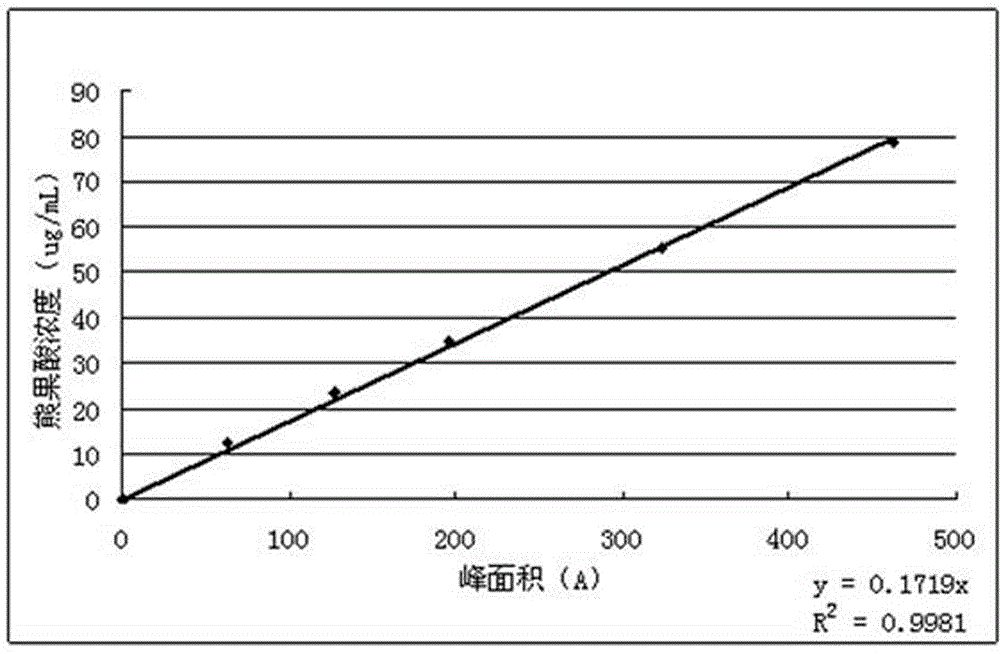
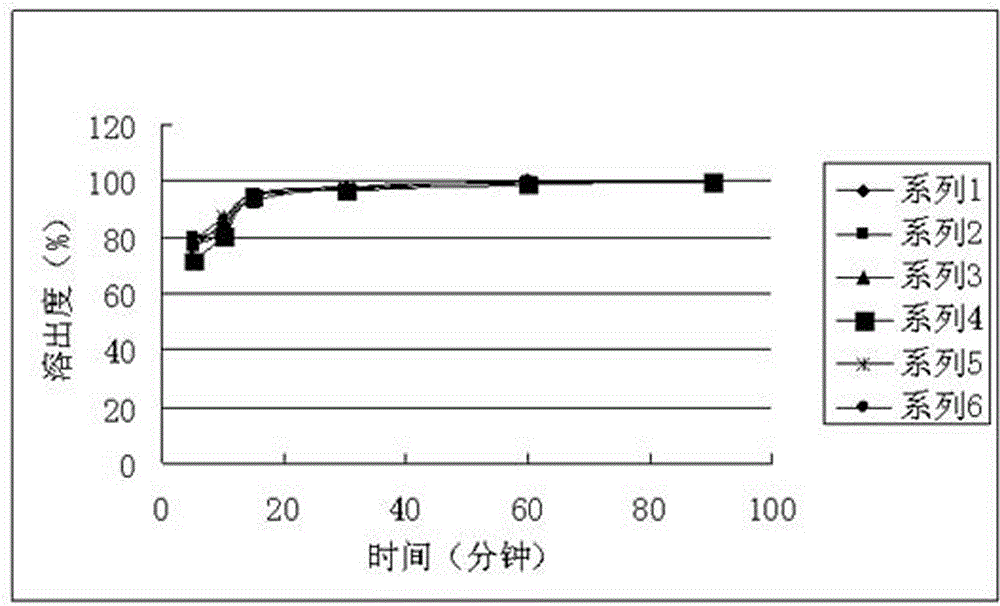
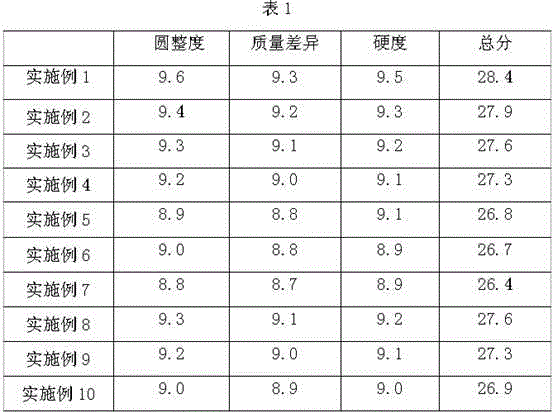
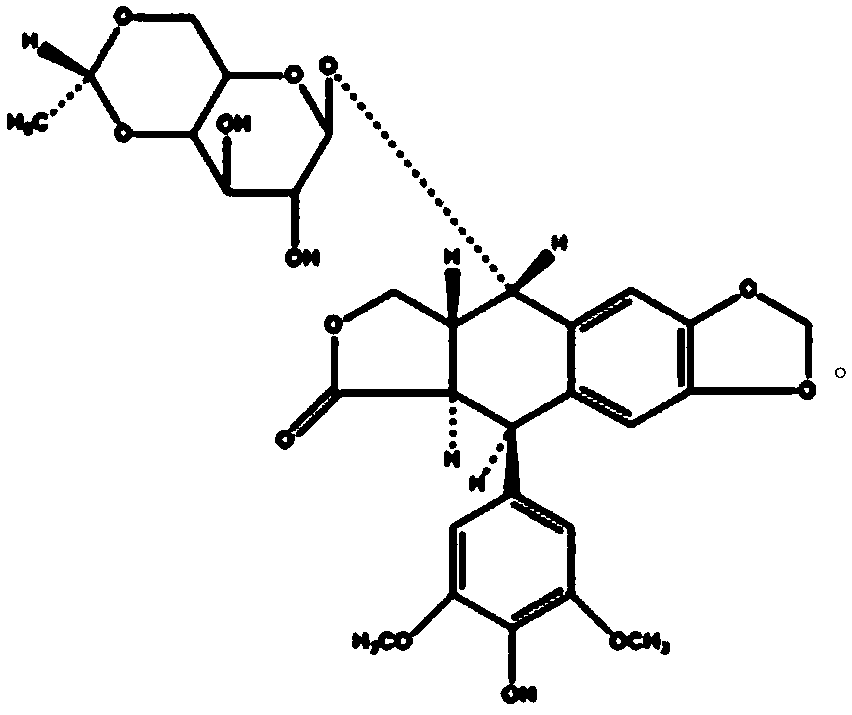
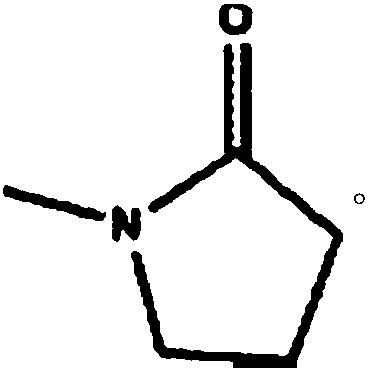
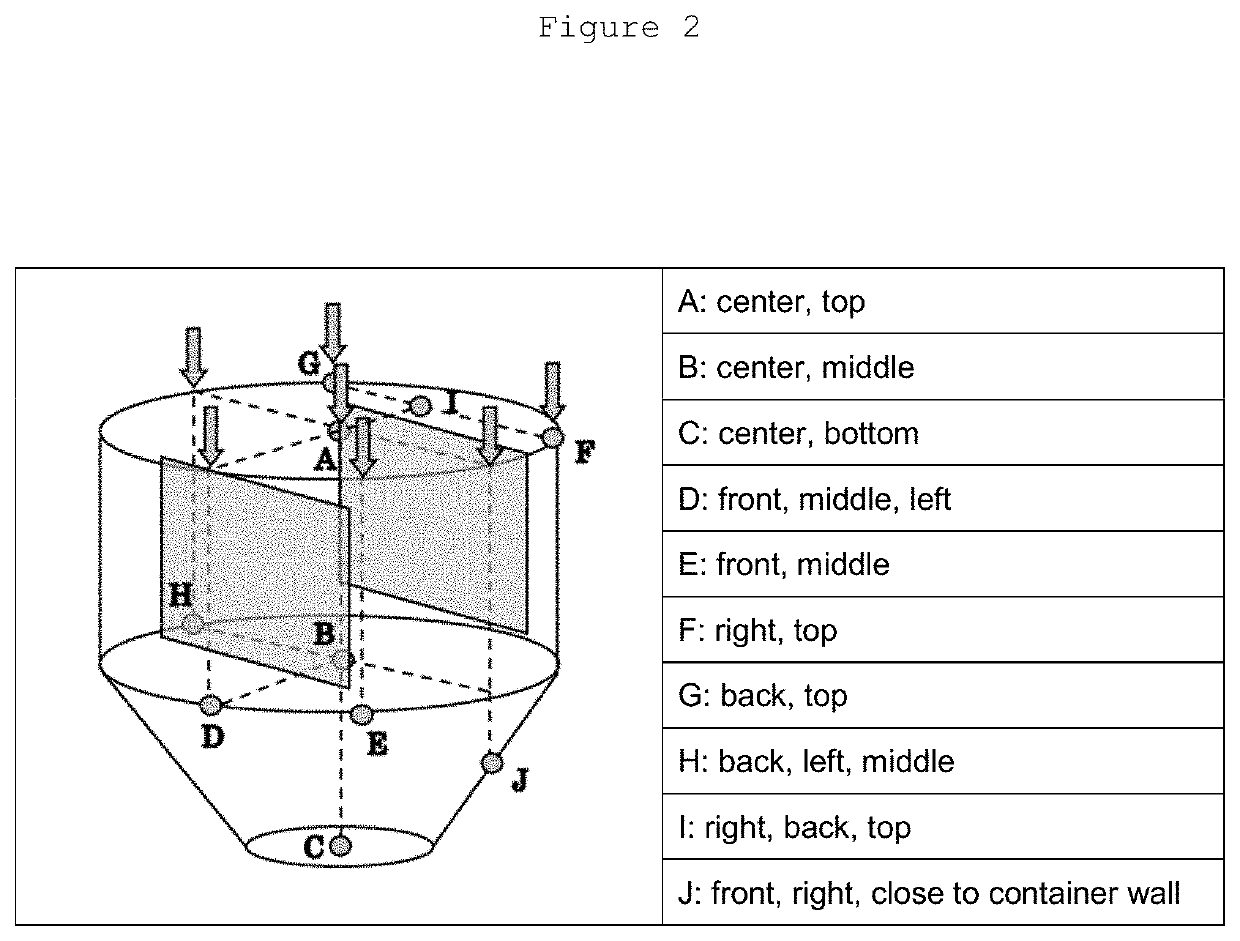
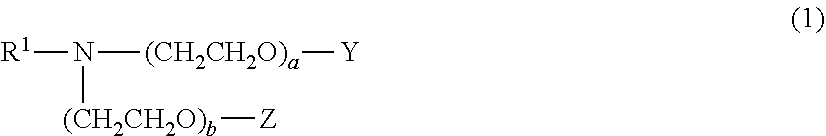Patents
Literature
30results about How to "Good dissolution properties" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
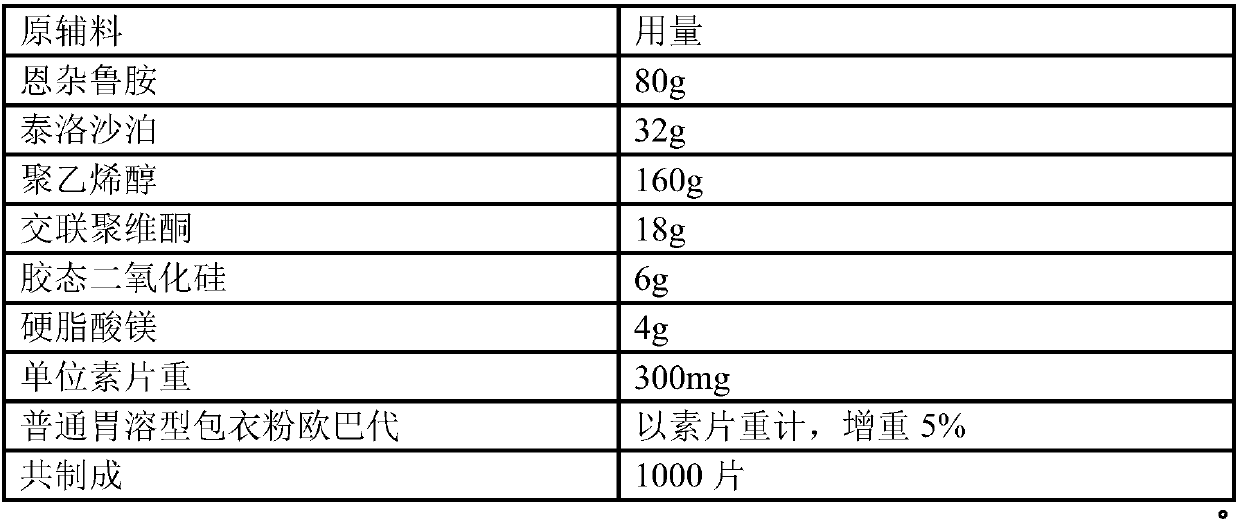
Orally-disintergrating solid preparation
ActiveUS20110091563A1Reduce breakageSufficient variationBiocideDigestive systemControlled releaseTableting
The present invention provides an orally-disintegrating solid preparation such as a tablet produced by tabletting fine granules showing controlled release of a pharmaceutically active ingredient and an additive, and the like, and the orally-disintegrating solid preparation containing fine granules coated with a coating layer containing a polymer affording a casting film having an elongation at break of about 100-about 700%. With the preparation, breakage of fine granules during tabletting can be suppressed in the production of an orally-disintegrating solid preparation containing fine granules showing controlled release of a pharmaceutically active ingredient.
