Enzalutamide nano crystal oral solid pharmaceutical composition
A technology for enzalutamide and nanocrystals, which is applied in the field of enzalutamide nanocrystal oral solid pharmaceutical compositions and the preparation thereof, can solve the problems of easy adhesion, high labor intensity, affecting product quality and yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
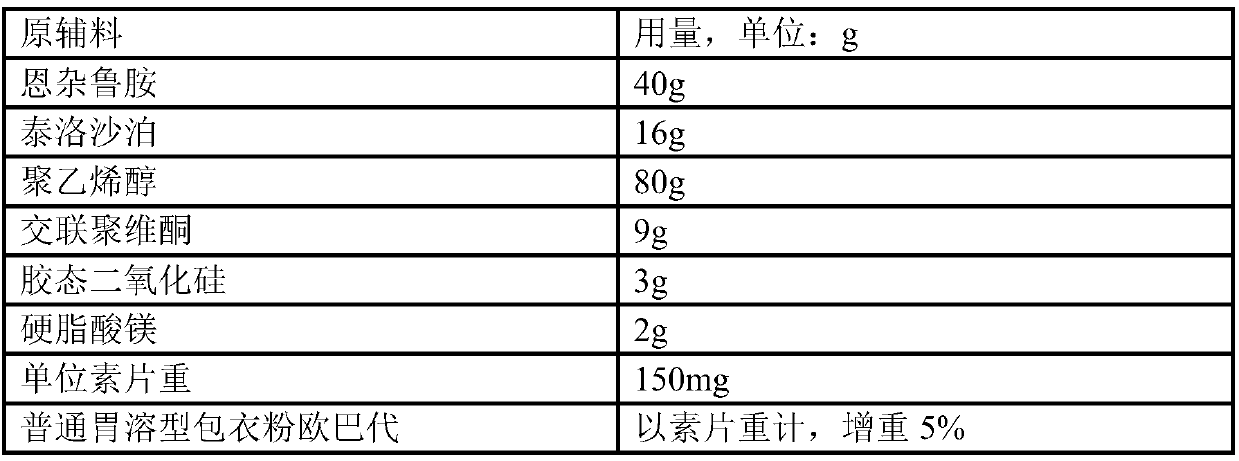
[0110] Example 1 Preparation of 40 mg Enzalutamide Tablets (Unit: g)
[0111] prescription:
[0112]
[0113] The pharmaceutical composition of the enzalutamide nanocrystals is further prepared into tablets by the following steps:
[0114] 1) Take the enzalutamide bulk drug and pulverize it, pass it through a 200-mesh sieve, and set aside;
[0115] 2) Take tyloxapol and polyvinyl alcohol and dissolve them in purified water successively, the concentrations are 2% and 10%;
[0116] 3) Disperse the enzalutamide bulk drug obtained in step 1) in the solution obtained in step 1), with a concentration of 5%;
[0117] 4) Add the suspension obtained in step 3) into a high-pressure homogenizer, and circulate it 7 times under a pressure of 100 bar to obtain a suspension of enzalutamide nanocrystals;
[0118] 5) Take the enzalutamide nanocrystal suspension obtained in step 4), and spray dry to obtain the enzalutamide nanocrystal;
[0119] 6) Take the enzalutamide nanocrystals obtai...
Embodiment 2
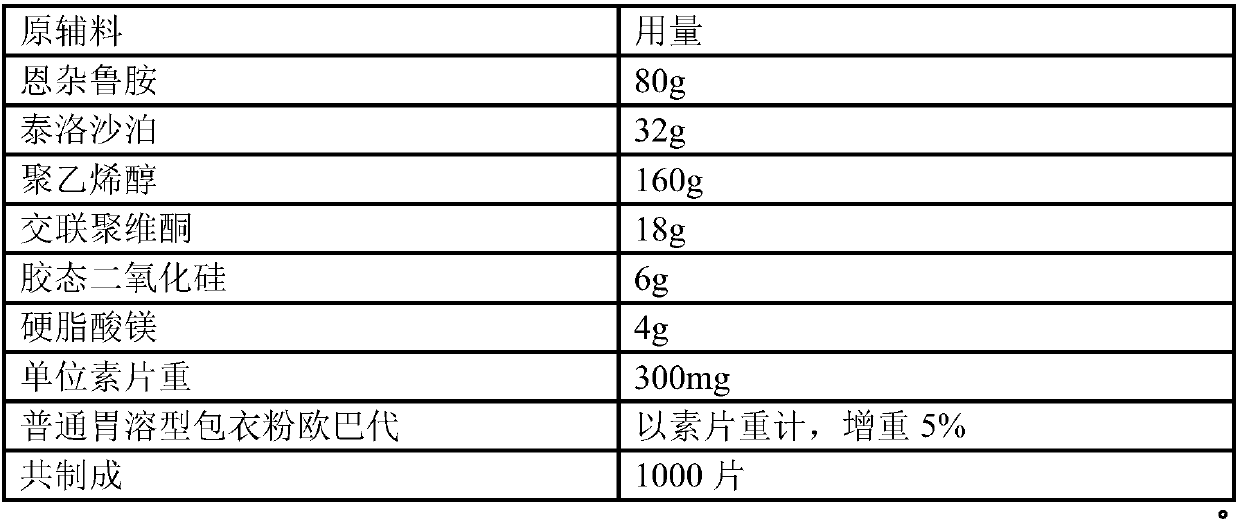
[0121] Example 2 Preparation of 80 mg Enzalutamide Tablets (Unit: g)
[0122] prescription:
[0123]
[0124]
[0125] The pharmaceutical composition of the enzalutamide nanocrystals is further prepared into tablets by the following steps:
[0126] 1) Take the enzalutamide bulk drug and pulverize it, pass it through a 200-mesh sieve, and set aside;
[0127] 2) Take tyloxapol and polyvinyl alcohol and dissolve them in purified water successively, the concentrations are 2% and 10%;
[0128] 3) Disperse the enzalutamide bulk drug obtained in step 1) in the solution obtained in step 1), with a concentration of 5%;
[0129] 4) Add the suspension obtained in step 3) into a high-pressure homogenizer, and circulate it 7 times under a pressure of 100 bar to obtain a suspension of enzalutamide nanocrystals;
[0130] 5) Take the enzalutamide nanocrystal suspension obtained in step 4), and spray dry to obtain the enzalutamide nanocrystal;
[0131] 6) Take the enzalutamide nanocr...
Embodiment 3
[0133] Example 3 Preparation of 160 mg Enzalutamide Tablets (Unit: g)
[0134] prescription:
[0135]
[0136] The pharmaceutical composition of the enzalutamide nanocrystals is further prepared into tablets by the following steps:
[0137] 1) Take the enzalutamide bulk drug and pulverize it, pass it through a 200-mesh sieve, and set aside;
[0138] 2) Take tyloxapol and polyvinyl alcohol and dissolve them in purified water successively, the concentrations are 2% and 10%;
[0139] 3) Disperse the enzalutamide bulk drug obtained in step 1) in the solution obtained in step 1), with a concentration of 5%;
[0140] 4) Add the suspension obtained in step 3) into a high-pressure homogenizer, and circulate it 7 times under a pressure of 100 bar to obtain a suspension of enzalutamide nanocrystals;
[0141] 5) Take the enzalutamide nanocrystal suspension obtained in step 4), and spray dry to obtain the enzalutamide nanocrystal;
[0142] 6) Take the enzalutamide nanocrystals obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com