Owner:TAKEDA PHARMA CO LTD
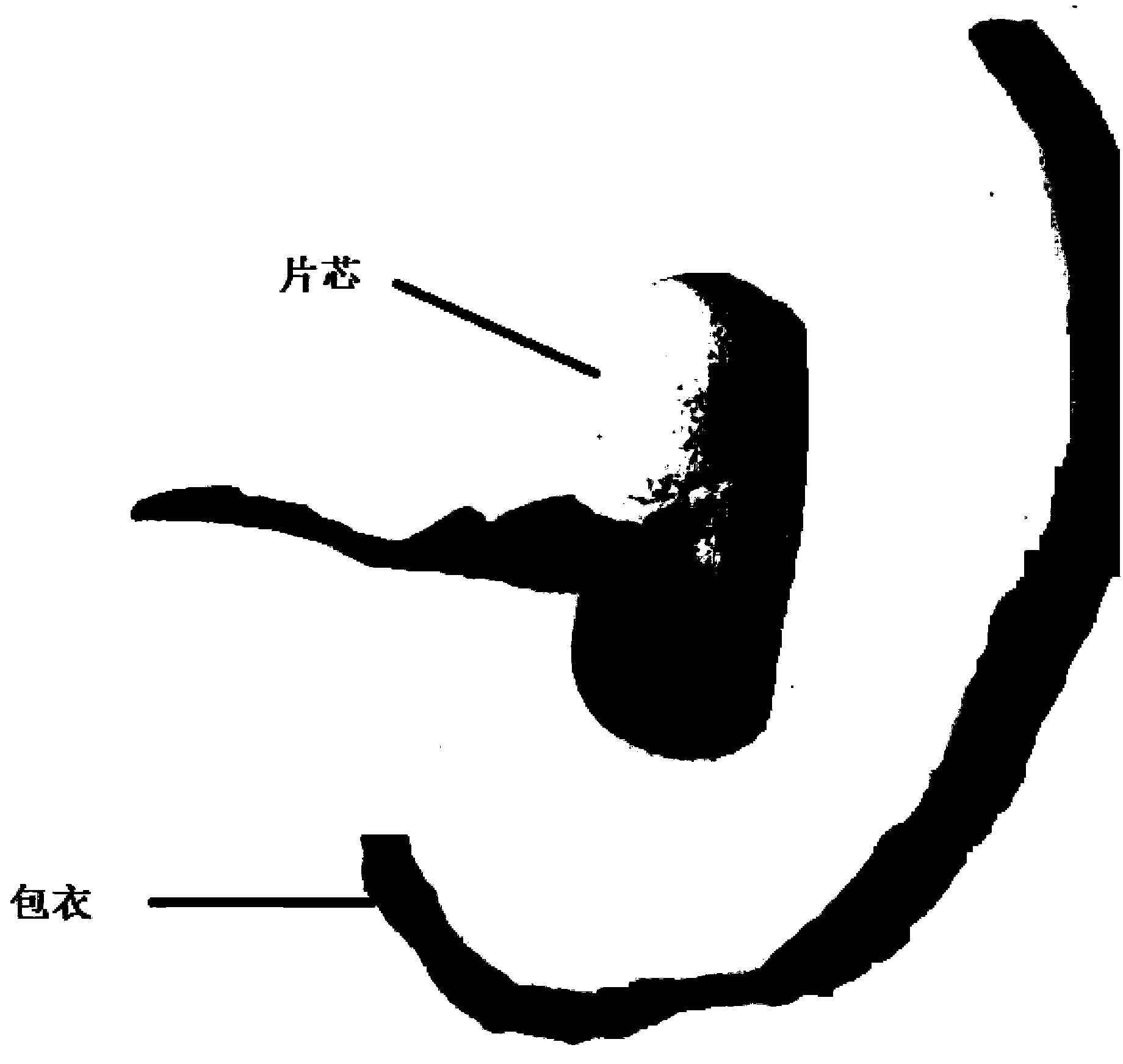
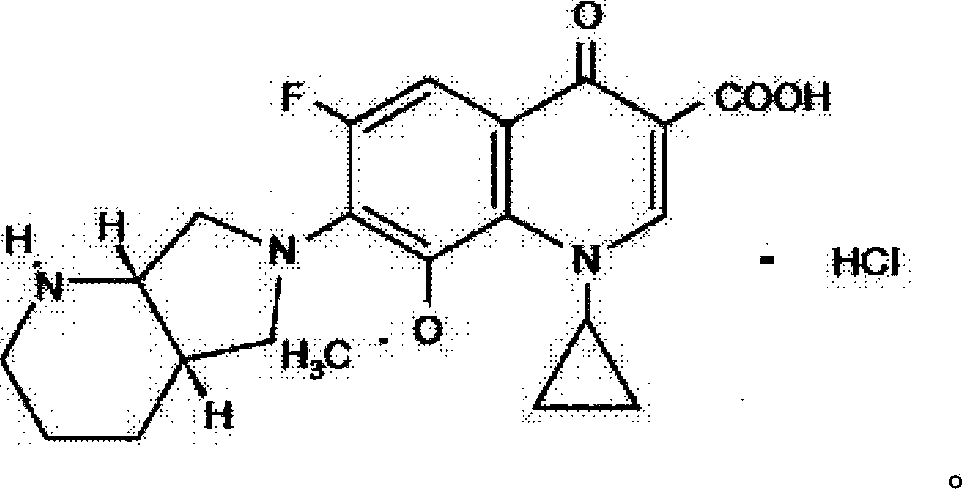
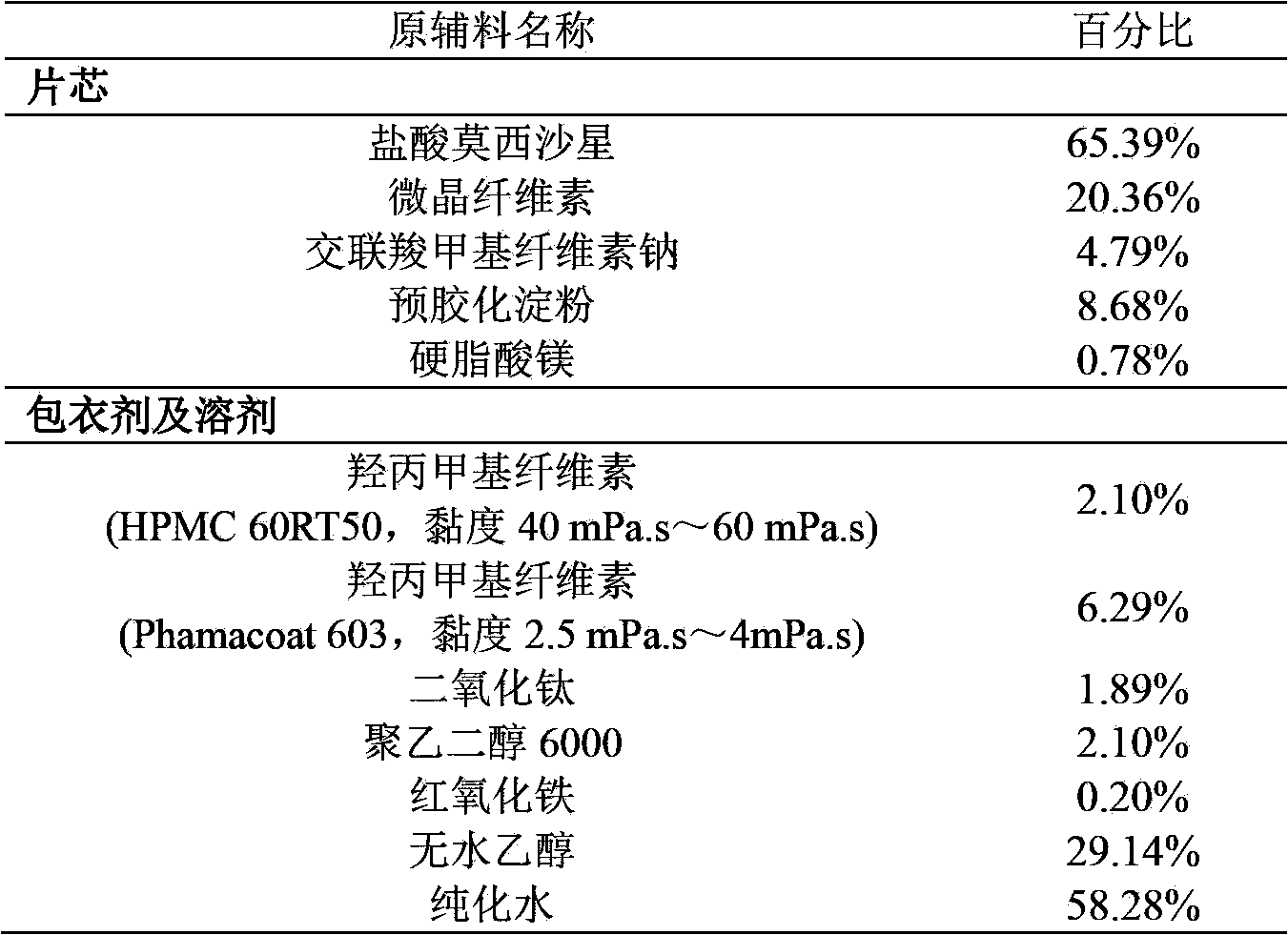
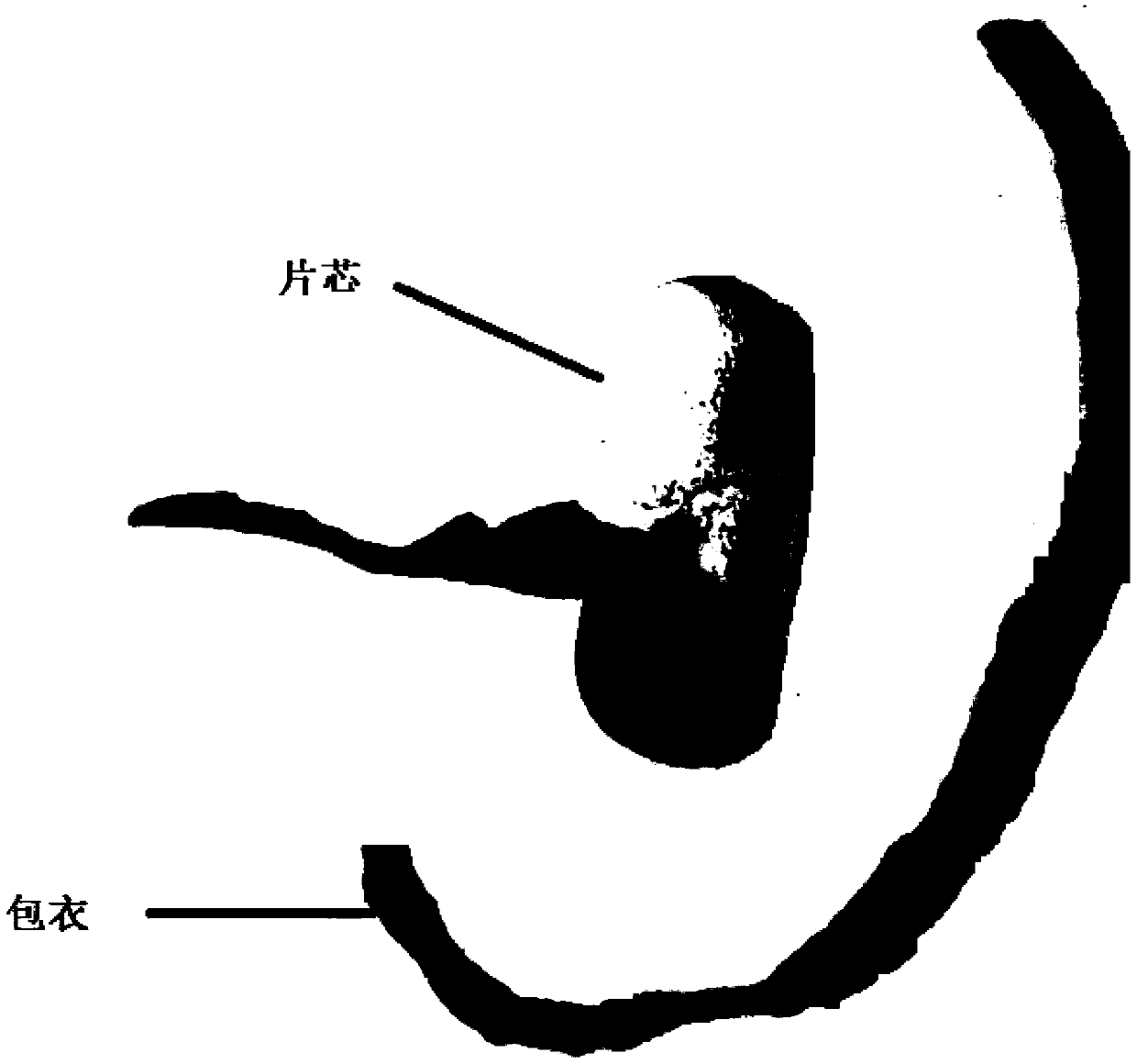
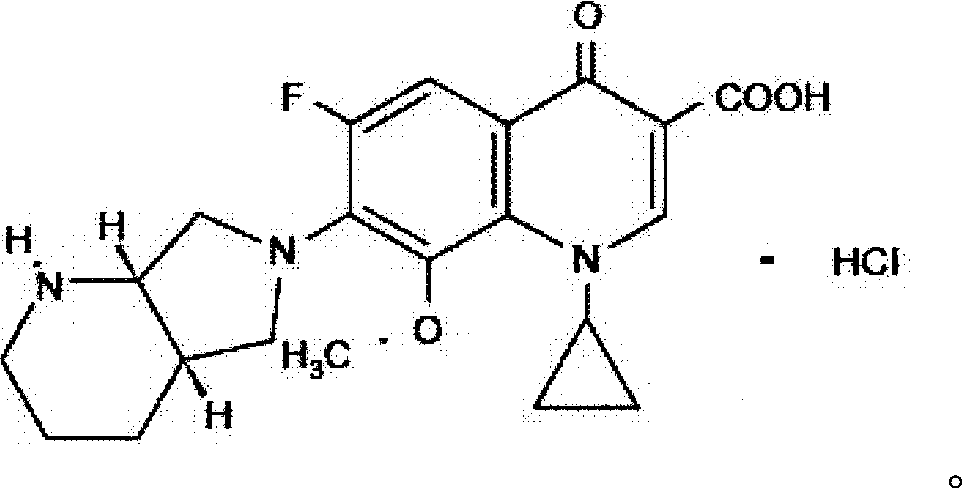
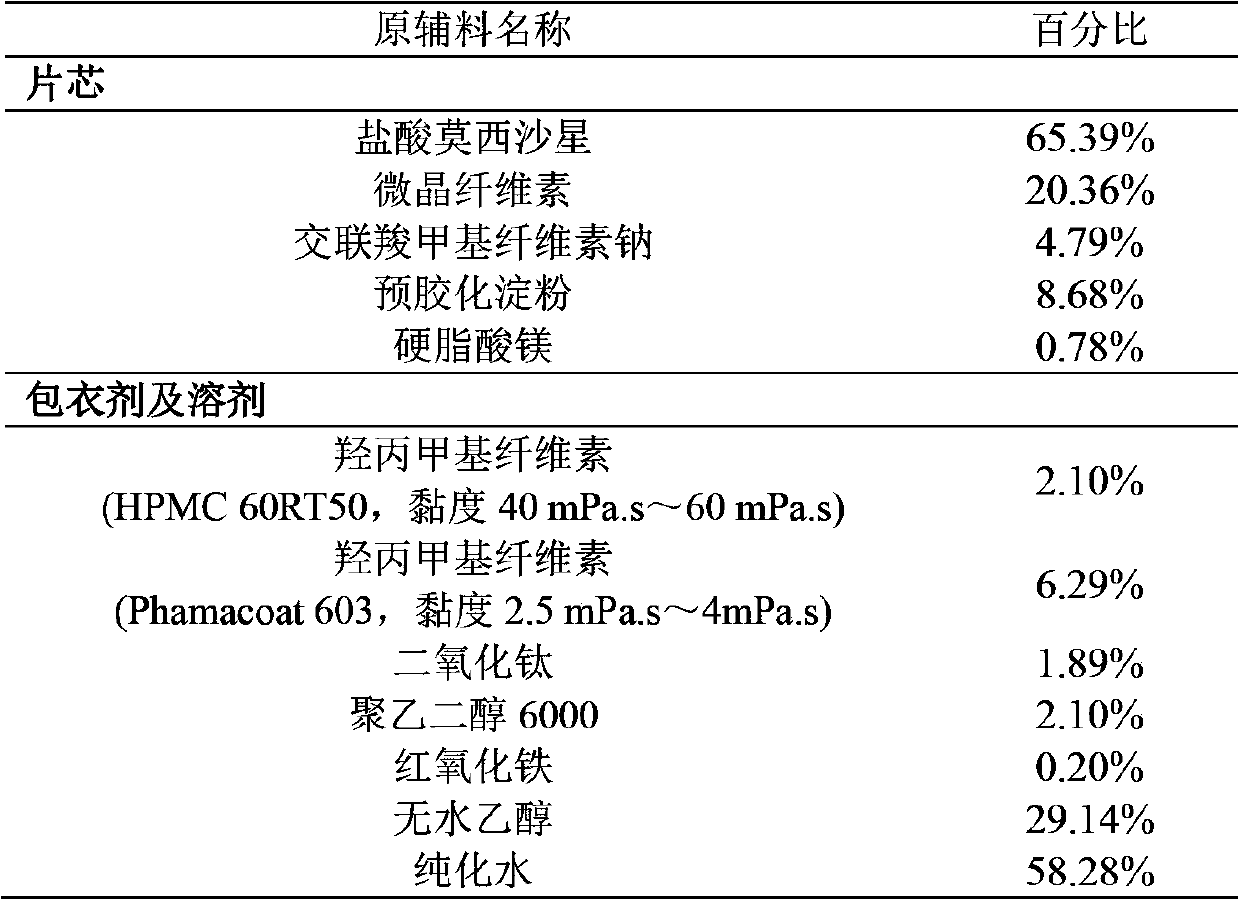
Moxifloxacin hydrochloride pharmaceutical composition and preparation method thereof
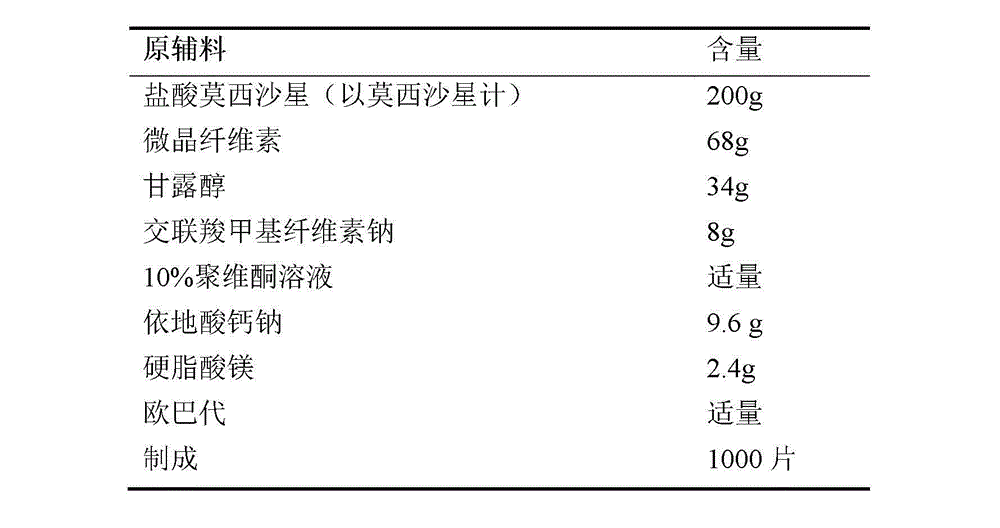
InactiveCN103768063ASave energySave costsAntibacterial agentsOrganic active ingredientsHypromelloseMoxifloxacin hydrochloride
The invention provides a moxifloxacin hydrochloride pharmaceutical composition and preparation method thereof. The invention is characterized in that: tablet core is prepared by moxifloxacin hydrochloride, more than one excipient and a coating material, wherein, the weight of moxifloxacin hydrochloride is 60-70% of that of the tablet core, and the weight of excipients is 30-40% of that of the tablet core, and the weight of the coating material which is used for coating the tablet core and is composed of hypromellose with two or more than two viscosities is 1.0-4.0% of that of the pharmaceutical composition tablet core, and the weight ratio between hypromellose with viscosity of 40-120mPa.s and hypromellose with viscosity of 2.5-1.8mPa.s is 1:5-1:2. The pharmaceutical composition has the advantages of good stability, simple preparation technology and easy reproduction; the preparation method is suitable for industrial production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Composition with improved dissolution property
ActiveCN111467501AGood dissolution propertiesImprove dissolution ratePharmaceutical non-active ingredientsAntineoplastic agentsMedicinal chemistryPolymer
The invention relates to a composition with an improved dissolution property. The composition comprises a salt formed by alkaline tinibs drug molecules and an acidic polymer. The composition with theimproved dissolution property comprises the salt formed by alkaline tinibs drug molecules and the acidic polymer, alkaline tinibs drugs are dispersed in the acidic polymer at a molecular level to forman amorphous solid dispersion, ionic bonds are formed between the alkaline tinibs drug molecules and the acidic polymer, the stability is good, and the dissolution property is improved. The dissolution rate and solubility of the composition are improved, so that the composition can be favorably absorbed by a patient.
Owner:NEOFORM BIOPHARMACEUTICAL LTD
Moxifloxacin-containing pharmaceutical composition
ActiveCN102908323AGood dissolution propertiesHigh hardnessAntibacterial agentsOrganic active ingredientsProcess qualityAdditive ingredient
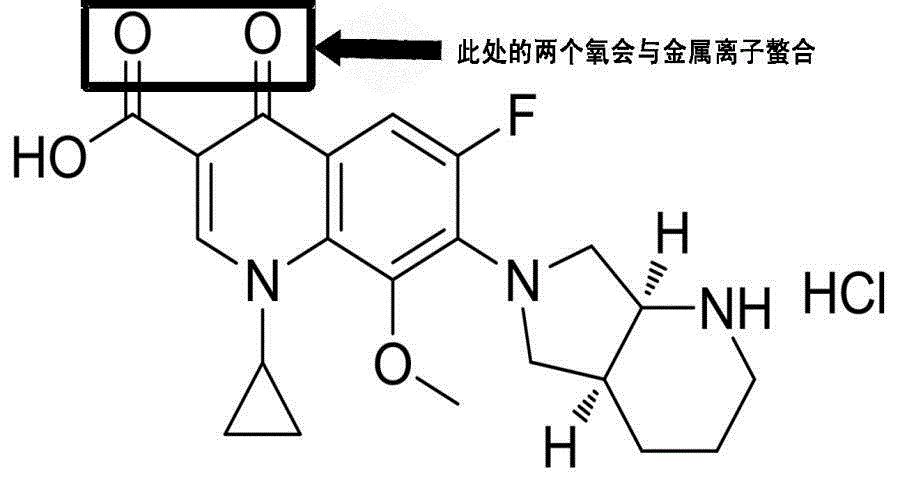
The invention relates to a moxifloxacin-containing pharmaceutical composition which is a solid pharmaceutical composition prepared from moxifloxacin and minor ingredients acceptable pharmaceutically. The composition can be prepared into orally-taken solid preparations according to the technical scheme, and metal ion chelating agents added into a formula avoid generation of red moxifloxacin metal ion chelates during production of the solid preparations, so that stability of moxifloxacin tablets is improved. Besides, the solid preparations have the dissolution behavior and hardness similar to those of imported tablets in the market. The preparation formula and process quality are stable and controllable.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Metformin-glibenclamide capsule and preparation method thereof
InactiveCN101757002AControllable content uniformityGood dissolution propertiesMetabolism disorderSulfonylurea active ingredientsMetformin HydrochlorideGlibenclamide
The invention discloses a metformin-glibenclamide capsule, which is prepared by the following steps: super-finely crushing 250 parts of metformin hydrochloride by weight and 1.25 parts of glibenclamide by weight or 250 parts of the metformin hydrochloride by weight and 2.5 parts of the glibenclamide by weight into superfine powder of 1-100 microns, adding 3-30 parts of disintegrant by weight and 0.15-2 parts of lubricating agent by weight, uniformly mixing, putting into a novel powder feeder and finally filling by using a novel capsule filler. Compared with the prior art, the metformin-glibenclamide capsule prepared by the invention has small weight difference, uniform content, excellent dissolution and stable quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Solid Preparation
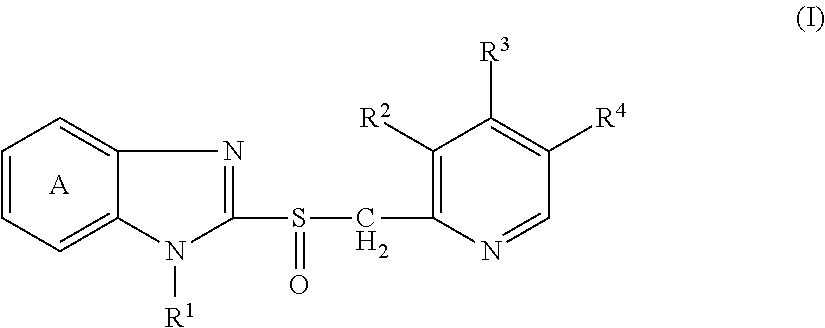
InactiveUS20120115837A1Stability is finely maintainedGood dissolution propertiesBiocideAnimal repellantsAlcohol sugarsMedicinal chemistry
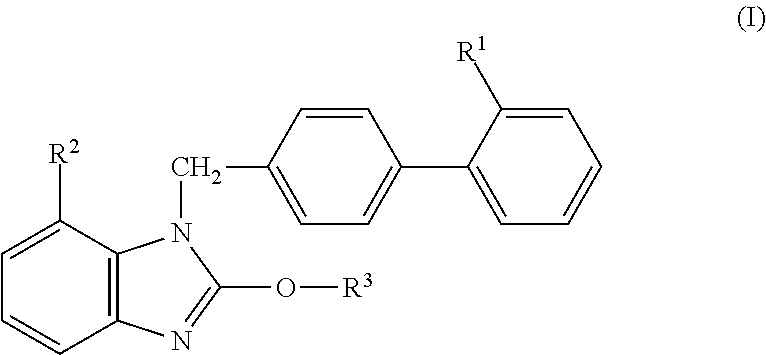
Provided is a solid preparation comprising (i) a compound represented by the formula (I): wherein each symbol is as defined in the specification, or a salt thereof, (ii) a sugar alcohol, and (iii) a calcium antagonist, which is superior in the dissolution property and stability.
Owner:TAKEDA PHARMACEUTICALS CO LTD
Method for improving dissolvability of anticoagulant
ActiveCN102791271AGood dissolution propertiesOrganic active ingredientsPharmaceutical non-active ingredientsBlood Coagulation Factor XAnticoagulant
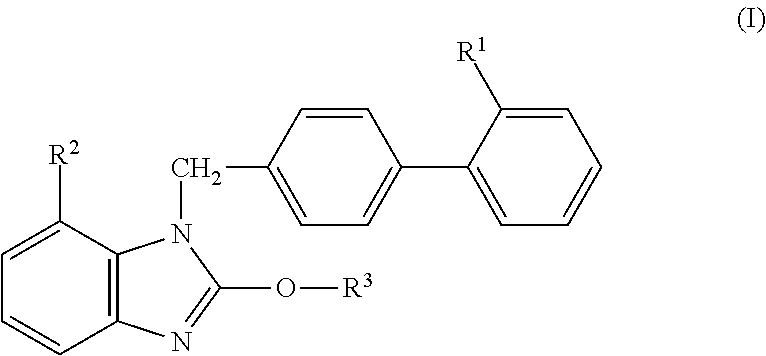
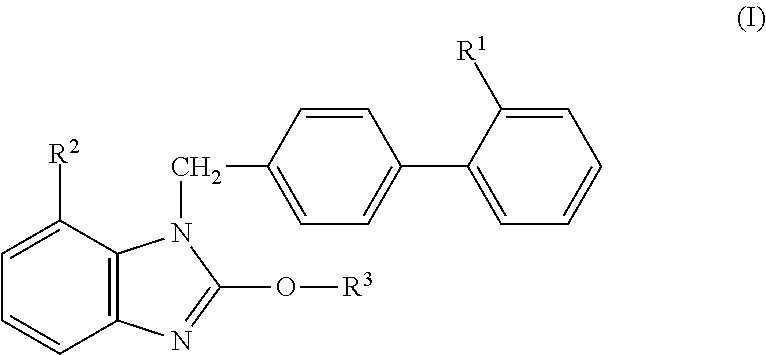
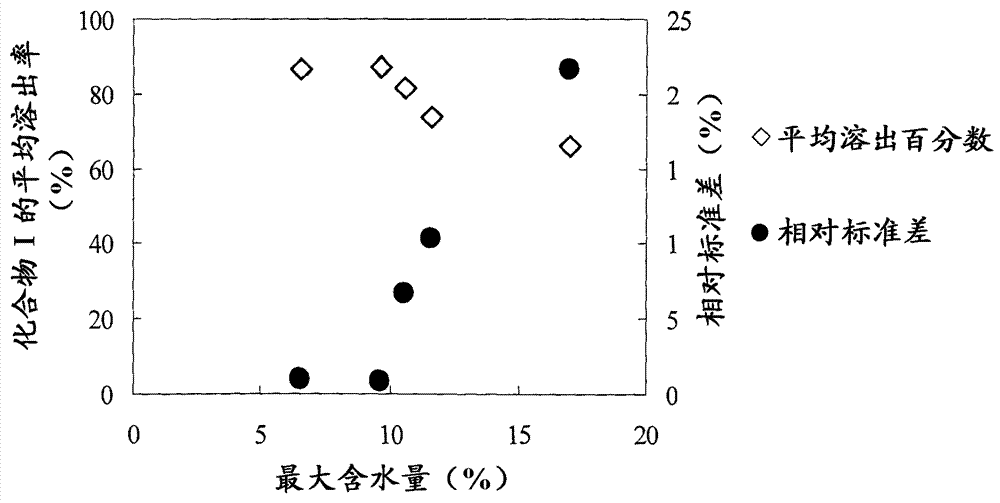
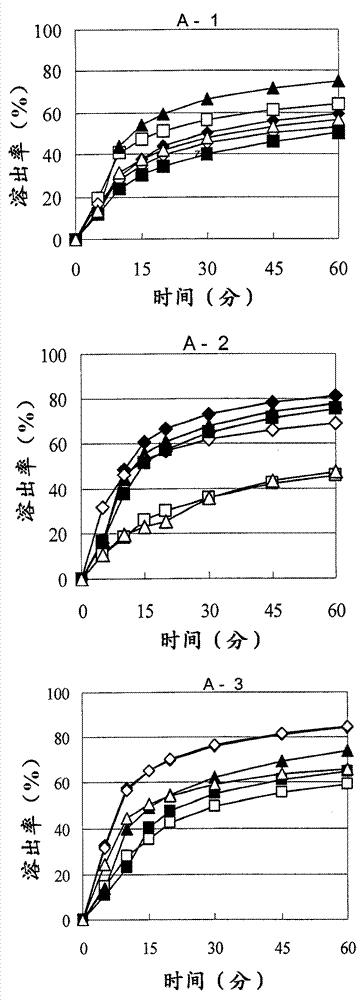
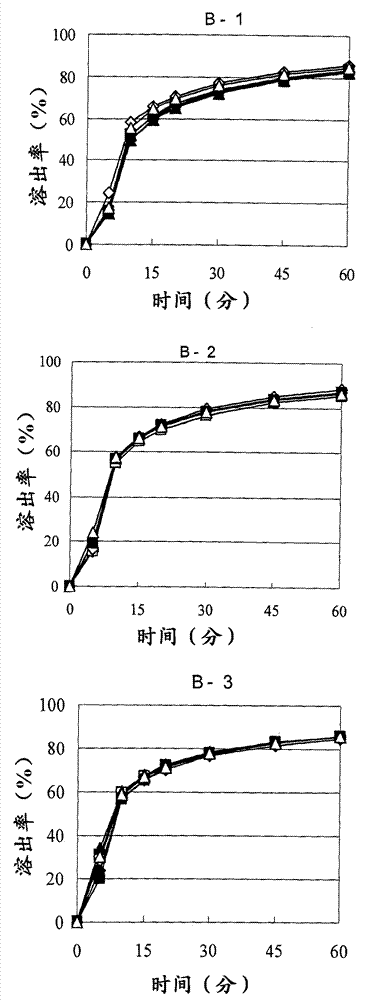
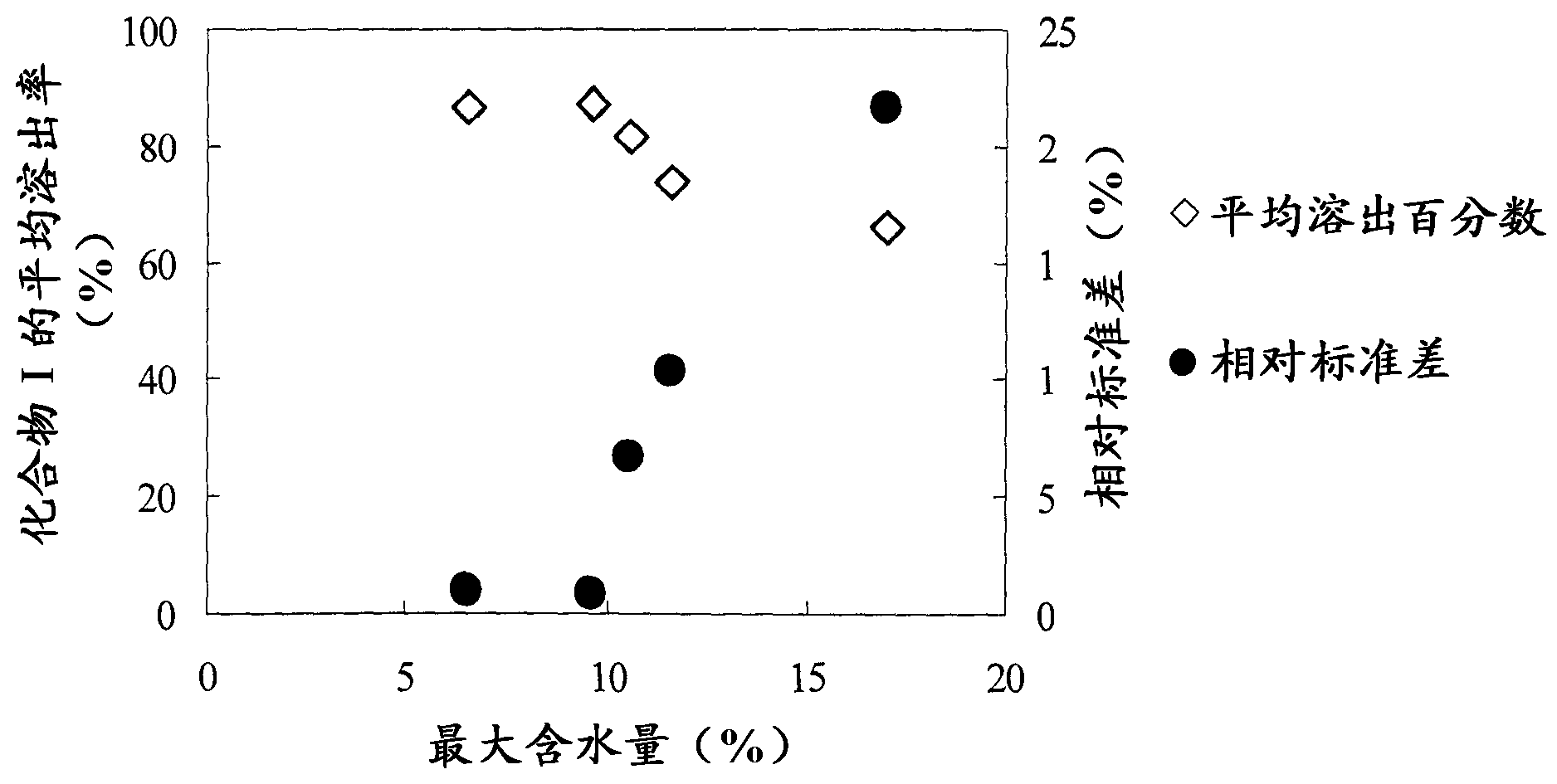
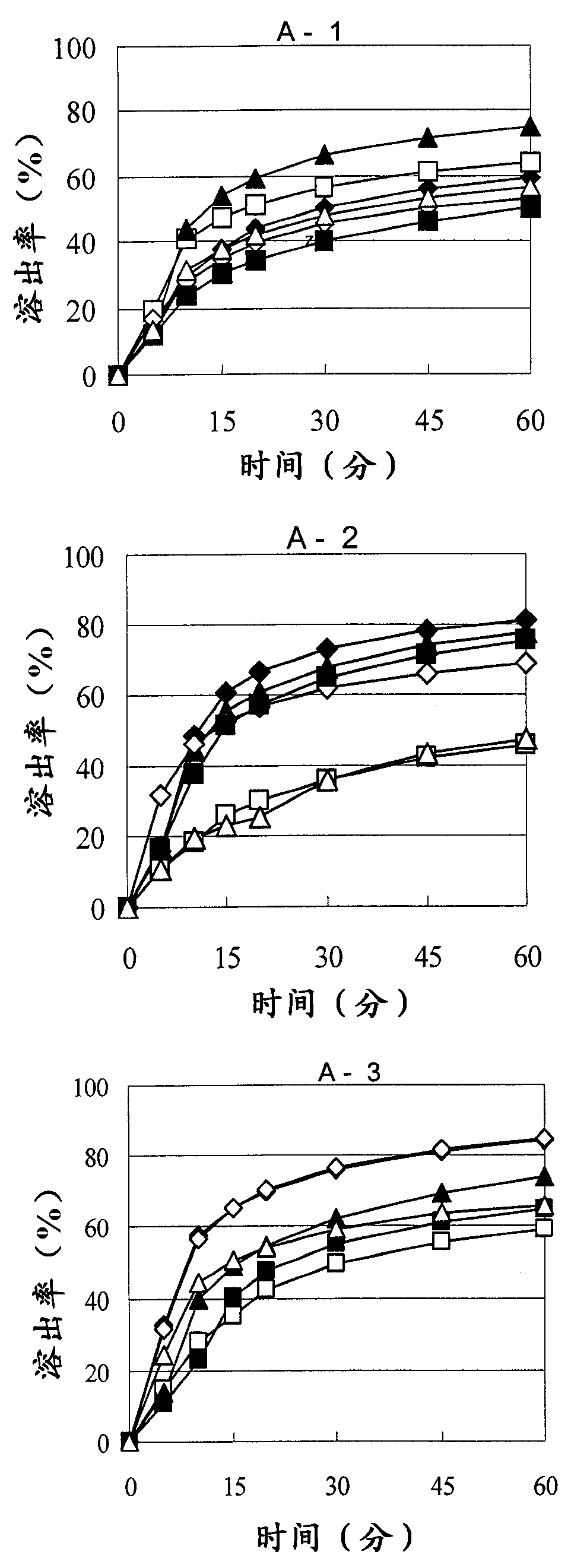
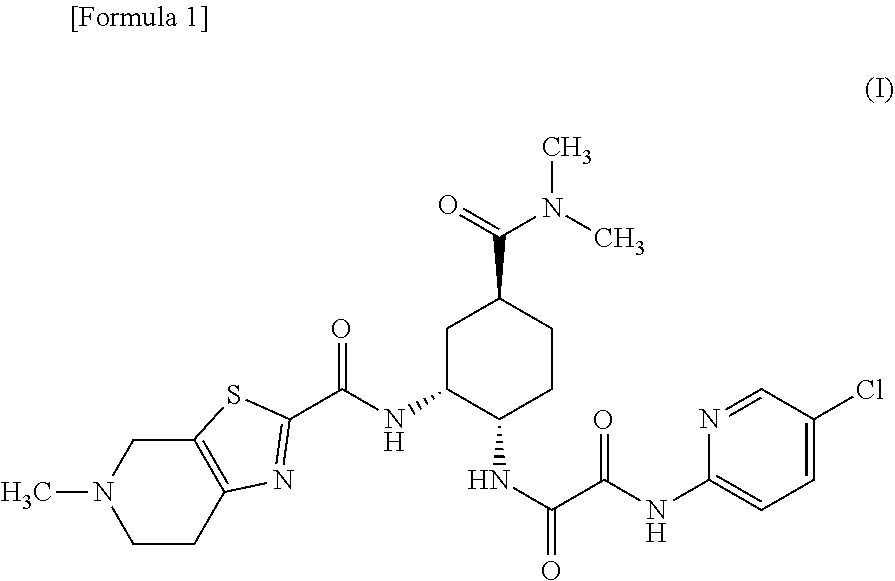
There have been demands for a pharmaceutical composition, which has good dissolvability and contains a compound represented by formula (I), a pharmaceutically acceptable salt thereof or a solvate of the compound or salt, and which has an inhibitory effect on activated blood coagulation factor X and is useful as a prophylactic and / or therapeutic agent for thrombotic diseases. Disclosed is a method for producing a pharmaceutical composition that contains a compound represented by formula (I), which comprises a step wherein a compound represented by formula (I), a pharmaceutically acceptable salt thereof or a solvate of the compound or salt, one or more excipients selected from the group consisting of sugar alcohols and water-swellable additives, a disintegrant and a binder are mixed under such conditions that the maximum water content in a granulated product is maintained at 10% or less during the granulation.
Owner:DAIICHI SANKYO CO LTD
Enzalutamide nano crystal oral solid pharmaceutical composition
InactiveCN108815129AImprove stabilityGood dissolution propertiesOrganic active ingredientsOrganic chemistry methodsSolubilityAdjuvant
The invention relates to an enzalutamide nano crystal oral solid pharmaceutical composition, and a preparation method and applications thereof. The raw materials of enzalutamide are processed by a nano technology to prepare enzalutamide nano crystals, and then an oral solid preparation is prepared from the enzalutamide nano crystals. The test results show that the effect is good. Through a seriesof tests, an enzalutamide nano crystal preparation technology is obtained finally, and enzalutamide nano crystals with good water solubility are obtained. By selecting the adjuvants, the degradation of amido bonds and lactam bonds of bulk drugs is inhibited, and the enzalutamide nano crystals are made into a common oral solid preparation. The test results show that the dissolving-out behavior of the oral solid preparation prepared by the technology is obviously improved and the bioavailability is enhanced at the same time. At the same time, the hydrolysis impurities are inhibited, the stability is improved, the preparation technology is simple and suitable for industrial production, and the quality is controllable.
Owner:天津双硕医药科技有限公司
Cleaning agent for removal of, removal method for, and cleaning method for water-soluble, lead-free solder flux
InactiveUS8877697B2Improve securityMaintain good propertiesPrinted circuit assemblingSurface-active detergent compositionsGlycol ethersWater soluble
Owner:ARAKAWA CHEM IND LTD
Method for improving dissolvability of anticoagulant
ActiveCN102791271BGood dissolution propertiesOrganic active ingredientsPill deliveryBlood Coagulation Factor XAnticoagulant
It is desired to provide a pharmaceutical composition containing a compound represented by formula (I) or a pharmaceutically acceptable salt thereof, or a solvate thereof, which exhibits an inhibitory effect on activated blood coagulation factor X, and is useful as an agent for preventing and / or treating thrombosis, wherein the pharmaceutical composition exhibits favorable dissolution properties. The present invention provides a method for producing a pharmaceutical composition containing a compound represented by formula (I), comprising the step of mixing a compound represented by formula (I) or a pharmaceutically acceptable salt thereof, or a solvate thereof, one or more excipients selected from the group consisting of a sugar alcohol and a water-swelling additive, a disintegrant, and a binder under conditions for keeping the maximum water content of the granules during granulation at 10% or less.
Owner:DAIICHI SANKYO CO LTD
Ezetimibe oral solid pharmaceutical composition
InactiveCN109864981AImprove stabilityGood dissolution propertiesOrganic active ingredientsMetabolism disorderSolubilitySolvent
The invention relates to an ezetimibe oral solid pharmaceutical composition, an inventor finds that an ezetimibe raw material medicine is poor in water solubility and relatively low in bioavailabilityin the research process of an ezetimibe preparation, and meanwhile, after an existing preparation is stored for a long time, an azacyclobutanone structure can be hydrolyzed to generate an open-loop impurity A; the inventor applies a surfactant solubilizing technology to the ezetimibe preparation. Bulk drug, the surfactant and the hydrophilic polymer are jointly dissolved in the solvent; when theezetimibe oral solid preparation is used for preparing a soft material, the raw material medicine and the surfactant are separated out, tightly combined and dispersed in the hydrophilic polymer at thesame time, the solubility of the medicine in water is effectively improved, and test results prove that the dissolution behavior of the ezetimibe oral solid preparation prepared through the technology is obviously improved, so that the bioavailability of the ezetimibe oral solid preparation is also improved. Meanwhile, the hydrolysis impurity A is inhibited, the stability is improved, and the preparation is simple in process, controllable in quality and suitable for industrial production.
Owner:董贵雨
Tablet
ActiveUS20180214460A1Seizure suppressionEasy to carryOrganic active ingredientsNervous disorderPotassiumSalicylic acid
The present invention provides a tablet showing high stability of the active ingredients (potassium-competitive acid blocker and acetylsalicylic acid) and stably and rapidly expressing the pharmacological effects of the active ingredients after administration.The present invention provides a tablet containing an inner core and an outer layer, wherein the inner core is an enteric-coated tablet containing acetylsalicylic acid, and the outer layer contains a potassium-competitive acid blocker free of enteric coating.
Owner:TAKEDA PHARMA CO LTD
Venetoclaxnanocrystal oral solid medicinal composition
InactiveCN108836947AImprove stabilityGood dissolution propertiesOrganic active ingredientsOrganic chemistrySolubilityMedicine
The invention relates to a venetoclaxnanocrystal oral solid medicinal composition and a preparation method and application thereof. The inventor applies a nanotechnology to a venetoclax raw material to prepare a venetoclaxnanocrystal so as to prepare an oral solid preparation. Ultimately, a preparation technology of the venetoclaxnanocrystal is obtained and a venetoclaxnanocrystal raw material with high water solubility is obtained. The venetoclaxnanocrystal raw material is further prepared into an ordinary oral solid preparation. Tests prove that the dissolution behavior of the venetoclax oral solid preparation prepared by the technology is significantly improved, so that the bioavailability is also improved. The preparation technology is simple, the quality is controllable, and the preparation technology is suitable for industrial production.
Owner:天津双硕医药科技有限公司
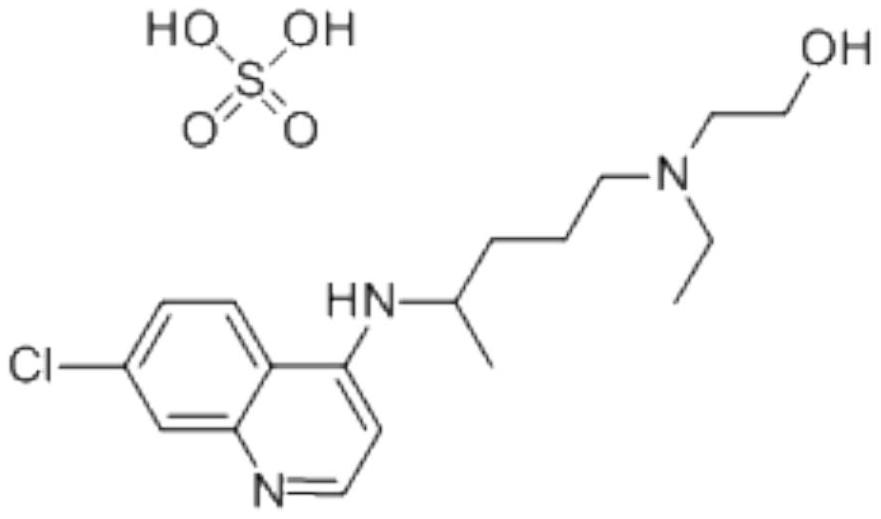
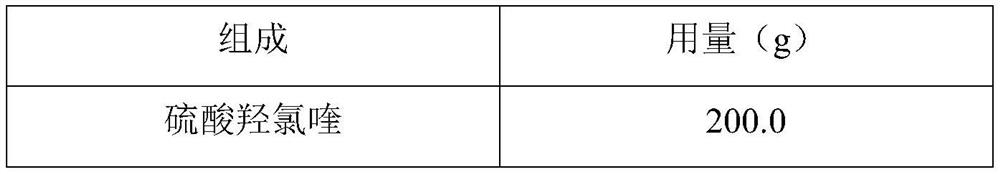
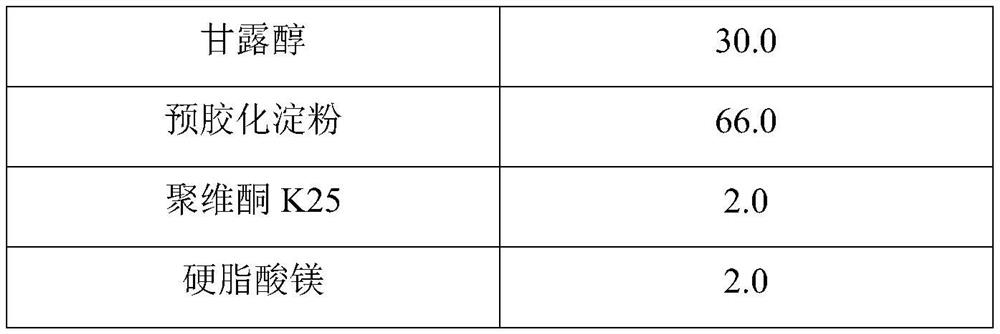
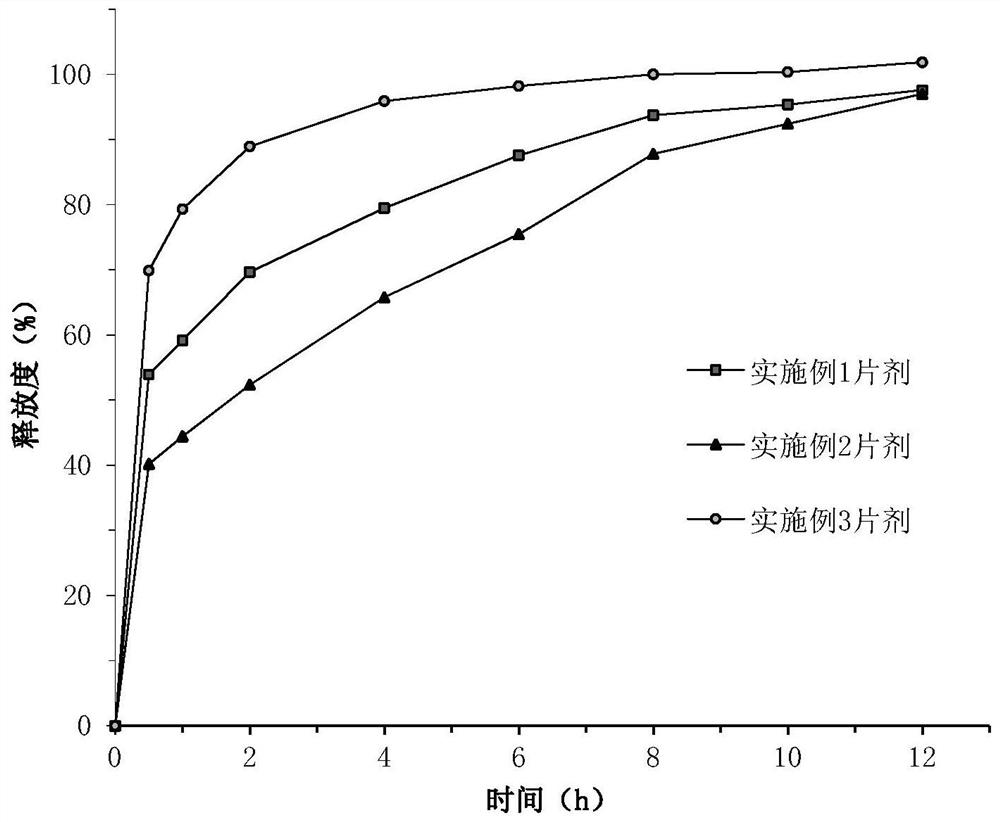
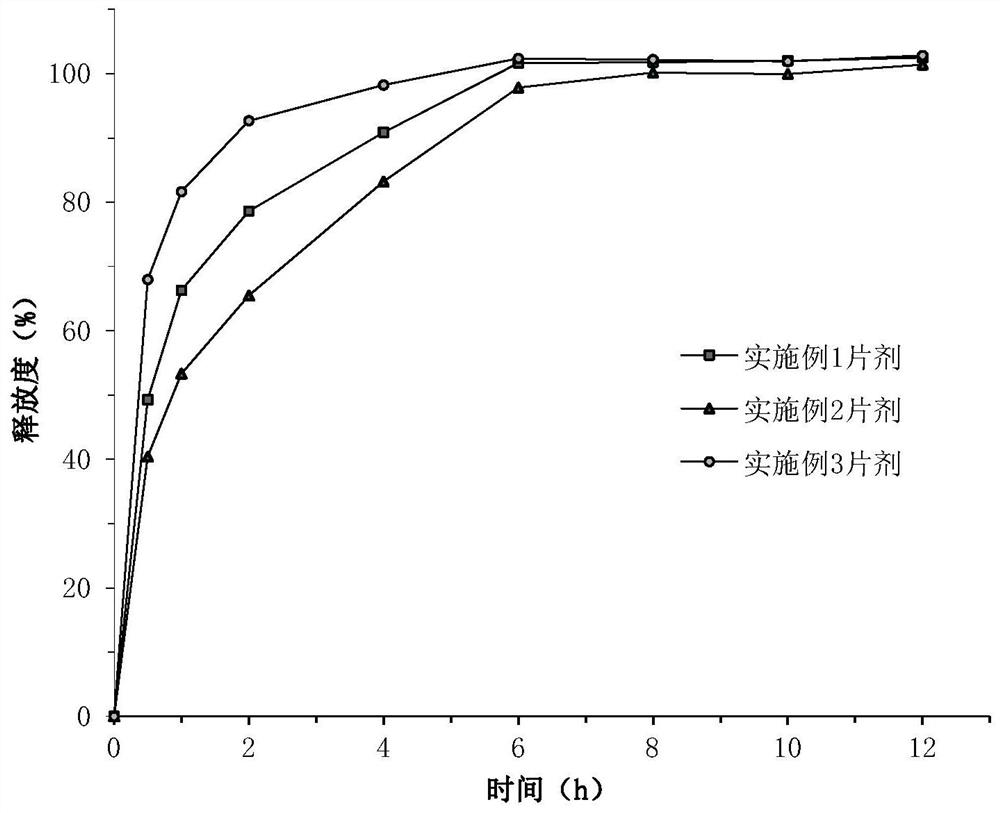
Hydroxychloroquine sulfate pharmaceutical preparation
PendingCN113244180ASolve the problem of easy stickingSolve the problem of poor compressibility caused by high friabilityOrganic active ingredientsAntipyreticDrugs preparationsHydroxychloroquine Sulfate
The invention provides a hydroxychloroquine sulfate pharmaceutical preparation. The hydroxychloroquine sulfate pharmaceutical preparation comprises the following components in percentage by weight: 50-75% of hydroxychloroquine sulfate, 5-15% of mannitol, 15-35% of pregelatinized starch, 0.5-2.0% of a water-soluble adhesive and 0.5-2.0% of a lubricant. According to the hydroxychloroquine pharmaceutical preparation disclosed by the invention, the mannitol and the pregelatinized starch are used as filling agents, and are matched with the adhesive and the lubricant, so that the problem that the hydroxychloroquine pharmaceutical preparation is extremely likely to stick and the problem of poor compressibility caused by high friability of tablets can be solved, the hydroxychloroquine pharmaceutical preparation has good dissolution property, and the dissolution effect is consistent with that of a reference reagent.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Pharmaceutical composition containing nitroxoline lysine salt as well as preparation method and application of the pharmaceutical composition
InactiveCN111773193AGood dissolution propertiesAntiinfectivesPharmaceutical non-active ingredientsNitroxolineQuinoline
The invention discloses a pharmaceutical composition containing nitroxoline lysine salt as well as a preparation method and application of the pharmaceutical composition. The pharmaceutical composition comprises a first layer and a second layer, wherein the first layer comprises 40%-70% of an active pharmaceutical ingredient, 10%-30% of a filling agent, 5%-12% of a disintegrating agent, 0.5%-2% ofa lubricating agent, 0.1%-1.5% of a flow aid and 10%-20% of an alkaline substance based on the total weight of the first layer; the second layer is prepared from the following components in percentage by total weight of the second layer: 40 to 70 percent of active pharmaceutical ingredient, 10 to 30 percent of filling agent, 10 to 35 percent of sustained-release material, 0.1 to 2 percent of lubricating agent and 0.1 to 2 percent of flow aid; and the active pharmaceutical ingredients are selected from one or more of nitroxoline lysine salt, a crystal form of the nitroxoline lysine salt and asolvate of the nitroxoline lysine salt. The pharmaceutical composition has a high dissolution property, and can achieve the purpose of suddenly releasing drugs in the early stage and continuously andslowly releasing drugs in the later stage.
Owner:JIANGSU YAHONG MEDITECH CO LTD +1
Orally disintegrating tablet
InactiveCN103402500AStay acid resistantControl releaseOrganic active ingredientsDigestive systemLansoprazoleControlled release
A orally disintegrating tablet is obtained by tableting fine granules showing controlled release of lansoprazole and an additive, which is capable of suppressing breakage of the fine granules during tableting, and can control the release of lansoprazole for a long time, and can maintain a therapeutically effective concentration for a prolonged time, and shows superior disintegration property in the oral cavity.
Owner:TAKEDA PHARMA CO LTD
Ursolic acid pills and preparation method thereof
InactiveCN102258491BConvenient for clinical operationGood dissolution propertiesPill deliveryPolyethylene glycolUrsolic acid
The invention discloses ursolic acid pills and a preparation method thereof. The ursolic acid pills comprise an ursolic acid active ingredient and a substrate, wherein the substrate is a mixture of polyethylene glycol and poloxamer, and the mass ratio of ursolic acid to substrate is 1:(4-15). Under the cooperative and synergetic effects of different substances, the dissolution property of the ursolic acid pills is greatly improved. The dissolution rate of the prepared ursolic acid pills within 15 minutes can be up to 95% or so; thus, compared with the ursolic acid pills reported at present, the ursolic acid pills disclosed by the invention have the advantages of higher dissolution speed and higher dissolution rate; and the ursolic acid pills disclosed by the invention also have the advantages of simple preparation process, stable quality and favorable forming property, and is suitable for industrial mass production.
Owner:SHANDONG ACAD OF CHINESE MEDICINE +1
An etoposide solid pharmaceutical composition
InactiveCN109200019AImprove stabilityGood dissolution propertiesPowder deliveryOrganic active ingredientsSolubilityWater soluble
The present application relates to an etoposide solid pharmaceutical composition, and the inventors have finally obtained an improved etoposide solid dispersion preparation process through a series ofexperiments, and obtained an improved etoposide solid dispersion with good water solubility. Furthermore, by choosing crosslinked polyvidone with low water content (no more than 5%) as disintegratingagent of solid preparation, the water content of the preparation is effectively reduced while the disintegrating effect is exerted, and the degradation caused by absorbing the water in the preparation is inhibited. Finally, etoposide is prepared into common oral solid preparation. The results showed that the dissolution behavior and bioavailability of etoposide oral solid preparation prepared bythis technique are significantly improved. At the same time, because of choosing the auxiliary material with low water content, the degradation impurity is restrained and the stability is improved. The preparation process is simple and the quality is controllable, which is suitable for industrial production.
Owner:天津双硕医药科技有限公司
3-((L-valyl)amino)-1-propanesulfonic acid crystal form, preparation method and applications thereof
PendingCN111170901AHigh purityHigh thermodynamic stabilityOrganic active ingredientsNervous disorderPropylsulfonic acidAmino radical
The invention relates to a crystal form of a compound 3-((L-valyl)amino)-1-propanesulfonic acid, a preparation method and applications thereof.
Owner:RISEN SUZHOU PHARMA TECH CO LTD
Pharmaceutical composition with improved dissolution properties and apparent solubility and preparation method thereof
ActiveCN113577029AGood dissolution propertiesImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsCaplet Dosage FormPharmaceutical drug
The invention discloses a pharmaceutical composition capable of improving the dissolution property and apparent solubility of insoluble drugs. The pharmaceutical composition comprises insoluble pharmaceutical ingredient particles and a stabilizer, wherein the mass of the insoluble drug component accounts for 0.1-95% of the mass of the whole pharmaceutical composition; and the particle size of the insoluble drug component particles is 10 to 2000 nm. The apparent solubility of the medicine can be remarkably improved through the interaction of the medicine colloid particles and the stabilizer. In addition, the pharmaceutical composition can be directly used as a medicine or used as a medicine intermediate for preparing other medicine dosage forms, such as capsules, tablets, suspensions, granules, inhalants, emulsions, ointments and the like. The invention also discloses a preparation method of the pharmaceutical composition with improved dissolution property and solubility.
Owner:CHINA PHARM UNIV
A kind of moxifloxacin hydrochloride pharmaceutical composition and preparation method thereof
InactiveCN103768063BSave energyGood film formingAntibacterial agentsOrganic active ingredientsMedicineHypromellose
The invention provides a moxifloxacin hydrochloride pharmaceutical composition and preparation method thereof. The invention is characterized in that: tablet core is prepared by moxifloxacin hydrochloride, more than one excipient and a coating material, wherein, the weight of moxifloxacin hydrochloride is 60-70% of that of the tablet core, and the weight of excipients is 30-40% of that of the tablet core, and the weight of the coating material which is used for coating the tablet core and is composed of hypromellose with two or more than two viscosities is 1.0-4.0% of that of the pharmaceutical composition tablet core, and the weight ratio between hypromellose with viscosity of 40-120mPa.s and hypromellose with viscosity of 2.5-1.8mPa.s is 1:5-1:2. The pharmaceutical composition has the advantages of good stability, simple preparation technology and easy reproduction; the preparation method is suitable for industrial production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
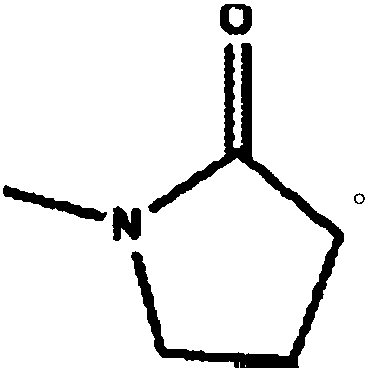
Cilostazol oral solid medicine composition
InactiveCN109157516AImprove stabilityGood dissolution propertiesPowder deliveryOrganic active ingredientsSolubilityWater soluble
The invention relates to a cilostazol oral solid medicine composition. Through research, the inventor obtains an improved cilostazo solid dispersion preparation process and an improved cilostazo soliddispersion with high water solubility as well. In addition, low substituted hydroxypropyl cellulose (of which the water content is less than 5%) of a low water content is selected as a disintegrant of a solid preparation, and the overall water content of the preparation is effectively reduced while a disintegration function is brought into play, so that disintegration caused by the situation thatmoisture in the preparation is absorbed by a raw material medicine can be inhibited. Finally, cilostazol is prepared into a common oral solid preparation. Tests show that the dissolution behavior ofa cilostazol oral solid preparation prepared by using the technique is remarkably improved, and furthermore, the bioavailability of the preparation can be also improved. Meanwhile, because of a low overall water content of auxiliary materials, degradation impurities caused by hydrolysis, of the raw material medicine, can be inhibited, the stability can be improved, and the preparation is simple inprocess, controllable in quality and applicable to industrial production.
Owner:天津双硕医药科技有限公司
A kind of ursolic acid dropping pill and preparation method thereof
InactiveCN102258491AConvenient for clinical operationGood dissolution propertiesAntibacterial agentsOrganic active ingredientsPolythylene glycolUrsolic acid
The invention discloses ursolic acid pills and a preparation method thereof. The ursolic acid pills comprise an ursolic acid active ingredient and a substrate, wherein the substrate is a mixture of polyethylene glycol and poloxamer, and the mass ratio of ursolic acid to substrate is 1:(4-15). Under the cooperative and synergetic effects of different substances, the dissolution property of the ursolic acid pills is greatly improved. The dissolution rate of the prepared ursolic acid pills within 15 minutes can be up to 95% or so; thus, compared with the ursolic acid pills reported at present, the ursolic acid pills disclosed by the invention have the advantages of higher dissolution speed and higher dissolution rate; and the ursolic acid pills disclosed by the invention also have the advantages of simple preparation process, stable quality and favorable forming property, and is suitable for industrial mass production.
Owner:SHANDONG ACAD OF CHINESE MEDICINE +1
Anti-pulmonary fibrosis composition with improved dissolution properties
ActiveCN111973596BGood dissolution propertiesImprove solubilityPowder deliveryOrganic active ingredientsOral medicationPulmonary fibroses
The present invention relates to an anti-pulmonary fibrosis composition with improved dissolution properties, comprising a salt formed of nintedanib and an acidic polymer. The salt formed by nintedanib and acidic polymer has higher solubility and certain stability. The composition of the invention can be used for oral administration, and helps the dissolution and absorption of drugs in the gastrointestinal tract.
Owner:NEOFORM BIOPHARMACEUTICAL LTD
A kind of rosiglitazone saccharin salt and preparation method thereof
ActiveCN109053718BGood dissolution propertiesGreat tasteOrganic active ingredientsMetabolism disorderPharmaceutical industryPhysical chemistry
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Granules containing diamine derivative
ActiveUS20210267954A1Improve solubilityReduce bitternessOrganic active ingredientsDispersion deliveryCarmellose SodiumCellulose
Provision of a granular preparation that contains edoxaban or a pharmacologically acceptable salt thereof, and has the property of being rapidly dissolved or suspended by the addition of water. A granular preparation comprising first granules containing (A) edoxaban or a pharmacologically acceptable salt thereof, (B) a sugar alcohol, and (C) a water-swelling additive, and second granules containing (D) 0.5 to 10% by weight of carmellose sodium with respect to the total weight of the preparation, and (E) 70 to 90% by weight of xylitol or sorbitol with respect to the total weight of the preparation.
Owner:DAIICHI SANKYO CO LTD
Weight losing tablet containing orlistat
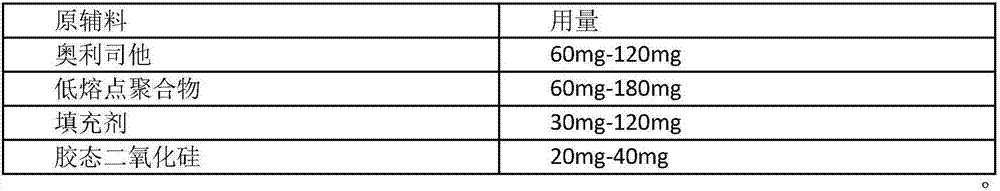
InactiveCN107412176AHigh dissolution rateAvoid thermal decompositionOrganic active ingredientsMetabolism disorderOrlistatDecomposition problem
The invention belongs to the technical field of medicines and particularly relates to a weight losing tablet containing orlistat and a preparation method thereof. A low-temperature hot-melt extrusion and cutting technology is designed by the inventor and used for preparing the orlistat tablet. In this way, an orlistat raw medicine is molten with a low-melting-point polymer jointly beside the melting point of the orlistat raw medicine to form a dispersion body, compared with technologies such as micronization, the dissolution degree of a final preparation is greatly increased, and meanwhile owing to the fact that low-temperature melting is conducted and water is not involved, medicine wet and heat decomposition is avoided; owing to the fact that a conventional tabletting technology is not adopted, the problem of sticking of the conventional tabletting technology does not exist; and finally, whole mixing is conducted, the melting process is conducted in a sealed cavity of a screw extruder and does not make contact with air, and therefore the heat decomposition problem in the air is avoided.
Owner:天津双硕医药科技有限公司
Pharmaceutical composition containing nitroxyquinoline prodrug and its preparation method and application
ActiveCN111514142BImprove stabilityGood dissolution propertiesInorganic non-active ingredientsAntiinfectivesAcyl groupPharmaceutical drug
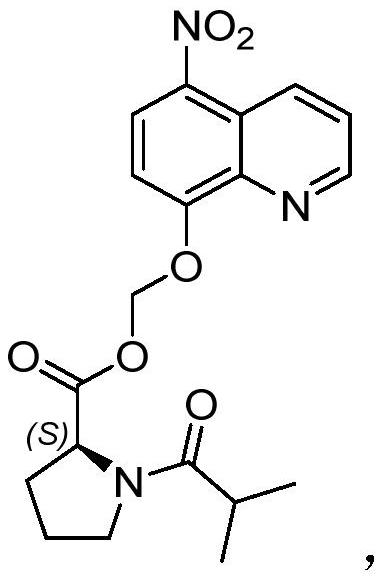
The invention discloses a pharmaceutical composition containing nitroxyquinoline prodrug, a preparation method and application thereof. The pharmaceutical composition comprises the following components in parts by weight: 100 parts of active pharmaceutical ingredients, 22.5-320 parts of fillers, 0-40 parts of disintegrants, 0-95 parts of binders and 2-30 parts of lubricants; Relative to every 100 parts by weight of the active pharmaceutical ingredient, the total amount of the filler, the disintegrant and the binder is 54-345 parts by weight; the active pharmaceutical ingredient is (S)-(5- Nitroquinoline-8-yloxy)methyl 1-isopropionylpyrrolidine-2-carboxylate. The pharmaceutical composition has good stability and dissolution properties, and has good pharmacokinetic characteristics, and can be advantageously used in the preparation and clinical application of medicines.
Owner:JIANGSU YAHONG MEDITECH CO LTD +1
Compositions with improved dissolution properties
ActiveCN111467501BGood dissolution propertiesImprove dissolution ratePharmaceutical non-active ingredientsAntineoplastic agentsMedicineBiology
The present invention relates to a composition with improved dissolution properties, comprising a salt formed of a basic tinib drug molecule and an acidic polymer. The composition with improved dissolution properties comprises a salt formed of a basic tinib drug molecule and an acidic polymer, the basic tinib drug is dispersed in the acidic polymer at a molecular level to form an amorphous solid dispersion, and the base An ionic bond is formed between the tinib drug molecule and the acidic polymer, which has good stability, improves the dissolution rate and solubility of the composition, and is beneficial to be absorbed by patients.
Owner:NEOFORM BIOPHARMACEUTICAL LTD
A kind of eutectic of 5-fluorouracil and kaempferol and preparation method thereof
ActiveCN112209887BLow hygroscopicityGood dissolution propertiesOrganic active ingredientsOrganic chemistry methodsSolubilityPharmaceutical drug
The invention discloses a co-crystal of 5-fluorouracil and kaempferol and a preparation method thereof. The molar ratio of 5-fluorouracil and kaempferol in the eutectic is 1:1, and the X-ray powder diffraction pattern of the eutectic is 12.2±0.2°, 13.9±0.2°, 14.8±0.2°, 16.5±0.2°, There are characteristic peaks at 17.5±0.2° and 26.7±0.2°. The eutectic preparation method provided by the invention has simple process, easy control of the crystallization process, good reproducibility, and is suitable for industrial production. This co-crystal can effectively reduce the hygroscopicity of kaempferol, optimize the dissolution properties of 5-fluorouracil and kaempferol, reduce the difference in solubility between the two, improve the compatibility of the preparations of the two drugs, and provide better performance for the two drugs. Anti-tumor synergy provides a research basis.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com